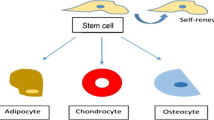
Abstract
The constantly evolving field of regenerative medicine deals with replacing, repairing, or regenerating tissue by using multidisciplinary scientific fields for humans and animals suffering from various injuries to severe diseases. Tissue engineering takes cell biology, materials science, and engineering principles to replace or repair damaged tissues. The scale of stem cell applications in regenerative medicine has increased extensively to develop various clinical-based treatments and potential stem cell-based therapies. This chapter provides an overview of different stem cell sources and their self-renewal, differentiation mechanisms in the regenerative medicine field with the importance of proteomics analyses to understand the stem cell biological processes. Additionally, the importance of biomaterials selection for stem cell-based regenerative medicine and tissue engineering applications is also discussed in this chapter.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Mason C, Dunnill P (2008) A brief definition of regenerative medicine. Regen Med 3:1–5
Vallée M, Côté JF, Fradette J (2009) Adipose-tissue engineering: taking advantage of the properties of human adipose-derived stem/stromal cells. Pathol Biol 57:309–317
Noël D, Caton D, Roche S et al (2008) Cell specific differences between human adipose-derived and mesenchymal-stromal cells despite similar differentiation potentials. Exp Cell Res 314:1575–1584
Pisu M, Concas A, Cao G (2007) A novel simulation model for stem cells differentiation. J Biotechnol 130:171–182
Gomillion CT, Burg KJ (2006) Stem cells and adipose tissue engineering. Biomaterials 27:6052–6063
Bonilla S, Silva A, Valdés L et al (2005) Functional neural stem cells derived from adult bone marrow. Neuroscience 133:85–95
Cancedda R, Dozin B, Giannoni P et al (2003) Tissue engineering and cell therapy of cartilage and bone. Matrix Biol 22:81–91
Jiang Y, Jahagirdar BN, Reinhardt RL et al (2002) Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418:41–49
Majumdar MK, Wang E, Morris EA (2001) BMP-2 and BMP-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of IL-1. J Cell Physiol 189:275–284
Reya T, Morrison SJ, Clarke MF et al (2001) Stem cells, cancer, and cancer stem cells. Nature 414:105–111
Devine SM, Hoffman R (2000) Role of mesenchymal stem cells in hematopoietic stem cell transplantation. Curr Opin Hematol 7:358–363
Furth ME, Atala A, Van Dyke ME (2007) Smart biomaterials design for tissue engineering and regenerative medicine. Biomaterials 28:5068–5073
Casteilla L, Dani C (2006) Adipose tissue-derived cells: from physiology to regenerative medicine. Diabetes Metab 32:393–401
Jagur-Grodzinski J (2006) Polymers for tissue engineering, medical devices, and regenerative medicine. Concise general review of recent studies. Polym Adv Technol 17:395–418
Thomas V, Dean DR, Vohra YK (2006) Nanostructured biomaterials for regenerative medicine. Curr Nanosci 2:155–177
Lutolf MP, Hubbell JA (2005) Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol 23:47–55
de Wert G, Mummery C (2003) Human embryonic stem cells: research, ethics and policy. Hum Reprod 18:672–682
Frontini-López YR, Gojanovich AD, Masone D et al (2018) Adipose-derived mesenchymal stem/stromal cells: from the lab bench to the basic concepts for clinical translation. Biocell 42:67–78
Evans MJ, Kaufman MH (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature 292:154–156
Spradling A, Drummond-Barbosa D, Kai T (2001) Stem cells find their niche. Nature 414:98–104
Pera MF, Reubinoff B, Trounson A (2000) Human embryonic stem cells. J Cell Sci 113:5–10
Bodnar AG, Ouellette M, Frolkis M et al (1998) Extension of life-span by introduction of telomerase into normal human cells. Science 279:349–352
Thomson JA, Itskovitz-Eldor J, Shapiro SS et al (1998) Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147
Gogarty B, Nicol D, Chalmers D (2002) Regulating biomedical advances: embryonic stem cell research. Macquarie Law J 2:31–59
Okita K, Ichisaka T, Yamanaka S (2007) Generation of germline-competent induced pluripotent stem cells. Nature 448:313–317
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676
Hockemeyer D, Jaenisch R (2016) Induced pluripotent stem cells meet genome editing. Cell Stem Cell 18:573–586
Friedenstein AJ, Chailakhjan RK, Lalykina KS (1970) The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet 3:393–403
Phinney DG, Prockop DJ (2007) Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells 25:2896–2902
Kargozar S, Mozafari M, Hamzehlou S et al (2019) Bone tissue engineering using human cells: a comprehensive review on recent trends, current prospects, and recommendations. Appl Sci 9:174
Tsuji W, Rubin JP, Marra KG (2014) Adipose-derived stem cells: Implications in tissue regeneration. World J Stem Cells 6:312–321
Feisst V, Meidinger S, Locke MB (2015) From bench to bedside: use of human adipose-derived stem cells. Stem Cells Cloning 8:149–162
Argentati C, Morena F, Montanucci P et al (2018) Surface hydrophilicity of poly(l-lactide) acid polymer film changes the human adult adipose stem cell architecture. Polymers 10:140
Romagnoli C, Zonefrati R, Galli G et al (2015) In vitro behavior of human adipose tissue-derived stem cells on poly(ε-caprolactone) film for bone tissue engineering applications. Biomed Res Int 2015:323571
Haimi S, Suuriniemi N, Haaparanta AM et al (2009) Growth and osteogenic differentiation of adipose stem cells on PLA/bioactive glass and PLA/beta-TCP scaffolds. Tissue Eng Part A 15:1473–1480
Frese L, Dijkman PE, Hoerstrup SP (2016) Adipose tissue-derived stem cells in regenerative medicine. Transfus Med Hemother 43:268–274
Ullah I, Subbarao RB, Rho GJ (2015) Human mesenchymal stem cells—current trends and future prospective. Biosci Rep 35:e00191
Bunnell BA, Flaat M, Gagliardi C et al (2008) Adipose-derived stem cells: isolation, expansion and differentiation. Methods 45:115–120
Zhang HT, Liu ZL, Yao XQ et al (2012) Neural differentiation ability of mesenchymal stromal cells from bone marrow and adipose tissue: a comparative study. Cytotherapy 14:1203–1214
Technau A, Froelich K, Hagen R et al (2011) Adipose tissue-derived stem cells show both immunogenic and immunosuppressive properties after chondrogenic differentiation. Cytotherapy 13:310–317
Schilling T, Küffner R, Klein-Hitpass L et al (2008) Microarray analyses of transdifferentiated mesenchymal stem cells. J Cell Biochem 103:413–433
Schilling T, Nöth U, Klein-Hitpass L et al (2007) Plasticity in adipogenesis and osteogenesis of human mesenchymal stem cells. Mol Cell Endocrinol 271:1–17
Taléns-Visconti R, Bonora A, Jover R et al (2007) Human mesenchymal stem cells from adipose tissue: differentiation into hepatic lineage. Toxicol In Vitro 21:324–329
Tatard VM, D’Ippolito G, Diabira S et al (2007) Neurotrophin-directed differentiation of human adult marrow stromal cells to dopaminergic-like neurons. Bone 40:360–373
Zuk PA, Zhu M, Ashjian P et al (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13:4279–4295
Woodbury D, Schwarz EJ, Prockop DJ et al (2000) Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res 61:364–370
Bryder D, Rossi DJ, Weissman IL (2006) Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am J Pathol 169:338–346
Hwang KC, Kim JY, Chang W et al (2008) Chemicals that modulate stem cell differentiation. Proc Natl Acad Sci U S A 105:7467–7471
Takahashi K, Tanabe K, Ohnuki M et al (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872
Pittenger MF, Mackay AM, Beck SC et al (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Cattaneo E, McKay R (1990) Proliferation and differentiation of neuronal stem cells regulated by nerve growth factor. Nature 347:762–765
Santos J (2013) A proteomic investigation of multi-lineage differentiated adult adipose-derived stem cells. Dissertation, Macquarie University
Mauney JR, Nguyen T, Gillen K et al (2007) Engineering adipose-like tissue in vitro and in vivo utilizing human bone marrow and adipose-derived mesenchymal stem cells with silk fibroin 3D scaffolds. Biomaterials 28:5280–5290
Cho SW, Kim I, Kim SH et al (2006) Enhancement of adipose tissue formation by implantation of adipogenic-differentiated preadipocytes. Biochem Biophys Res Commun 345:588–594
Hong L, Peptan IA, Colpan A et al (2006) Adipose tissue engineering by human adipose-derived stromal cells. Cells Tissues Organs 183:133–140
Parsons WJ, Ramkumar V, Stiles GL et al (1988) Isobutylmethylxanthine stimulates adenylate cyclase by blocking the inhibitory regulatory protein, Gi. Mol Pharmacol 34:37–41
van Calker D, Müller M, Hamprecht B (1979) Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem 33:999–1005
Petersen RK, Madsen L, Pedersen LM et al (2008) Cyclic AMP (cAMP)-mediated stimulation of adipocyte differentiation requires the synergistic action of Epac- and cAMP-dependent protein kinase-dependent processes. Mol Cell Biol 28:3804–3816
Darlington GJ, Ross SE, MacDougald OA (1998) The role of C/EBP genes in adipocyte differentiation. J Biol Chem 273:30057–30060
Chawla A, Schwarz EJ, Dimaculangan DD et al (1994) Peroxisome proliferator-activated receptor (PPAR) gamma: adipose-predominant expression and induction early in adipocyte differentiation. Endocrinology 135:798–800
Lehmann JM, Lenhard JM, Oliver BB et al (1997) Peroxisome proliferator-activated receptors alpha and gamma are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J Biol Chem 272:3406–3410
Prawitt J, Niemeier A, Kassem M et al (2008) Characterization of lipid metabolism in insulin-sensitive adipocytes differentiated from immortalized human mesenchymal stem cells. Exp Cell Res 314:814–824
Strem BM, Hicok KC, Zhu M et al (2005) Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med 54:132–141
Enomoto H, Furuichi T, Zanma A et al (2004) Runx2 deficiency in chondrocytes causes adipogenic changes in vitro. J Cell Sci 117:417–425
Dicker A, Le Blanc K, Aström G et al (2005) Functional studies of mesenchymal stem cells derived from adult human adipose tissue. Exp Cell Res 308:283–290
Rubin JP, Bennett JM, Doctor JS et al (2007) Collagenous microbeads as a scaffold for tissue engineering with adipose-derived stem cells. Plast Reconstr Surg 120:414–424
von Heimburg D, Zachariah S, Heschel I et al (2001) Human preadipocytes seeded on freeze-dried collagen scaffolds investigated in vitro and in vivo. Biomaterials 22:429–438
Woolf AD, Pfleger B (2003) Burden of major musculoskeletal conditions. Bull World Health Organ 81:646–656
Wagner W, Wein F, Seckinger A et al (2005) Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol 33:1402–1416
Muschler GF, Nitto H, Boehm CA et al (2001) Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J Orthop Res 19:117–125
Liu Y, Zhou Y, Feng H et al (2008) Injectable tissue-engineered bone composed of human adipose-derived stromal cells and platelet-rich plasma. Biomaterials 29:3338–3345
Flynn L, Prestwich GD, Semple JL et al (2007) Adipose tissue engineering with naturally derived scaffolds and adipose-derived stem cells. Biomaterials 28:3834–3842
Jing W, Xiong Z, Cai X et al (2010) Effects of gamma-secretase inhibition on the proliferation and vitamin D(3) induced osteogenesis in adipose derived stem cells. Biochem Biophys Res Commun 392:442–447
Liu G, Zhou H, Li Y et al (2008) Evaluation of the viability and osteogenic differentiation of cryopreserved human adipose-derived stem cells. Cryobiology 57:18–24
Im GI, Shin YW, Lee KB (2005) Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthritis Cartilage 13:845–853
Matsubara H, Hogan DE, Morgan EF et al (2012) Vascular tissues are a primary source of BMP2 expression during bone formation induced by distraction osteogenesis. Bone 51:168–180
Dragoo JL, Choi JY, Lieberman JR et al (2003) Bone induction by BMP-2 transduced stem cells derived from human fat. J Orthop Res 21:622–629
Kramer J, Hegert C, Guan K et al (2000) Embryonic stem cell-derived chondrogenic differentiation in vitro: activation by BMP-2 and BMP-4. Mech Dev 92:193–205
Bell TD, Demay MB, Burnett-Bowie SA (2010) The biology and pathology of vitamin D control in bone. J Cell Biochem 111:7–13
Phillips JE, Gersbach CA, Wojtowicz AM et al (2006) Glucocorticoid-induced osteogenesis is negatively regulated by Runx2/Cbfa1 serine phosphorylation. J Cell Sci 119:581–591
Hidalgo AA, Trump DL, Johnson CS (2010) Glucocorticoid regulation of the vitamin D receptor. J Steroid Biochem Mol Biol 121:372–375
Suh JH, Lee HW, Lee JW et al (2008) Hes1 stimulates transcriptional activity of Runx2 by increasing protein stabilization during osteoblast differentiation. Biochem Biophys Res Commun 367:97–102
Nakashima K, de Crombrugghe B (2003) Transcriptional mechanisms in osteoblast differentiation and bone formation. Trends Genet 19:458–466
Ducy P (2000) Cbfa1: a molecular switch in osteoblast biology. Dev Dyn 219:461–471
Coelho MJ, Fernandes MH (2000) Human bone cell cultures in biocompatibility testing. Part II: effect of ascorbic acid, beta-glycerophosphate and dexamethasone on osteoblastic differentiation. Biomaterials 21:1095–1102
Park JB (2012) The effects of dexamethasone, ascorbic acid, and β-glycerophosphate on osteoblastic differentiation by regulating estrogen receptor and osteopontin expression. J Surg Res 173:99–104
Franceschi RT, Iyer BS, Cui Y (1994) Effects of ascorbic acid on collagen matrix formation and osteoblast differentiation in murine MC3T3-E1 cells. J Bone Miner Res 9:843–854
Fratzl-Zelman N, Fratzl P, Hörandner H et al (1998) Matrix mineralization in MC3T3-E1 cell cultures initiated by beta-glycerophosphate pulse. Bone 23:511–520
Chung CH, Golub EE, Forbes E et al (1992) Mechanism of action of beta-glycerophosphate on bone cell mineralization. Calcif Tissue Int 51:305–311
Heng BC, Cao T, Lee EH (2004) Directing stem cell differentiation into the chondrogenic lineage in vitro. Stem Cells 22:1152–1167
Hardingham T, Tew S, Murdoch A (2002) Tissue engineering: chondrocytes and cartilage. Arthritis Res 4(Suppl 3):S63–S68
Koga H, Engebretsen L, Brinchmann JE et al (2009) Mesenchymal stem cell-based therapy for cartilage repair: a review. Knee Surg Sports Traumatol Arthrosc 17:1289–1297
Bartlett W, Skinner JA, Gooding CR et al (2005) Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br 87:640–645
Peterson L, Brittberg M, Kiviranta I et al (2002) Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med 30:2–12
Brittberg M, Lindahl A, Nilsson A et al (1994) Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331:889–895
Csaki C, Schneider PR, Shakibaei M (2008) Mesenchymal stem cells as a potential pool for cartilage tissue engineering. Ann Anat 190:395–412
Lee SH, Shin H (2007) Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv Drug Deliv Rev 59:339–359
Ponticiello MS, Schinagl RM, Kadiyala S et al (2000) Gelatin-based resorbable sponge as a carrier matrix for human mesenchymal stem cells in cartilage regeneration therapy. J Biomed Mater Res 52:246–255
Wu SC, Chang JK, Wang CK et al (2010) Enhancement of chondrogenesis of human adipose derived stem cells in a hyaluronan-enriched microenvironment. Biomaterials 31:631–640
Hui TY, Cheung KM, Cheung WL et al (2008) In vitro chondrogenic differentiation of human mesenchymal stem cells in collagen microspheres: influence of cell seeding density and collagen concentration. Biomaterials 29:3201–3212
Betre H, Ong SR, Guilak F et al (2006) Chondrocytic differentiation of human adipose-derived adult stem cells in elastin-like polypeptide. Biomaterials 27:91–99
Li WJ, Tuli R, Okafor C et al (2005) A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials 26:599–609
Liu X, Sun H, Yan D et al (2010) In vivo ectopic chondrogenesis of BMSCs directed by mature chondrocytes. Biomaterials 31:9406–9414
Her GJ, Wu HC, Chen MH et al (2013) Control of three-dimensional substrate stiffness to manipulate mesenchymal stem cell fate toward neuronal or glial lineages. Acta Biomater 9:5170–5180
Nii M, Lai JH, Keeney M et al (2013) The effects of interactive mechanical and biochemical niche signaling on osteogenic differentiation of adipose-derived stem cells using combinatorial hydrogels. Acta Biomater 9:5475–5483
Banka S, Mukudai Y, Yoshihama Y et al (2012) A combination of chemical and mechanical stimuli enhances not only osteo- but also chondro-differentiation in adipose-derived stem cells. J Oral Biosci 54:188–195
Morgan EF, Hussein AI, Al-Awadhi BA et al (2012) Vascular development during distraction osteogenesis proceeds by sequential intramuscular arteriogenesis followed by intraosteal angiogenesis. Bone 51:535–545
Wang L, Wang ZH, Shen CY et al (2010) Differentiation of human bone marrow mesenchymal stem cells grown in terpolyesters of 3-hydroxyalkanoates scaffolds into nerve cells. Biomaterials 31:1691–1698
Wong M, Tuan RS (1993) Nuserum, a synthetic serum replacement, supports chondrogenesis of embryonic chick limb bud mesenchymal cells in micromass culture. In Vitro Cell Dev Biol Anim 29A:917–922
Shih DT, Chen JC, Chen WY et al (2011) Expansion of adipose tissue mesenchymal stromal progenitors in serum-free medium supplemented with virally inactivated allogeneic human platelet lysate. Transfusion 51:770–778
Chin AC, Padmanabhan J, Oh SK et al (2010) Defined and serum-free media support undifferentiated human embryonic stem cell growth. Stem Cells Dev 19:753–761
Mendelson A, Frank E, Allred C et al (2011) Chondrogenesis by chemotactic homing of synovium, bone marrow, and adipose stem cells in vitro. FASEB J 25:3496–3504
Kawamura M, Urist MR (1988) Growth factors, mitogens, cytokines, and bone morphogenetic protein in induced chondrogenesis in tissue culture. Dev Biol 130:435–442
Ng F, Boucher S, Koh S et al (2008) PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood 112:295–307
Sekiya I, Vuoristo JT, Larson BL et al (2002) In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc Natl Acad Sci U S A 99:4397–4402
De Luca F, Barnes KM, Uyeda JA et al (2001) Regulation of growth plate chondrogenesis by bone morphogenetic protein-2. Endocrinology 142:430–436
Hunziker EB, Driesang IM, Morris EA (2001) Chondrogenesis in cartilage repair is induced by members of the transforming growth factor-beta superfamily. Clin Orthop Relat Res. https://doi.org/10.1097/00003086-200110001-00017
Derynck R, Zhang YE (2003) Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425:577–584
Grönroos E, Hellman U, Heldin CH et al (2002) Control of Smad7 stability by competition between acetylation and ubiquitination. Mol Cell 10:483–493
Liu D, Black BL, Derynck R (2001) TGF-beta inhibits muscle differentiation through functional repression of myogenic transcription factors by Smad3. Genes Dev 15:2950–2966
Chubinskaya S, Hurtig M, Rueger DC (2007) OP-1/BMP-7 in cartilage repair. Int Orthop 31:773–781
Pang EK, Im SU, Kim CS et al (2004) Effect of recombinant human bone morphogenetic protein-4 dose on bone formation in a rat calvarial defect model. J Periodontol 75:1364–1370
Hanada K, Solchaga LA, Caplan AI et al (2001) BMP-2 induction and TGF-beta 1 modulation of rat periosteal cell chondrogenesis. J Cell Biochem 81:284–294
Sakaguchi Y, Sekiya I, Yagishita K et al (2005) Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum 52:2521–2529
Huang JI, Zuk PA, Jones NF et al (2004) Chondrogenic potential of multipotential cells from human adipose tissue. Plast Reconstr Surg 113:585–594
Nakayama N, Duryea D, Manoukian R et al (2003) Macroscopic cartilage formation with embryonic stem-cell-derived mesodermal progenitor cells. J Cell Sci 116:2015–2028
Pereira RC, Economides AN, Canalis E (2000) Bone morphogenetic proteins induce gremlin, a protein that limits their activity in osteoblasts. Endocrinology 141:4558–4563
Delcroix GJ, Schiller PC, Benoit JP et al (2010) Adult cell therapy for brain neuronal damages and the role of tissue engineering. Biomaterials 31:2105–2120
Kingham PJ, Kalbermatten DF, Mahay D et al (2007) Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp Neurol 207:267–274
Ishii K, Katayama M, Hori K et al (1993) Effects of 2-mercaptoethanol on survival and differentiation of fetal mouse brain neurons cultured in vitro. Neurosci Lett 163:159–162
Santos J, Milthorpe BK, Herbert BR et al (2017) Proteomic analysis of human adipose derived stem cells during small molecule chemical stimulated pre-neuronal differentiation. Int J Stem Cells 10:193–217
Choi CB, Cho YK, Prakash KV et al (2006) Analysis of neuron-like differentiation of human bone marrow mesenchymal stem cells. Biochem Biophys Res Commun 350:138–146
Romero-Ramos M, Vourc’h P, Young HE et al (2002) Neuronal differentiation of stem cells isolated from adult muscle. J Neurosci Res 69:894–907
Safford KM, Hicok KC, Safford SD et al (2002) Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem Biophys Res Commun 294:371–379
Kim BJ, Seo JH, Bubien JK et al (2002) Differentiation of adult bone marrow stem cells into neuroprogenitor cells in vitro. NeuroReport 13:1185–1188
Fajardo J, Milthorpe BK, Santos J (2020) Molecular mechanisms involved in neural substructure development during phosphodiesterase inhibitor treatment of mesenchymal stem cells. Int J Mol Sci 21:4867
Santos J, Hubert T, Milthorpe BK (2020) Valproic acid promotes early neural differentiation in adult mesenchymal stem cells through protein signalling pathways. Cells 9:619
Santos J, Dolai S, O’Rourke MB et al (2020) Quantitative proteomic profiling of small molecule treated mesenchymal stem cells using chemical probes. Int J Mol Sci 22:160
di Summa PG, Kingham PJ, Raffoul W et al (2010) Adipose-derived stem cells enhance peripheral nerve regeneration. J Plast Reconstr Aesthet Surg 63:1544–1552
Ferraro F, Celso CL, Scadden D (2010) Adult stem cels and their niches. Adv Exp Med Biol 695:155–168
Abazova N, Krijgsveld J (2017) Advances in stem cell proteomics. Curr Opin Genet Dev 46:149–155
Hughes CS, Moggridge S, Müller T et al (2019) Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nat Protoc 14:68–85
Baharvand H, Fathi A, van Hoof D et al (2007) Concise review: trends in stem cell proteomics. Stem Cells 25:1888–1903
Levchenko A (2005) Proteomics takes stem cell analyses to another level. Nat Biotechnol 23:828–830
van Hoof D, Krijgsveld J, Mummery C (2012) Proteomic analysis of stem cell differentiation and early development. Cold Spring Harb Perspect Biol 4:a008177
Humphrey SJ, Karayel O, James DE et al (2018) High-throughput and high-sensitivity phosphoproteomics with the EasyPhos platform. Nat Protoc 13:1897–1916
Unwin RD, Griffiths JR, Whetton AD (2010) Simultaneous analysis of relative protein expression levels across multiple samples using iTRAQ isobaric tags with 2D nano LC-MS/MS. Nat Protoc 5:1574–1582
Williamson AJ, Smith DL, Blinco D et al (2008) Quantitative proteomics analysis demonstrates post-transcriptional regulation of embryonic stem cell differentiation to hematopoiesis. Mol Cell Proteomics 7:459–472
Ong SE, Blagoev B, Kratchmarova I et al (2002) Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol Cell Proteomics 1:376–386
Dayon L, Pasquarello C, Hoogland C et al (2010) Combining low- and high-energy tandem mass spectra for optimized peptide quantification with isobaric tags. J Proteomics 73:769–777
Thompson A, Schäfer J, Kuhn K et al (2003) Tandem mass tags: a novel quantification strategy for comparative analysis of complex protein mixtures by MS/MS. Anal Chem 75:1895–1904
Paulo Davim J (ed) (2013) Biomaterials and medical tribology, 1st edn. Woodhead Publishing series in biomaterials. Woodhead Publishing, Cambridge
Pruitt LA, Chakravartul AM (eds) (2011) Mechanics of biomaterials: fundamental principles for implant design. Cambridge University Press, Cambridge
Merolli A, Joyce T (eds) (2009) Biomaterials in hand surgery. Springer, Milan
Shah FA, Trobos M, Thomsen P et al (2016) Commercially pure titanium (cp-Ti) versus titanium alloy (Ti6Al4V) materials as bone anchored implants—is one truly better than the other? Mater Sci Eng C Mater Biol Appl 62:960–966
de Viteri VS, Fuentes E (2013) Titanium and titanium alloys as biomaterials. In: Gegner J (ed) Tribology—fundamentals and advancements. IntechOpen, London. https://doi.org/10.5772/55860
Bruni S, Martinesi M, Stio M et al (2005) Effects of surface treatment of Ti-6Al-4V titanium alloy on biocompatibility in cultured human umbilical vein endothelial cells. Acta Biomater 1:223–234
Singh R, Dahotre NB (2007) Corrosion degradation and prevention by surface modification of biometallic materials. J Mater Sci Mater Med 18:725–751
Rath PC, Besra L, Singh BP et al (2012) Titania/hydroxyapatite bi-layer coating on Ti metal by electrophoretic deposition: characterization and corrosion studies. Ceram Int 38:3209–3216
Paital SR, Dahotre NB (2009) Calcium phosphate coatings for bio-implant applications: materials, performance factors, and methodologies. Mater Sci Eng R Rep 66:1–70
Choi AH, Ben-Nissan B (2017) Calcium phosphate nanocomposites for biomedical and dental applications: recent developments. In: Thakur VK, Thakur MK, Kessler MR (eds) Handbook of composites from renewable materials. Wiley, New Jersey, pp 423–450
Choi AH, Ben-Nissan B, Bendavid A (2017) Thin films and nanocoatings of hydroxyapatite on titanium implants: production methods and adhesion testing. Lambert Academic Publishing, Germany
Choi AH, Cazalbou S, Ben-Nissan B (2015) Nanobiomaterial coatings in dentistry. In: Deb S (ed) Biomaterials for oral and craniomaxillofacial applications, frontiers of oral biology, vol 17. Karger, Basel, pp 49–61
Baino F, Novajra G, Vitale-Brovarone C (2015) Bioceramics and scaffolds: a winning combination for tissue engineering. Front Bioeng Biotechnol 3:202
Planell JA (ed) (2009) Bone repair biomaterials. Woodhead Publishing, Cambridge
Heness GL, Ben-Nissan B (2004) Innovative bioceramics. Mater Forum 27:104–114
Hench LL, Polak JM (2002) Third-generation biomedical materials. Science 295:1014–1017
Hench LL (1998) Bioceramics. J Am Ceram Soc 81:1705–1728
Mahyudin F, Widhiyanto L, Hermawan H (2016) Biomaterials in orthopaedics. In: Mahyudin F, Hermawan H (eds) Biomaterials and medical devices. Advanced structured materials, vol 58. Springer, Cham, pp 161–181
Guillemot F (2005) Recent advances in the design of titanium alloys for orthopedic applications. Expert Rev Med Dev 2:741–748
Hench LL (1980) Biomaterials. Science 208:826–831
Macha IJ, Charvillat C, Cazalbou S et al (2016) Comparative study of coral conversion, part 3: intermediate products in the first half an hour. J Aust Ceram Soc 52:177–182
Gunduz O, Sahin YM, Agathopoulos S et al (2014) A new method for fabrication of nanohydroxyapatite and TCP from the sea snail Cerithium vulgatum. J Nanomaterials. https://doi.org/10.1155/2014/382861
Ben-Nissan B, Pezzotti G (2003) Bioceramics processing routes and mechanical evaluation. J Ceram Soc Jpn 110:601–608
Hench LL, Jones JR (eds) (2005) Biomaterials, artificial organs and tissue engineering. Woodhead Publishing, Cambridge
Franklin-Ford TW, Suarez-Gonzalez D, Lee JS et al (2013) Biomimetic hydroxyapatite materials for therapeutic delivery. In: Zhang S (ed) Hydroxyapatite coatings for biomedical applications. CRC Press, Boca Raton, pp 201–238
Roest R, Latella BA, Heness G et al (2011) Adhesion of sol-gel derived hydroxyapatite nanocoatings on anodised pure titanium and titanium (Ti6Al4V) alloy substrates. Surf Coat Technol 205:3520–3529
Zreiqat H, Valenzuela SM, Ben-Nissan B et al (2005) The effect of surface chemistry modification of titanium alloy on signalling pathways in human osteoblasts. Biomaterials 26:7579–7586
Karacan I, Ben-Nissan B, Sinutok S (2019) Marine-based calcium phosphates from hard coral and calcified algae for biomedical applications. In: Choi AH, Ben-Nissan B (eds) Marine-derived biomaterials for tissue engineering applications. Springer series in biomaterials science and engineering, vol 14. Springer, Singapore, pp 137–153
Macha IJ, Ozyegin LS, Oktar FN et al (2015) Conversion of ostrich eggshells (Struthio camelus) to calcium phosphates. J Aust Ceram Soc 51:125–133
Andrés-Vergés M, Fernández-González C, Martínez-Gallego M (1998) Hydrothermal synthesis of calcium deficient hydroxyapatites with controlled size and homogeneous morphology. J Eur Ceram Soc 18:1245–1250
Chou J, Hao J, Ben-Nissan B et al (2013) Coral exoskeletons as a precursor material for the development of a calcium phosphate drug delivery system for bone tissue engineering. Biol Pharm Bull 36:1662–1665
Mann S (1988) Molecular recognition in biomineralization. Nature 332:119–124
Wong JY, Bronzino JD, Peterson DR (eds) (2012) Biomaterials: principles and practices. CRC Press, Boca Raton
Ratner BD, Hoffman AS, Schoen FJ et al (eds) (2013) Biomaterials science, 3rd edn. Academic Press, Massachusetts
Nair LS, Laurencin CT (2007) Biodegradable polymers as biomaterials. Prog Polym Sci 32:762–798
Thavornyutikarn B, Chantarapanich N, Sitthiseripratip K et al (2014) Bone tissue engineering scaffolding: computer-aided scaffolding techniques. Prog Biomater 3:61–102
Naahidi S, Jafari M, Edalat F et al (2013) Biocompatibility of engineered nanoparticles for drug delivery. J Control Release 166:182–194
Rezwan K, Chen QZ, Blaker JJ et al (2006) Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 27:3413–3431
Narayanan G, Vernekar VN, Kuyinu EL et al (2016) Poly (lactic acid)-based biomaterials for orthopaedic regenerative engineering. Adv Drug Deliv Rev 107:247–276
Ferrández-Montero A, Lieblich M, González-Carrasco JL et al (2019) Development of biocompatible and fully bioabsorbable PLA/Mg films for tissue regeneration applications. Acta Biomater 98:114–124
Armentano I, Gigli M, Morena F et al (2018) Recent advances in nanocomposites based on aliphatic polyesters: design, synthesis, and applications in regenerative medicine. Appl Sci 8:1452
Argentati C, Morena F, Bazzucchi M et al (2018) Adipose stem cell translational applications: from bench-to-bedside. Int J Mol Sci 19:3475
Badyra B, Sułkowski M, Milczarek O et al (2020) Mesenchymal stem cells as a multimodal treatment for nervous system diseases. Stem Cells Transl Med 9:1174–1189
Iaquinta MR, Mazzoni E, Bononi I et al (2019) Adult stem cells for bone regeneration and repair. Front Cell Dev Biol 7:268
Choi AH, Karacan I, Ben-Nissan B (2020) Surface modifications of titanium alloy using nanobioceramic-based coatings to improve osseointegration: a review. Mater Technol 35:742–751
Gao C, Peng S, Feng P et al (2017) Bone biomaterials and interactions with stem cells. Bone Res 5:17059
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Karacan, I., Milthorpe, B., Ben-Nissan, B., Santos, J. (2022). Stem Cells and Proteomics in Biomaterials and Biomedical Applications. In: Choi, A.H., Ben-Nissan, B. (eds) Innovative Bioceramics in Translational Medicine I. Springer Series in Biomaterials Science and Engineering, vol 17. Springer, Singapore. https://doi.org/10.1007/978-981-16-7435-8_4
Download citation
DOI: https://doi.org/10.1007/978-981-16-7435-8_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-7434-1
Online ISBN: 978-981-16-7435-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)