Abstract
The genus Closterium is the best characterized charophycean green alga with respect to the process of sexual reproduction. Two sex pheromones , named PR-IP Inducer and PR-IP , that are involved in the progress of these processes were physiologically and biochemically characterized and the corresponding genes were cloned. These pheromones function in most steps of sexual reproduction. The timing after mixing, appropriate concentrations of the pheromones, and conditions of the cells are all essential for pheromones to be functional. To elucidate the molecular mechanisms of sexual reproduction in detail, molecular tools such as expressed sequence tag (EST), microarray analysis, and genetic transformation systems have been established. These methods will enable us to clarify the details of sexual reproduction in the near future.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
In the process of sexual reproduction, two sexually competent cells recognize each other, followed by conjugation or fertilization . In some algae, dormant zygospores are formed as a result of sexual reproduction and show resistance to severe environmental conditions, such as drought stress. In the case of Chlamydomonas reinhardtii , one of the best characterized models in green algae, sexual adhesion between the gametes is mediated by agglutinin molecules on their flagellar membranes. The plus and minus agglutinins are sex specifically displayed by nitrogen-starved mating-type plus (mt+) and mating-type minus (mt−) gametes, respectively (Adair et al. 1983; Goodenough et al. 1985; Ferris et al. 2005). Once an agglutinin molecule directly binds to the agglutinin molecule on the flagellum of an opposite mating type as a consequence of agglutination, a gamete-specific flagellar adenylyl cyclase is activated and the intracellular cAMP level is elevated nearly tenfold, triggering dramatic alterations in the cell (Pasquale and Goodenough 1987; Saito et al. 1993; Zhang and Snell 1994). First, flagellar motility is altered, and the adhesiveness of the flagellar surface is increased (Saito et al. 1985; Goodenough 1989; Hunnicutt et al. 1990). Second, a matrix-degrading enzyme is activated (Buchanan et al. 1989; Snell et al. 1989; Kinoshita et al. 1992) and the cell wall is degraded so that the gametes are able to fuse. Third, mt+ gametes erect an actin-filled microvillus (“fertilization tube”) as a mating structure and the mt− gametes also erect a small, dome-like, actin-free mating structure. Cell fusion initiates with an adhesive interaction between mt+ and mt− mating structures, followed by localized membrane fusion. Two proteins, FUS1 and GCS1/HAP2 , are known to be essential for the membrane fusion reaction (Ferris et al. 1996; Misamore et al. 2003; Liu et al. 2008; Mori et al. 2006). Both proteins are degraded rapidly upon fusion, as would be expected for a block to polygamy (Liu et al. 2010).
The desmid Closterium, which belongs to Zygnematophyceae, is the most successfully characterized unicellular charophycean in terms of the maintenance of strains and sexual reproduction (Ichimura 1971). Charophyceans, which are most closely related to land plants, form a relevant monophyly with land plants. Recently, it was suggested that either the Zygnematophyceae or a clade consisting of Zygnematophyceae and Coleochaetophyceae might be the most likely sister group of land plants (Turmel et al. 2006; Wodniok et al. 2011).
In this review, the sexual reproductive processes of Closterium peracerosum-strigosum-littorale complex (C. psl. complex) are described in detail. Molecular tools for analyses of the processes are also presented.
2 Sexual Reproduction Controlled by Specific Sex Pheromones in the C. psl. Complex
2.1 Overview of Sexual Reproduction in Closterium
The sexual reproduction of species in the genus Closterium has been of interest to many investigators for more than 100 years, and the morphological details and modes of sexual reproduction are well documented (Cook 1963; Ichimura 1973; Lippert 1967; Noguchi 1988; Noguchi and Ueda 1985; Pickett-Heaps and Fowke 1971). Closterium has no flagellum-like machinery for active movement. Therefore, it is thought that the cells of this alga exploit some diffusible substances for the intercellular communication.
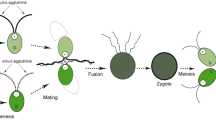
In the case of C. psl. complex, there are two types of conjugation to produce zygospores: that between two complementary mating-type cells (mt+ and mt−) and that between clonal cells. The former is called heterothallism and the latter is called homothallism (Graham and Wilcox 2000). The conjugation process can be divided into several steps: sexual cell division (SCD ), which produces sexually competent gametangial cells; pairing, formation of conjugation papillae; condensing of their cytoplasm; release and fusion of gametic protoplasts (gametes); and formation of zygospores (Fig. 28.1). Zygospores become dormant and acquire resistance to dry conditions. Exposure to dry conditions and subsequent water supply lead to the start of meiosis. Two non-sister nuclei of the second meiotic division survive and the other two degenerate. As a result, the two surviving nuclei carry opposite mating-type genes in the absence of crossing over, and a pair of mt+ and mt− cells arise from one zygospore, in the case of heterothallic strains (Brandham and Godward 1965; Hamada et al. 1982; Lippert 1967; Watanabe and Ichimura 1982).
Schematic illustrations of the sexual reproduction of Closterium peracerosum-strigosum-littorale complex: (1) mucilage secretion, (2) sexual cell division, (3) sexual pair formation induced by unknown chemoattractant pheromone(s), (4) protoplast release, (5) zygospore. Most processes are induced by the protoplast-release-inducing protein (PR-IP) and the PR-IP Inducer. Gray arrows indicate pheromonal communication
2.2 Characters of Sex Pheromones in the Heterothallic C. psl. Complex
2.2.1 Sex Pheromones Responsible for Protoplast Release During Sexual Reproduction
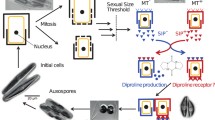
When mt+ and mt− cells are mixed together in a nitrogen-depleted mating medium in the light, cells of both types differentiate into gametangial cells as a result of SCD and become paired. Then, paired cells release their protoplasts to form zygospores (Fig. 28.1). Sekimoto et al. (1990) successfully isolated the first Closterium pheromone from the C. psl. complex and designated it as protoplast-release-inducing protein (PR-IP) (Sekimoto et al. 1990). The PR-IP is a glycoprotein that consists of subunits of 42 and 19 kDa: it is released by mt+ cells (NIES-67) and is responsible for inducing the release of protoplasts from mt− cells (NIES-68) (Fig. 28.2). PR-IP receptors have not yet been isolated; however, specific binding of the biotinylated 19-kDa subunit of PR-IP to sexually competent mt− cells has been clearly demonstrated (Sekimoto and Fujii 1992; Sekimoto et al. 1993b).
Secretion of PR-IP by mt+ cells is induced in medium in which only mt− cells have been cultured (Sekimoto et al. 1993a). Another pheromone that induces the synthesis and release of PR-IP was detected and named PR-IP Inducer (Sekimoto et al. 1993a). The pheromone is also a glycoprotein with a molecular mass of 18.7 kDa (Nojiri et al. 1995). PR-IP Inducer is released constitutively from mt− cells in the presence of light and directly induces the production and release of PR-IP from mt+ cells.
cDNAs encoding the subunits of PR-IP (Sekimoto et al. 1994a, b) and PR-IP Inducer (Sekimoto et al. 1998) have been isolated. A computer search using the nucleotide sequences and deduced amino-acid sequences failed to reveal any homologies to known proteins. Genes for these pheromones can be detected in cells of both mating types using genomic Southern hybridization analysis, but they are only expressed in cells of the respective mating type, suggesting the presence of sex-specific regulation of gene expression by sex-limited trans-acting factors (Sekimoto et al. 1998; Sekimoto et al. 1994c; Endo et al. 1997).
2.2.2 Sex Pheromones Involved in SCD and Mucilage Secretion
In the sexual reproductive processes of Closterium species, gametangial cells are produced from haploid vegetative cells. Ichimura (1971) reported that vegetative cells of the C. psl. complex divided at once before formation of sexual pairs when the two mating-type cells were mixed (Ichimura 1971). This type of cell division is SCD.
The SCD-inducing activities specific to the two mating-type cells have been detected and characterized physiologically (Tsuchikane et al. 2003). Mt− cells release an SCD-inducing pheromone (SCD-IP ) specific for mt+ cells, and are designated SCD-IP-minus, whereas an mt−-specific pheromone released from mt+ cells is designated SCD-IP-plus. Recent time-lapse video analyses revealed that SCD was not always required for successful pairing (Y. Tsuchikane, M. Sato, H. Sekimoto, personal communication).
Closterium exhibits gliding locomotory behavior, mediated by the forceful extrusion of mucilage from one pole of the cell that causes the cell to glide in the opposite direction (Domozych et al. 1993). Substances with the ability to stimulate secretion of uronic acid-containing mucilage from mt+ and mt− cells were detected in media in which mt− and mt+ cells had been cultured separately and were designated mucilage secretion-stimulating pheromone (MS-SP)-minus and MS-SP-plus, respectively (Akatsuka et al. 2003).
2.2.3 Multifunction of Sex Pheromones
Both MS-SP-minus and SCD-IP-minus show quite similar characteristics to PR-IP Inducer, whereas both MS-SP-plus and SCD-IP-plus show quite similar characteristics to PR-IP, with respect to molecular weight, heat stability, and dependency on light for their secretion and function, indicating close relationships among these pheromones. Recombinant PR-IP Inducer produced in yeast cells was assayed for both production of PR-IP and induction of SCD (Sekimoto 2002; Tsuchikane et al. 2005). Although both biological activities were observed by treating recombinant pheromone with mt+ cells, SCD could be induced by exposure to a relatively lower concentration of recombinant PR-IP Inducer. Moreover, SCD was induced by a shorter period of treatment with the pheromone than the production of PR-IP (Tsuchikane et al. 2005). In addition, purified native PR-IP Inducer showed mucilage secretion-stimulating activity against mt+ cells (Akatsuka et al. 2003). These results strongly indicate that previously characterized PR-IP Inducer has mucilage secretion-stimulating, SCD-inducing, and PR-IP-inducing activities for mt+ cells, although the induction mechanisms seem to differ.
Purified PR-IP showed not only protoplast-releasing activity, but also mucilage secretion-stimulating and SCD-inducing activities against mt− cells (Akatsuka et al. 2006). Minimum concentrations required for the respective activities were quite different: 5 × 10−16 M PR-IP stimulated mucilage secretion, 5 × 10−10 M PR-IP was required for protoplast release, and 5 × 10−11 M PR-IP resulted in the induction of SCD as well as mucilage secretion. These results strongly suggest that PR-IP is also a multifunctional pheromone that independently promotes multiple steps in conjugation at the appropriate times through different induction mechanisms.
2.3 Summary of Sexual Reproduction in the Heterothallic C. psl. Complex
Based on the results described here, sexual reproductive events, postulated at this time, are summarized (Fig. 28.1). The PR-IP Inducer is released from mt− cells when cells are exposed to nitrogen-depleted conditions in the light. Then, mt+ cells receive the signal and begin to release the PR-IP into the medium. During this communication, mucilage is secreted into the surrounding medium. Concentrations of these pheromones are gradually elevated, leading to the induction of SCD and the respective formation of gametangial cells. Then, mt+ and mt− gametangial cells move together and become paired through the effect of unknown chemotactic pheromones. After the final communication by PR-IP and PR-IP Inducer, mt− cells begin to release their protoplasts. Then the release of protoplasts from mt+ cells is eventually induced by direct adhesion of cells, and these protoplasts fuse to form a zygospore.
Information concerning physical cell–cell recognition and fusion of cells involved in conjugation processes has not yet been clarified; however, fluorescein isothiocyanate (FITC)-labeled lectins, Lycopersicon esculentum lectin (LEL) and Concanavalin A (ConA), accumulated on the conjugation papillae and inhibited the progress of zygote formation (Hori et al. 2012). These results suggest that different carbohydrates specifically recognized by these lectins are involved in cell recognition or fusion during conjugation processes in the C. psl. complex.
3 Molecular Biological Approaches to Sexual Reproduction
3.1 Expressed Sequence Tag (EST) and Microarray Analyses
To elucidate the molecular mechanism of intercellular communication during sexual reproduction, a normalized cDNA library was established from a mixture of cDNA libraries prepared from cells at various stages of sexual reproduction and from a mixture of vegetative mt+ and mt− cells. The aim was to reduce redundancy, and 3,236 ESTs were generated, which were classified into 1,615 nonredundant groups (Sekimoto et al. 2003; Sekimoto et al. 2006). The EST sequences were compared with nonredundant protein sequence databases in the public domain using the BLASTX program, and 1,045 nonredundant sequences displaying similarity to previously registered genes in the public databases were confirmed. The source group with the highest similarity was land plants, including Arabidopsis thaliana.
A cDNA microarray was then constructed and expression profiles were obtained using mRNA isolated from cells in various stages of the life cycle. Finally, 88 pheromone-inducible, conjugation-related, or sex-specific genes were identified (Sekimoto et al. 2006), although their functions during sexual reproduction have not been characterized.
Of the 88 genes identified, a gene encoding receptor-like protein kinase (RLK ) was the most notable and was named CpRLK1 . The gene is expressed during sexual reproduction, and treatment of mt+ cells with the PR-IP Inducer also induces its expression, indicating that the CpRLK1 protein probably functions during sexual reproduction (Sekimoto et al. 2006). The full-length cDNA has been isolated, and an amino-acid sequence containing an extracellular domain (ECD) was obtained (unpublished data). In A. thaliana, the RLK family is the largest gene family with more than 600 family members (Shiu and Bleecker 2001, 2003; Shiu et al. 2004), although the functions of most of these genes are still unknown. Only two RLK genes have been found in the genome of Chlamydomonas reinhardtii; however, the predicted proteins do not have recognizable ECDs. No RLK gene was found in the genome of Ostreococcus tauri (Lehti-Shiu et al. 2009). In contrast, RLKs having transmembrane domains or ECDs have been isolated from two charophyceans (Nitella axillaris and Closterium ehrenbergii) (Sasaki et al. 2007), indicating that the receptor configuration was likely established before the divergence of land plants from charophyceans but after the divergence of charophyceans from chlorophytes (Graham and Wilcox 2000; Karol et al. 2001). The receptor configuration is likely to function for intercellular communication, especially during sexual reproduction; however, the confirmation of genomic information from early diversified nonsexual charophyceans such as Klebsormidiophyceae and Chlorokybophyceae is necessary to confirm this assumption.
A gene named CpRLP1 (receptor-like protein-1) was also fascinating. Several leucine-rich repeats and a transmembrane domain were found in the deduced protein, but a kinase domain was not involved. As in the case of the CLV2 protein of A. thaliana, the CpRLP1 protein may form a heterodimer with another protein, such as a receptor-like protein kinase (Zhu et al. 2010a; Zhu et al. 2010b), to transduce the unknown extracellular signal into the intracellular compartment.
3.2 Genetic Transformation
Establishment of a nuclear transformation system for genes of interest obtained from transcriptome analyses greatly enhances the understanding of molecular mechanisms for sexual reproduction in C. psl. complex. Particle bombardment was used for gene delivery into C. psl. complex cells. In general, it is most important to choose efficient promoters to drive the introduced genes. However, expression using the CaMV 35S promoter in the C. psl. complex was quite low (Abe et al. 2008a). Two endogenous promoters derived from the highly and constitutively expressed genes CpHSP70 and CpCAB1, encoding a heat shock protein 70 (HSP70) and a chlorophyll a/b-binding protein (CAB) in the C. psl. complex, respectively, were selected and isolated to drive the transgenes. In the C. psl. complex, codons are highly biased in G and C, resulting in synonymous codons favoring G and C at the third position (Abe et al. 2008a). Because this feature is very similar to that of Chlamydomonas reinhardtii, the marker and reporter genes used in Chlamydomonas reinhardtii are applicable to transformation in the C. psl. complex.
Two constructs, pSA006 and pSA106, were successfully transformed in the C. psl. complex (Fig. 28.3). These constructs consisted of the Chlamydomonas selectable marker gene ble encoding a phleomycin-resistant protein (Stevens et al. 1996) and the cgfp gene encoding a Chlamydomonas-adapted green fluorescent protein (GFP) (Fuhrmann et al. 1999). These genes were mutually fused in-frame and linked either to the CpHSP70 (pSA006) or the CpCAB1 (pSA106) promoters. Finally, approximately 250 and 100 of the transiently GFP-expressed cells were obtained in a plate (in one trial of particle bombardment) using plasmid pSA006 and pSA106, respectively (Abe et al. 2008a).
Phleomycin is a useful antibiotic for the selection of stable transformants in the C. psl. complex because the drug inhibits cell proliferation at low concentrations both in liquid media and on solid media (Abe et al. 2008b). The overexpression vector pSA1102, which allowed direct selection by phleomycin and the overexpression of the arbitrary genes, was constructed (Fig. 28.3, Abe et al. 2011). In the case of CpPI (encoding PR-IP Inducer), the expression level in transformed mt+ cells displayed about a 16-fold increase compared with wild-type cells. In addition, both transcripts encoding the respective PR-IP subunits (Cp19ksu and Cp42ksu) also displayed an approximately 67-fold increase in the same transformants, indicating that the ectopically expressed PR-IP Inducer would be functional in vivo in the C. psl. complex. Further improvements such as the selection of more powerful promoters and the application of gene silencing will provide useful information to enhance our understanding of sexual reproduction in Closterium.
4 Perspective
In this chapter, regulation of sexual reproduction in the unicellular charophycean alga C. psl. complex was described in detail. In the sexual reproduction processes, two sex pheromones (PR-IP Inducer and PR-IP), released from mt− and mt+ cells, respectively, were indispensable. These exerted multiple functions, such as stimulation of mucilage secretion, induction of SCD, and release of PR-IP from mt+ cells or release of protoplasts from mt− cells. Moreover, timing after mixing, appropriate concentrations of the pheromones, and conditions of the cells are all essential for pheromones to be functional.
Using microarray analyses, cDNAs encoding a receptor-like kinase (CpRLK1) and a leucine-rich repeat containing receptor-like protein (CpRLP1) were identified, which may function as sex-specific receptors for recognition of unknown signals from opposite mating-type cells. To characterize sex-specific and sexual reproduction-related genes, including CpRLK1 and CpRLP1, genetic transformation systems have recently been established. Further improvements, such as selection of more powerful promoters, will enable us to analyze the function of unknown genes in the near future. In addition, large-scale EST analysis and draft genome sequencing of the C. psl. complex are now in progress.
Using the C. psl. complex as a model, the problem of speciation of organisms can be approached. One species of the C. psl. complex can be subclassified into several reproductively isolated groups (biological species). The reasons for the isolation could be partly explained as the loss of pheromonal communication (Tsuchikane et al. 2008; Sekimoto et al. 2012). Pheromones are also involved in the sexual reproduction of the homothallic strain (Tsuchikane et al. 2010a). In this strain, conjugation of two sister gametangial cells derived from one vegetative cell was predominant (Tsuchikane et al. 2010b). SCD of one vegetative cell into two sister gametangial cells seemed to be a segregative process that was required for the production of complementary mating types observed in the heterothallic cells (Tsuchikane et al. 2012).
As mentioned previously, the algal genus Closterium is one of the closest living organisms to land plants. The present studies concerning sexual reproduction of C. psl. complex are useful when considering the mechanisms and evolution of sexual reproduction in terrestrial plants.
References
Abe J, Hiwatashi Y, Ito M, Hasebe M, Sekimoto H (2008a) Expression of exogenous genes under the control of endogenous HSP70 and CAB promoters in the Closterium peracerosum-strigosum-littorale complex. Plant Cell Physiol 49:625–632
Abe J, Sakayori K, Sekimoto H (2008b) Effect of antibiotics on cell proliferation in the Closterium peracerosum-strigosum-littorale complex (Charophyceae, Chlorophyta). Biologia 63:932–936
Abe J, Hori S, Tsuchikane Y, Kitao N, Kato M, Sekimoto H (2011) Stable nuclear transformation of the Closterium peracerosum-strigosum-littorale complex. Plant Cell Physiol 52:1676–1685. doi:10.1093/pcp/pcr103
Adair WS, Hwang C, Goodenough UW (1983) Identification and visualization of the sexual agglutinin from the mating-type plus flagellar membrane of Chlamydomonas. Cell 33:183–193
Akatsuka S, Sekimoto H, Iwai H, Fukumoto R, Fujii T (2003) Mucilage secretion regulated by sex pheromones in Closterium peracerosum-strigosum-littorale complex. Plant Cell Physiol 44:1081–1087
Akatsuka S, Tsuchikane Y, Fukumoto R, Fujii T, Sekimoto H (2006) Physiological characterization of the sex pheromone protoplast-release-inducing protein from the Closterium peracerosum-strigosum-littorale complex (Charophyta). Phycol Res 54:116–121
Brandham PE, Godward MBE (1965) The inheritance of mating type in desmids. New Phytol 64:428–435
Buchanan MJ, Imam SH, Eskue WA, Snell WJ (1989) Activation of the cell wall degrading protease, lysin, during sexual signalling in Chlamydomonas: the enzyme is stored as an inactive, higher relative molecular mass precursor in the periplasm. J Cell Biol 108:199–207
Cook PA (1963) Variation in vegetative and sexual morphology among the small curved species of Closterium. Phycologia 3:1–18
Domozych CR, Plante K, Blais P (1993) Mucilage processing and secretion in the green alga Closterium. 1. Cytology and biochemistry. J Phycol 29:650–659
Endo B, Fujii T, Kamiya Y, Sekimoto H (1997) Analysis of genomic sequences encoding a sex pheromone from the Closterium peracerosum-strigosum-littorale complex. J Plant Res 110:463–467
Ferris PJ, Woessner JP, Goodenough UW (1996) A sex recognition glycoprotein is encoded by the plus mating-type gene fus1 of Chlamydomonas reinhardtii. Mol Biol Cell 7:1235–1248
Ferris P, Waffenschmidt S, Umen JG, Lin H, Lee J-H, Ishida K, Kubo T, Lau J, Goodenough UW (2005) Plus and minus sexual agglutinins from Chlamydomonas reinhardtii. Plant Cell 17:597–615
Fuhrmann M, Oertel W, Hegemann P (1999) A synthetic gene coding for the green fluorescent protein (GFP) is a versatile reporter in Chlamydomonas reinhardtii. Plant J 19:353–361
Goodenough UW (1989) Cyclic AMP enhances the sexual agglutinability of Chlamydomonas flagella. J Cell Biol 109:247–252
Goodenough UW, Adair WS, Collin-Osdoby P, Heuser JE (1985) Structure of the Chlamydomonas agglutinin and related flagellar surface proteins in vitro and in situ. J Cell Biol 101:924–941
Graham LE, Wilcox LW (2000) Algae. Prentice-Hall, Upper Saddle River
Hamada J, Yoshizawa-Katoh T, Tsunewaki K (1982) Genetic study on mating type genes by a new type of tetrad analysis in Closterium ehrenbergii. Bot Mag Tokyo 95:101–108
Hori S, Sekimoto H, Abe J (2012) Properties of cell surface carbohydrates in sexual reproduction of the Closterium peracerosum–strigosum–littorale complex (Zygnematophyceae, Charophyta). Phycol Res 60:254–260. doi:10.1111/j.1440-1835.2012.00656.x
Hunnicutt GR, Kosfiszer MG, Snell WJ (1990) Cell body and flagellar agglutinins in Chlamydomonas reinhardtii: the cell body plasma membrane is a reservoir for agglutinins whose migration to the flagella is regulated by a functional barrier. J Cell Biol 111:1605–1616
Ichimura T (1971) Sexual cell division and conjugation-papilla formation in sexual reproduction of Closterium strigosum. In: Nishizawa K (ed) Proceedings of the 7th international seaweed symposium. University of Tokyo Press, Tokyo, pp 208–214
Ichimura T (1973) The life cycle and its control in some species of Closterium, with special reference to the biological species problem. D. Science thesis, University of Tokyo
Karol KG, McCourt RM, Cimino MT, Delwiche CF (2001) The closest living relatives of land plants. Science 294:2351–2353. doi:10.1126/science.1065156
Kinoshita T, Fukuzawa H, Shimada T, Saito T, Matsuda Y (1992) Primary structure and expression of a gamete lytic enzyme in Chlamydomonas reinhardtii: similarity of functional domains to matrix metalloproteases. Proc Natl Acad Sci USA 89:4693–4697
Lehti-Shiu MD, Zou C, Hanada K, Shiu SH (2009) Evolutionary history and stress regulation of plant receptor-like kinase/pelle genes. Plant Physiol 150:12–26. doi:10.1104/pp. 108.134353
Lippert BE (1967) Sexual reproduction in Closterium moniliferum and Closterium ehrenbergii. J Phycol 3:182–198
Liu Y, Tewari R, Ning J, Blagborough AM, Garbom S, Pei J, Grishin NV, Steele RE, Sinden RE, Snell WJ, Billker O (2008) The conserved plant sterility gene HAP2 functions after attachment of fusogenic membranes in Chlamydomonas and Plasmodium gametes. Genes Dev 22:1051–1068. doi:10.1101/gad.1656508
Liu Y, Misamore MJ, Snell WJ (2010) Membrane fusion triggers rapid degradation of two gamete-specific, fusion-essential proteins in a membrane block to polygamy in Chlamydomonas. Development (Camb) 137:1473–1481. doi:10.1242/dev.044743
Misamore MJ, Gupta S, Snell WJ (2003) The Chlamydomonas Fus1 protein is present on the mating type plus fusion organelle and required for a critical membrane adhesion event during fusion with minus gametes. Mol Biol Cell 14:2530–2542
Mori T, Kuroiwa H, Higashiyama T, Kuroiwa T (2006) Generative cell specific 1 is essential for angiosperm fertilization. Nat Cell Biol 8:64–71. doi:10.1038/ncb1345
Noguchi T (1988) Numerical and structural changes in dictyosomes during zygospore germination of Closterium ehrenbergii. Protoplasma 147:135–142
Noguchi T, Ueda K (1985) Cell walls, plasma membranes, and dictyosomes during zygote maturation of Closterium ehrenbergii. Protoplasma 128:64–71
Nojiri T, Fujii T, Sekimoto H (1995) Purification and characterization of a novel sex pheromone that induces the release of another sex pheromone during sexual reproduction of the heterothallic Closterium peracerosum-strigosum-littorale complex. Plant Cell Physiol 36:79–84
Pasquale SM, Goodenough UW (1987) Cyclic AMP functions as a primary sexual signal in gametes of Chlamydomonas reinhardtii. J Cell Biol 105:2279–2292
Pickett-Heaps JD, Fowke LC (1971) Conjugation in the desmid Closterium littorale. J Phycol 7:37–50
Saito T, Tsubo Y, Matsuda Y (1985) Synthesis and turnover of cell body agglutinin as a pool of flagellar surface agglutinin in Chlamydomonas reinhardtii gamete. Arch Microbiol 142:207–210
Saito T, Small L, Goodenough UW (1993) Activation of adenylyl cyclase in Chlamydomonas reinhardtii by adhesion and by heat. J Cell Biol 122:137–147
Sasaki G, Katoh K, Hirose N, Suga H, Kuma K, Miyata T, Su ZH (2007) Multiple receptor-like kinase cDNAs from liverwort Marchantia polymorpha and two charophycean green algae, Closterium ehrenbergii and Nitella axillaris: Extensive gene duplications and gene shufflings in the early evolution of streptophytes. Gene (Amst) 401:135–144. doi:10.1016/j.gene.2007.07.009
Sekimoto H (2002) Production and secretion of a biologically active Closterium sex pheromone by Saccharomyces cerevisiae. Plant Physiol Biochem 40:789–794
Sekimoto H, Fujii T (1992) Analysis of gametic protoplast release in the Closterium peracerosum-strigosum-littorale complex (Chlorophyta). J Phycol 28:615–619
Sekimoto H, Satoh S, Fujii T (1990) Biochemical and physiological properties of a protein inducing protoplast release during conjugation in the Closterium peracerosum-strigosum-littorale complex. Planta (Berl) 182:348–354
Sekimoto H, Inoki Y, Fujii T (1993a) Detection and evaluation of an inducer of diffusible mating pheromone of heterothallic Closterium peracerosum-strigosum-littorale complex. Plant Cell Physiol 37:991–996
Sekimoto H, Satoh S, Fujii T (1993b) Analysis of binding of biotinylated protoplast-release-inducing protein that induces release of gametic protoplasts in the Closterium peracerosum-strigosum-littorale complex. Planta (Berl) 189:468–474
Sekimoto H, Sone Y, Fujii T (1994a) cDNA cloning of a 42-kilodalton subunit of protoplast-release-inducing protein from Closterium. Plant Physiol 104:1095–1096
Sekimoto H, Sone Y, Fujii T (1994b) A cDNA encoding a 19-kilodalton subunit of protoplast-release-inducing protein from Closterium. Plant Physiol 105:447
Sekimoto H, Sone Y, Fujii T (1994c) Regulation of expression of the genes for a sex pheromone by an inducer of the sex pheromone in the Closterium peracerosum-strigosum-littorale complex. Planta (Berl) 193:137–144
Sekimoto H, Fukumoto R, Dohmae N, Takio K, Fujii T, Kamiya Y (1998) Molecular cloning of a novel sex pheromone responsible for the release of a different sex pheromone in Closterium peracerosum-strigosum-littorale complex. Plant Cell Physiol 39:1169–1175
Sekimoto H, Tanabe Y, Takizawa M, Ito N, Fukumoto R, Ito M (2003) Expressed sequence tags from the Closterium peracerosum-strigosum-littorale complex, a unicellular charophycean alga, in the sexual reproduction process. DNA Res 10:147–153
Sekimoto H, Tanabe Y, Tsuchikane Y, Shirosaki H, Fukuda H, Demura T, Ito M (2006) Gene expression profiling using cDNA microarray analysis of the sexual reproduction stage of the unicellular charophycean alga Closterium peracerosum-strigosum-littorale complex. Plant Physiol 141:271–279
Sekimoto H, Abe J, Tsuchikane Y (2012) New insights into the regulation of sexual reproduction in Closterium. Int Rev Cell Mol Biol 297:309–338. doi:10.1016/B978-0-12-394308-8.00014-5
Shiu SH, Bleecker AB (2001) Plant receptor-like kinase gene family: diversity, function, and signaling. Sci STKE 2001:re22. doi:10.1126/stke.2001.113.re22
Shiu SH, Bleecker AB (2003) Expansion of the receptor-like kinase/Pelle gene family and receptor-like proteins in Arabidopsis. Plant Physiol 132:530–543. doi:10.1104/pp. 103.021964
Shiu SH, Karlowski WM, Pan R, Tzeng YH, Mayer KF, Li WH (2004) Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant Cell 16:1220–1234. doi:10.1105/tpc.020834
Snell WJ, Eskue WA, Buchanan MJ (1989) Regulated secretion of a serine protease that activates an extracellular matrix-degrading metalloprotease during fertilization in Chlamydomonas. J Cell Biol 109:1689–1694
Stevens DR, Rochaix JD, Purton S (1996) The bacterial phleomycin resistance gene ble as a dominant selectable marker in Chlamydomonas. Mol Gen Genet 251:23–30
Tsuchikane Y, Fukumoto R, Akatsuka S, Fujii T, Sekimoto H (2003) Sex pheromones that induce sexual cell division in the Closterium peracerosum-strigosum-littorale complex (Charophyta). J Phycol 39:303–309
Tsuchikane Y, Ito M, Fujii T, Sekimoto H (2005) A sex pheromone, protoplast-release-inducing protein (PR-IP) Inducer, induces sexual cell division and production of PR-IP in Closterium. Plant Cell Physiol 46:1472–1476
Tsuchikane Y, Ito M, Sekimoto H (2008) Reproductive isolation by sex pheromones in the Closterium peracerosum-strigosum-littorale complex (Zynematales, Charophyceae). J Phycol 44:1197–1203
Tsuchikane Y, Kokubun Y, Sekimoto H (2010a) Characterization and molecular cloning of conjugation-regulating sex pheromones in homothallic Closterium. Plant Cell Physiol 51:1515–1523
Tsuchikane Y, Sato M, Ootaki T, Kokubun Y, Nozaki H, Ito M, Sekimoto H (2010b) Sexual processes and phylogenetic relationships of a homothallic strain in the Closterium peracerosum-strigosum-littorale complex (Zygnematales, Charophyceae). J Phycol 46:278–284
Tsuchikane Y, Tsuchiya M, Hindak F, Nozaki H, Sekimoto H (2012) Zygospore formation between homothallic and heterothallic strains of Closterium. Sex Plant Reprod 25:1–9. doi:10.1007/s00497-011-0174-z
Turmel M, Otis C, Lemieux C (2006) The chloroplast genome sequence of Chara vulgaris sheds new light into the closest green algal relatives of land plants. Mol Biol Evol 23:1324–1338. doi:10.1093/molbev/msk018
Watanabe MM, Ichimura T (1982) Biosystematic studies of the Closterium peracerosum-strigosum-littorale complex. IV. Hybrid breakdown between two closely related groups, group II-A and group II-B. Bot Mag Tokyo 95:241–247
Wodniok S, Brinkmann H, Glockner G, Heidel AJ, Philippe H, Melkonian M, Becker B (2011) Origin of land plants: do conjugating green algae hold the key? BMC Evol Biol 11:104. doi:10.1186/1471-2148-11-104
Zhang YH, Snell WJ (1994) Flagellar adhesion-dependent regulation of Chlamydomonas adenylyl cyclase in vitro: a possible role for protein kinases in sexual signaling. J Cell Biol 125:617–624
Zhu Y, Wan Y, Lin J (2010a) Multiple receptor complexes assembled for transmitting CLV3 signaling in Arabidopsis. Plant Signal Behav 5:300–302
Zhu Y, Wang Y, Li R, Song X, Wang Q, Huang S, Jin JB, Liu CM, Lin J (2010b) Analysis of interactions among the CLAVATA3 receptors reveals a direct interaction between CLAVATA2 and CORYNE in Arabidopsis. Plant J 61:223–233. doi:10.1111/j.1365-313X.2009.04049.x
Acknowledgments
The research projects were partly supported by Grants-in-Aid for Scientific Research (nos. 22405014, 23657161, 24370038, and 24247042 to H.S., no. 23770277 to J.A., no. 23770093 to Y.T.) from the Japan Society for the Promotion of Science, Japan, Grants-in-Aid for Scientific Research on Innovative Areas “Elucidating common mechanisms of allogenic authentication” (nos. 22112521 and 24112713 to H.S.) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, a grant from the New Technology Development Foundation to Y.T.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
This chapter is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
Copyright information
© 2014 The Author(s)
About this paper
Cite this paper
Sekimoto, H., Tsuchikane, Y., Abe, J. (2014). Sexual Reproduction of a Unicellular Charophycean Alga, Closterium peracerosum-strogosum-littorale Complex. In: Sawada, H., Inoue, N., Iwano, M. (eds) Sexual Reproduction in Animals and Plants. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54589-7_28
Download citation
DOI: https://doi.org/10.1007/978-4-431-54589-7_28
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54588-0
Online ISBN: 978-4-431-54589-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)