Abstract
Toxicants still represent a relevant stressor in river ecosystems. We describe the entry paths of toxicants into rivers and discuss the processes that determine the levels of exposure. Subsequently, the impacts of chemical toxicants on different levels of biological organisation, ranging from individuals to meta-communities, are portrayed with a special emphasis on the propogation of effects through biotic interactions and the modulation of effects through environmental conditions and potentially co-occurring stressors. We outline mitigation measures for different input paths that may reduce the concentrations of chemical toxicants, and consequently their risks to organisms, in river ecosystems. Finally, we discuss challenges and promising tools for a more realistic assessment of risks, which is the first step to an efficient management of chemicals in river ecosystems
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
1 Introduction
Chemicals are used widely in all spheres of modern society (Table 12.1), for example, in industrial production (e.g., solvents, coolants), medicine (e.g., pharmaceuticals), agriculture (e.g., pesticides), and consumer products (e.g., sunscreens). In 2015, more than 33 million chemicals were commercially available (Chemical Abstracts Service 2015) with approximately 100,000 estimated to be in current use and 30,000 produced with more than 1 ton per year in the European Union (EU) (Breithaupt 2006). The production of chemicals with harmful impacts to the aquatic environment in the EU totaled more than 130,000,000 tons in 2013 (EUROSTAT 2015). The widespread use of such chemicals comes with their intentional (e.g., pesticide spraying) and unintentional (e.g., leaching or gassing out) release into the environment, where they may enter river ecosystems via different paths depending on geological, hydrological, climatic and use patterns, often associated with land use, in the river’s catchment (see Chap. 13). These paths include (1) direct discharge, such as from industrial facilities, mining, or wastewater treatment plants (WWTPs), but also accidental spills; (2) runoff from the land surface or subsurface flows, often after precipitation; (3) erosion or disposal of waste, which can lead to the re-suspension, desorption, or diffusion of chemicals into the water phase; and (4) atmospheric deposition of chemicals.
The entry paths can be classified as point sources (e.g., path 1) and nonpoint (also diffuse, e.g., paths 2 and 4) sources, a distinction that is important with respect to management actions. The relevance of the entry paths is compound specific (i.e., influenced by physicochemical properties such as solubility, lipophilicity, vapor pressure) and depends on the environmental context of the river in terms of pH, temperature, ultraviolet radiation, and soil type. For example, compounds with high water solubility and a high vapor pressure (e.g., urea herbicides) enter aquatic systems predominantly via discharge, runoff, or subsurface flows, whereas compounds with a low water solubility and low vapor pressure (e.g., polychlorinated biphenyls) primarily enter aquatic systems via atmospheric deposition. Furthermore, compounds with a low water solubility and high vapor pressure (e.g., pyrethroid insecticides) enter aquatic systems primarily adsorbed or bound to particulate matter, often during runoff events. Moreover, the environmental context influences the movement and, in the case of organic compounds, degradation and bioavailability of chemicals. Similarly, several environmental variables (e.g., pH, temperature, ionic composition) govern the speciation of metals and consequently their ecotoxicological effects.
Catchment hydrology is another important driver of the chemical exposure regime. The magnitude, timing, frequency, and duration of spates as well as the lateral and longitudinal connectivity influence transport and concentration levels of chemicals in rivers (see Chap. 3, 5, 9). For example, the river discharge determines the dilution potential for micropollutants (i.e., organic and inorganic chemical stressors detected at concentrations of μg/L or lower) released from WWTPs (Englert et al. 2013). Thus, discharge minima may be associated with the highest chemical concentrations from point sources. Moreover, discharge maxima are typically associated with heavy precipitation events that can induce surface runoff or subsurface drainage leading to the diffuse entry of agrochemicals (e.g., pesticides and nutrients) and of chemicals from roads and urban environments [e.g., biocides, metals, nanomaterials, and polycyclic aromatic hydrocarbons (PAHs)]. Again this can lead to transient peak concentrations, particularly in streams, and in rivers with a strong variability in discharge.
Depending on the compound, peak concentrations can be a more important driver of ecological effects than the average concentration (see next section). Consequently, the hydrological profile should also be considered when designing monitoring programs for chemicals in a river. An adequate exposure characterization relies on sampling methods that allow for the sampling at discharge minima and maxima. However, current governmental monitoring is largely independent from such considerations and is conducted at fixed temporal intervals. For example, an analysis of European river monitoring data showed that the sampling frequency is monthly or lower in 80% of sampling sites (Malaj et al. 2014). Such a monitoring strategy is likely to miss short-term exposure events such as runoff, which result in peak concentrations in streams and in rivers with a strong variability in discharge. For streams, a modeling study revealed that monthly monitoring would miss almost all peak exposure events of pesticides, which lasted from several hours to a few days (Stehle et al. 2013).
Multiple sampling methods that could be used in river systems have been developed to capture peak exposure events. These methods include automated sampling devices that can take event-triggered or flow-dependent samples (Mortimer et al. 2007). Passive sampling, i.e., the deployment of devices with receiving phases into which chemicals passively diffuse or adsorb to (for an overview, see Vrana et al. 2005), can also be tailored to obtain estimates of peak exposures. However, the abovementioned methods need adaptation to the spectrum of chemicals. In the case of passive sampling, for example, a suitable receiving phase for the chemicals of interest needs to be identified (Vrana et al. 2005). In this context, the analysis of land use in the catchment can aid in selecting target chemicals for the chemical monitoring (see Chap. 13): Agricultural land use is the dominant driver of pesticide input in water bodies, whereas biocides rather enter surface waters through urban runoff or via WWTPs (Wittmer et al. 2010). Nonetheless, a complete characterization of chemical exposure remains a formidable challenge, especially in catchments with mixed land use, where several hundred or thousand compounds may be present in the different environmental media, while most monitoring programs are limited to a few tens to hundreds of chemicals. Biomonitoring in concert with stressor-specific biotic metrics, bioassays that indicate toxic exposure, and effect-directed analysis and related approaches therefore represent important complementary tools for a more holistic characterization of the chemical exposure regime (Brack et al. 2015).
2 Impacts
2.1 Propagation of Impacts Across Levels of Biological Organization
Chemical exposure can impact individual freshwater organisms and higher levels of biological organization (e.g., populations, species). Several examples of effects from compounds representative for different chemical groups on ecosystem properties are provided in Table 12.1. In this context, a toxic chemical acts initially on the physiological level of an organism (Fig. 12.1). For example, polychlorinated biphenyls and other organochlorine chemicals have been associated with changes in enzyme activity and chemical signaling such as induction of vitellogenin or reduced plasma levels of 11-ketotestosterone (Table 12.1). Such physiological effects represent the basis for effects on higher biological levels (Fig. 12.1): Changes in the metabolism and chemical signaling can lead to individual-level effects such as reduced fitness or mortality. This in turn, depending on the magnitude of effects on individuals, translates to a reduction in the population size or, in the case of endocrine-disrupting chemicals, to a change in the gender ratio (males to females). On the next hierarchical level, impacts on populations may change the composition of communities, as reported for microorganisms in response to antibiotics, to the biocide triclosan, and to metals such as copper (Table 12.1). Community changes can result in a reduction of ecosystem functioning, for example, of organic matter processing (Table 12.1), if community-level impacts are not buffered by functional redundancy. In other words, a reduction in functioning is induced by the loss of sensitive organisms, which is not compensated by an increase in tolerant organisms. Finally, ecosystem-level impacts can influence meta-ecosystem dynamics and the spatial and temporal distribution of species as well as macroecological characteristics, such as species-abundance distributions (Fig. 12.2). The ecological relevance of an impact increases from the physiological level toward the meta-ecosystem and landscape level, whereas understanding of impacts is highest on the lower levels of biological organization (e.g., physiological and individual). This discrepancy between relevance and understanding is partly due to the fact that most ecotoxicological studies focus on these lower levels of organization (Beketov and Liess 2012).
2.2 Relevance of Chemical Input into River Ecosystems
The relevance of chemical impacts on streams and rivers has been highlighted in several large-scale studies. Toxic chemicals, organic and inorganic, were listed among the most important pollutants in the Millennium Ecosystem Assessment for Rivers (Fynlayson and D’Cruz 2005). Similarly, a global analysis identified water pollution and water resource development as major ecological threats (Vörösmarty et al. 2010), with pesticides, nutrients, and metals comprising the dominant drivers of water pollution. Recent studies on insecticides on the global scale and on organic chemicals on the European scale estimated that approximately 70 and 42% of water bodies are at risk of adverse effects (Stehle and Schulz 2015; Malaj et al. 2014). However, all of the abovementioned studies relied on simplified risk assessment approaches, which involve the comparison of a measured or predicted environmental concentration of chemicals to an effect metric (e.g., effect concentration, threshold concentration), which is often related to ecotoxicological laboratory or mesocosm (e.g., artificial outdoor ponds) experiments. Such experiments mainly capture acute toxic effects of chemicals, e.g., direct mortality. Thus, the majority of experimental data refer to acute toxic effects, whereas data coverage is considerably lower for long-term chronic effects (Malaj et al. 2014) or indirect effects within food webs, which are further discussed below.
Combining this widespread availability of reliable acute toxicity data for many organic chemicals and metals with the fact that their toxicity in aquatic ecosystems varies strongly, the risk and exposure are often standardized based on the compounds’ acute toxicity, especially in scientific studies. However, this biases risk assessments, such as those mentioned above, toward chemicals that are likely to cause short-term acute toxicity, such as pesticides and metals. Notwithstanding, several other chemicals that occur widely in the environment can also have strong impacts on populations and communities (Table 12.1) through other modes of action than those linked to direct acute toxicity. Endocrine disruption, i.e., effects on the hormone system, may compromise long-term population viability (Kidd et al. 2007), and long-term chronic exposure from hydrophobic chemicals can have dramatic effects similar to acute short-term toxicity (see discussion in Malaj et al. 2014). For instance, exposure to low levels of estrogens, which can occur downstream from WWTPs, induced a collapse of a fish population after 3 years of chronic exposure (Kidd et al. 2007). Thus, such chemicals and their potential to affect the integrity of aquatic ecosystems over the long term may have been overlooked in these larger-scale risk assessment studies. Moreover, chemical monitoring has been tailored toward pesticides and metals that are long-known culprits of chemical impacts, and exposure data on other micropollutants (e.g., pharmaceuticals, biocides, nanoparticles) is rather scarce. Nevertheless, chemical monitoring programs have started to include pharmaceuticals and biocides over the last decade. To sum up, although metal and pesticide toxicity are important drivers of chemical effects and related risks are widespread, several other chemical groups that occur widely and can affect riverine organisms have not received sufficient recognition due to a lack of exposure and effect data, especially related to real-world conditions. As outlined above, effect-directed analysis and related approaches represent important tools to unravel emerging toxicants (Brack et al. 2015), but they are rather eligible for application in case studies than for large-scale routine monitoring.
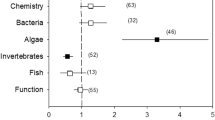
The relevance of chemical groups varies with land use (see above and Chap. 13) and, more broadly, with the management of chemical inputs. For example, many European countries strongly regulate certain chemicals, and wastewater is usually treated before discharge into surface water bodies. Consequently, in most European regions, chemical risks from nonpoint sources are presumably highest for invertebrates and primary producers (see discussion in Malaj et al. 2014), whereas fish are at low risk, albeit the efficiency of wastewater treatment varies with the compound group and related discharges may entail ecotoxicological risks (see below). Contrasting examples occur in several South Asian countries, where water regulation, and enforcement of the latter, is weaker, leading to higher chemical pollution levels, which can pose serious risks for all aquatic organisms including fish but also for human health, if the contaminated river water is used for human consumption (Vörösmarty et al. 2010). In addition, the catchment hydrology, climate, and geology influence the relevance of chemicals. For example, particularly in arid and semiarid regions, irrigation agriculture can result in increasing ion concentrations in water bodies and changes in ion ratios with associated adverse ecological effects (Cañedo-Argüelles et al. 2013). Furthermore, acid rain is particularly relevant in regions with hard-rock geology, such as granite, because of the lack of buffer capacity. The influence of the environmental context on impacts of chemicals is discussed in more detail in the next section.
2.3 Assessing and Predicting Impacts of Chemicals in River Systems
The assessment and prediction of chemical impacts in river systems are complicated by a range of context-dependent factors that act on different spatial scales (Clements et al. 2012). Here, we focus particularly on factors that influence the impacts of chemicals in river systems on the site and landscape scales (Fig. 12.2). Several of the factors listed in Fig. 12.2 can influence the impacts of chemicals on freshwater organisms twofold: firstly, through their influence on chemical concentrations, bioavailability, and speciation in the case of metals or ionizable organic compounds and, secondly, as filters that shape the composition and sensitivity of populations and communities on which chemical toxicants act. Above, we have already outlined that catchment hydrology, climate, land use, and geology influence the exposure patterns of chemicals. However, they also influence the impact on aquatic organisms by determining which species are present and potentially modulating their physiological fitness and ultimately sensitivity. For example, hydrology, in terms of water level fluctuations, resulted in a tenfold increase in the sensitivity of invertebrate communities to an insecticide in a study with artificial ponds (Stampfli et al. 2013). In fact, a wide range of additional stressors, including the abiotic variables such as pH and temperature, have been identified as modulators of chemical impacts (Laskowski et al. 2010). This is hardly surprising given that other pairs of stressors in rivers such as habitat degradation (see Chap. 3) and eutrophication (Chap. 10) are also known to interact in their impact on freshwater organisms (Jackson et al. 2015). Consequently, prediction of the impacts of a chemical in a river would require understanding of the interactions of chemicals with potentially co-occurring stressors or the environmental context in general (Clements et al. 2012).
This is highly relevant for risk assessment as it implies that the same chemical concentration can have different impacts depending on the context (Fig. 12.2). Indeed, the multiple-stressor situation is rather the rule than the exception. An analysis of four stressor classes in German rivers, namely, habitat degradation, eutrophication, organic chemicals, and invasive species, found that almost all river sampling sites (97%) were simultaneously subjected to two or more stressor classes (Schäfer et al. 2016). Thus, the multiple-stressor situation has stimulated large research efforts, such as the EU project MARS involving 24 research institutions (http://www.mars-project.eu), though chemicals play a minor role in this project. However, chemicals typically occur as mixtures and estimation of mixture effects of chemicals in real-world ecosystems still represents a major challenge (Brack et al. 2015).
Besides co-occurring stressors and abiotic variables, a wide range of context-dependent variables govern the impacts on populations and communities. These include, for instance, the history of disturbance, where communities with a long history of chemical exposure may embody a history of adaptations (Johnson and Sumpter 2015). Furthermore, the capacity to cope with or adapt to stress is related to the energetic state of the community. Riparian vegetation contributes to the energetic state of a river through provisioning of organic matter. This energetic state depends also on the position in the river network as conceptualized by the river continuum concept (Vannote et al. 1980). At the same time, the local populations and communities are linked to meta-population and meta-community dynamics, which also depend on river network connectivity and overland distances to other network branches as well as in-stream distances to recolonization pools (Kärnä et al. 2015). These aspects influence the resilience of river ecosystems to chemical effects, which is further discussed below in the context of mitigation.
An additional source of complexity when aiming to predict impacts based on chemical exposure results from species interactions inside communities: If a chemical affects a species A that interacts with another species B, the chemical impact can propagate onto species B, although this species was not directly affected by the toxicant. Analogous to general ecology, this phenomenon is called an indirect effect, though the distinction between trait-mediated and density-mediated indirect effects is rarely made in ecotoxicological studies due to their strong laboratory orientation (see Schäfer et al. 2016). An example for an indirect (density-mediated) effect is represented by an herbicide input that eradicates periphyton leading to a reduction of invertebrate grazers that had not been directly impacted by the herbicide.
Overall, we have outlined that several factors influence the effects of a chemical under real-world conditions. Rivers often integrate, depending on the size of the upstream catchment area, the outputs of different land use types leading to exposure from point sources such as industrial or wastewater effluents with micropollutants, pesticides from agriculture, and potentially biocides and salts from roads and urban areas. These mixtures of chemicals can have a variety of direct and indirect effects on the different trophic levels in a river, which, as highlighted above, also depend on the general environmental context. This complexity may explain the mismatch between effect thresholds based on laboratory or semi-field studies under simplified conditions and those from field studies that have been observed for pesticides and metals (Schäfer 2014). However, the assessment and prediction of chemical effects are crucial for river management, taking also into account that affected river sections are interconnected longitudinally with other sections and rivers as well as with the surrounding landscape.
Given that both chemical exposure and effects in rivers are currently incompletely captured, the true risk is difficult to predict. Therefore, the sheer amount of chemicals in use that can potentially occur as mixtures in rivers requires intelligent prioritization based on adapted monitoring strategies that enable a realistic characterization of exposure and effects. The establishment of exposure scenarios, based on land use and point sources, through joint modeling and monitoring approaches might aid in defining priority substances and priority mixtures that are likely to occur and promote a more realistic exposure characterization. Such scenarios could also help to assess potential mixture effects as well as the interactions with co-occurring stressors, which currently hamper a realistic evaluation of the contribution of chemicals to the ecological degradation of rivers. The actual situation is partly due to the too narrow focus of current ecotoxicological risk assessment approaches (Beketov and Liess 2012). More holistic and field-realistic ecotoxicological approaches are required and may include tools that detect effects in rivers on different biological levels such as biomarkers, bioassays, and trait-based community, meta-population, and meta-community approaches. Moreover, tools that allow for the detection of effects on ecosystem processes and in turn for the evaluation of the consequences of such effects for ecosystem services are relevant for river managers to balance management measures and to highlight the relevance of measures to the public and stakeholders potentially involved in decision-making. Additional research in the abovementioned areas is needed to deliver such tools, though several promising avenues exist (Brack et al. 2015; Segner et al. 2014).
3 Mitigation
With the goal to reduce the load of chemical stressors in ecosystems, various mitigation measures of different categories have been proposed that range from substitution and reduction of chemicals to effluent treatment and landscape design. Ideally, chemicals would only be active at their place of intended action (e.g., pharmaceuticals inside the organism, pesticides on the crop area) and would be harmless by design when entering nontarget ecosystems. This could, for example, be achieved through rapid and complete degradation in the case of organic chemicals, which has been characterized as “benign by design” (Kümmerer 2007). However, implementation of this concept seems challenging for chemicals such as biocides and pesticides that are used in products with an intended high longevity (e.g., paint) or that are released to crop areas to harm specific target organisms but subsequently often enter other environments, where nontarget organisms that are physiologically similar to the target organisms are exposed.
Use reduction and substitution with compounds that have a lower environmental risk are mitigation measures to decrease exposure and impacts in ecosystems such as rivers. Apart from measures that aim at reducing the amount of a chemical released into a receiving ecosystem at its source (e.g., discharge from industrial facilities and mines), many “end-of-pipe” technologies and measures are currently under discussion to decrease the chemical load of rivers. These can be separated into measures targeting point sources and nonpoint sources. Regarding point sources, the most widely used technique is the treatment of the effluent before it enters a water body, for example, in municipal WWTPs. However, such treatments currently are incomplete for several chemicals including metals and organic micropollutants. Consequently, advanced treatment technologies have been developed to reduce the concentrations of metals or organic micropollutants, the latter for which WWTP effluents are considered the major source (Schwarzenbach et al. 2006). For metals, chemical precipitation has widely been employed to reduce metal concentrations in effluents. This process, however, produces sludge that needs to be disposed. Alternative methods include adsorption to new adsorbents (bio-sorption), membrane filtration, and electrodialysis that have a higher removal efficiency than conventional chemical precipitation but, depending on the method, also lead to toxic waste or incur high operational costs (Barakat 2011). For organic micropollutants, activated carbon filtration and ozonation can reduce the concentrations on average by approximately 80%, where the efficiency depends on the chemical properties of the individual chemical (Margot et al. 2013). Such reductions in the chemical concentrations are also reflected by alleviation of various ecotoxicological measures at subcellular, organism, and population levels, as documented by a meta-analysis (Bundschuh et al. 2011).
While they do offer increases in treatment efficacy, both advanced treatment technologies increase treatment costs and can also impact aquatic organisms. Activated carbon, for instance, may adsorb, besides organic micropollutants, essential trace elements. This results in negative, sublethal effects on aquatic life. The impacts of these effects in the receiving ecosystem may be limited if the incoming wastewater is sufficiently enriched with nutrients from the receiving ecosystem (Bundschuh et al. 2011). Moreover, ozonation may induce the formation of toxic oxidation by-products such as aldehydes and carboxylic acids that can adversely affect fish and other organisms. These by-products can eventually be removed by means of sand filtration, thus detoxifying the wastewater prior to its release into the receiving stream or river ecosystem (Bundschuh et al. 2011). Furthermore, treatment technologies have been developed to remove salt concentrations in effluents originating from resource extraction activities such as mining or hydraulic fracturing (Cañedo-Argüelles et al. 2013).
Although empirical evidence suggests that advanced treatment technologies improve the chemical quality of the wastewater, it has been argued that aquatic communities downstream of such point sources are adapted to the environmental conditions including pollution after several decades of chronic exposure. Consequently, Johnson and Sumpter (2015) cautioned that a modification of water quality, albeit an improvement from the human perspective, might have negative consequences for communities downstream from WWTP potentially forcing an ecosystem to leave its steady state by undergoing a regime shift. These considerations call for a careful monitoring of the responses of wildlife within the receiving aquatic ecosystem to understand the long-term consequences of implementing advanced treatment technologies.
In contrast to these point sources, nonpoint sources of pesticides as well as metals and salts require different approaches to achieve a reasonable level of mitigation. In particular pesticides are intentionally released to the environment when applied on crops to control for weeds (i.e., herbicides) as well as for fungal and for insect pests (i.e., fungicides and insecticides). During or following their application, they non-intentionally enter off-crop areas, such as aquatic ecosystems, via spray drift and runoff (Schulz 2004). Similarly, salts are intentionally released into the environment as road de-icers and can run off from roads and urban areas and in turn contribute to the salinization of freshwater ecosystems (Cañedo-Argüelles et al. 2013). Another nonpoint source of salts is agriculture, where, especially in arid and semiarid regions, irrigation dissolves soil-borne salts and the clearing of natural vegetation, especially trees, can lead to rising groundwater tables of saline water (see Jolly et al. 2001 for examples in the Murray-Darling basin in Australia). Both processes potentially cause diffuse inputs of saline effluents into water bodies. Similar to salt input from de-icing, nonpoint sources of metals are primarily runoff from roads and urban areas following heavy precipitation. Vegetated buffer strips are one mitigation measure that can reduce the concentrations of toxicants (pesticides, metals, and salts from agricultural fields, roads, and urban areas) in runoff flowing into aquatic ecosystems (Schulz 2004), though erosion rills within these buffer strips can jeopardize their retention efficiency. Moreover, such buffer strips can substantially decrease the input of pesticides into rivers via spray drift, depending on the density of the vegetation, with dense shrubbery being most efficient (Schulz 2004).
Within the aquatic environment, natural and constructed wetlands are decentralized mitigation measures that can strongly reduce the concentrations and associated effects of hydrophobic organic substances and metals [partly up to 90% (Schulz 2004)]. Within these wetland systems, various processes contribute to the reduced load of pesticides and metals in the water phase. The primary processes are adsorption to sediments and macrophytes as well as trapping of the suspended particles carrying the insecticides. The secondary contributions of photolysis, hydrolysis, and microbial degradation to mitigation efficacy of wetlands are lower (Stehle et al. 2011). Recent studies showed that wetlands can also mitigate the peak concentrations of rather hydrophilic chemicals, albeit to a lower degree compared to hydrophobic substances (Stehle et al. 2011). Hence, wetlands but also buffer strips seem suitable low-tech solutions for environmental managers to mitigate loads and effects of nonpoint source inputs of chemicals, at least for compounds that are subjected to degradation processes (Fenner et al. 2013).
Other chemicals such as PAHs and phenomena such as acidification are linked to the wet and dry deposition of chemicals from the atmosphere. In such cases, the reduction of chemical emissions, for example, in the case of acidification of sulfur dioxide and nitrogen oxides, and treatment before emission are the most feasible mitigation measure. Finally, given that chemical effects such as diffuse inputs from extreme weather events cannot always be avoided and, in fact, are partly acceptable within the framework of current pesticide regulation, as long as recovery occurs, fostering the resilience of the river ecosystem represents an important measure. This can be achieved through improving the connectivity of river systems (Chap. 9) as well as through preservation and creation of recolonization pools on the landscape level. Indeed, several studies in lentic and lotic water bodies emphasized the importance of external recolonization from such pools for recovery from toxicant effects (Schäfer 2012; Trekels et al. 2011). The importance of healthy and intact pools and wetlands as nurseries and refugia on the floodplain was amply demonstrated following a massive cyanide spill on the Tisza river in 2000. Whereas all life in the river channel was extinguished by the cyanide plume, recolonization from the neighboring floodplain habitats was swift (weeks and months) and efficient (Sáyli et al. 2000).
4 Conclusions
Toxicants still represent a relevant stressor in river ecosystems, despite major improvements of the situation over the last decades, at least in regions with a strong governmental regulation. Intelligent strategies are required to deal with the complex exposure situation in terms of mixtures, multiple stressors, and other features of the environmental context that all influence the magnitude of potential negative effects. The identification of land use-specific exposure paths, the subsequent prioritization of compounds and adaptation of monitoring strategies, as well as the development of more holistic and field-realistic ecotoxicological tools would enable a reliable characterization of exposure and effects, which is of paramount importance. Notwithstanding, a variety of mitigation measures is available to address point and nonpoint source pollution that range from local effluent treatment to the enhancement of resilience of river ecosystems through landscape approaches. Several of these approaches have the advantage that they would not only alleviate the problem of chemical toxicants in rivers but also, for instance, reduce excessive nutrient inputs and enhance riverine connectivity.
References
Barakat MA (2011) New trends in removing heavy metals from industrial wastewater. Arab J Chem 4:361–377
Beketov MA, Liess M (2012) Ecotoxicology and macroecology – time for integration. Environ Pollut 162:247–254
Brack W, Altenburger R, Schüürmann G, Krauss M, López HD, van Gils J, Slobodnik J, Munthe J, Gawlik BM, van Wezel A, Schriks M, Hollender J, Tollefsen KE, Mekenyan O, Dimitrov S,Bunke D, Cousins I, Posthuma L, van den Brink PJ, López de Alda M, Barceló D, Faust M, Kortenkamp A, Scrimshaw M, Ignatova S, Engelen G, Massmann G, Lemkine G, Teodorovic I, Walz K-H, Dulio V, Jonker MTO, Jäger F, Chipman K, Falciani F, Liska I, Rooke D, Zhang X, Hollert H, Vrana B, Hilscherova K, Kramer K, Neumann S, Hammerbacher R, Backhaus T, Mack J, Segner H, Escher B, de Aragão Umbuzeiro G (2015) The SOLUTIONS project: challenges and responses for present and future emerging pollutants in land and water resources management. Sci Total Environ 503–504:22–31
Breithaupt H (2006) The costs of REACH. EMBO Rep 7:968–971
Bundschuh M, Gessner MO, Ternes TA, Sögding C, Schulz R (2011) Ecotoxicologial evaluation of wastewater ozonation based on detritus-detritivore interactions. Chemosphere 82:355–361
Cañedo-Argüelles M, Kefford B, Piscart C, Prat N, Schäfer RB, Schulz C-J (2013) Salinisation of rivers: an urgent ecological issue. Environ Pollut 173:157–167
Chemical Abstracts Service (2015) CHEMCATS – chemical suppliers database. http://www.cas.org/content/chemical-suppliers
Clements WH, Carlisle DM, Lazorchak JM, Johnson PC (2000) Heavy metals structure benthic communities in Colorado mountain streams. Ecol Appl 10:626–638
Clements WH, Hickey CW, Kidd KA (2012) How do aquatic communities respond to contaminants? It depends on the ecological context. Environ Toxicol Chem 31:1932–1940
Drury B, Scott J, Rosi-Marshall EJ, Kelly JJ (2013) Triclosan exposure increases triclosan resistance and influences taxonomic composition of benthic bacterial communities. Environ Sci Technol 47:8923–8930
Englert D, Zubrod JP, Schulz R, Bundschuh M (2013) Effects of municipal wastewater on aquatic ecosystem structure and function in the receiving stream. Sci Total Environ 454–455:401–410
EUROSTAT (2015) Production of environmentally harmful chemicals. http://ec.europa.eu/eurostat/tgm/table.do?tab=table&init=1&language=de&pcode=ten00011&plugin=1
Fenner K, Canonica S, Wackett LP, Elsner M (2013) Evaluating pesticide degradation in the environment: blind spots and emerging opportunities. Science 341:752–758
Fent K, Kunz PY, Gomez E (2008) UV filters in the aquatic environment induce hormonal effects and affect fertility and reproduction in Fish. CHIMIA Int J Chem 62:368–375
Floehr T, Scholz-Starke B, Xiao H, Hercht H, Wu L, Hou J, Schmidt-Posthaus H, Segner H, Kammann U, Yuan X, Roß-Nickoll M, Schäffer A, Hollert H (2015) Linking Ah receptor mediated effects of sediments and impacts on fish to key pollutants in the Yangtze Three Gorges Reservoir, China — a comprehensive perspective. Sci Total Environ 538:191–211
Fynlayson C, D’Cruz R (2005) Inland water systems. In: Millenium ecosystem assessment. Island Press, Washington, DC, pp 551–583
Jackson MC, Loewen CJG, Vinebrooke RD, Chimimba CT (2015) Net effects of multiple stressors in freshwater ecosystems: a meta-analysis. Glob Chang Biol 22:180–189
Johnson AC, Sumpter JP (2015) Improving the quality of wastewater to tackle trace organic contaminants: think before you act! Environ Sci Technol 49:3999–4000
Jolly ID et al (2001) Historical stream salinity trends and catchment salt balances in the Murray–Darling Basin, Australia. Mar Freshw Res 52(1):53–63
Kärnä O-M, Grönroos M, Antikainen H, Hjort J, Ilmonen J, Paasivirta L, Heino J (2015) Inferring the effects of potential dispersal routes on the metacommunity structure of stream insects: as the crow flies, as the fish swims or as the fox runs? J Anim Ecol 84:1342–1353
Kidd KA, Blanchfield PJ, Mills KH, Palace VP, Evans RE, Lazorchak JM, Flick RW (2007) Collapse of a fish population after exposure to a synthetic estrogen. Proc Natl Acad Sci USA 104:8897–8901
Kümmerer K (2007) Sustainable from the very beginning: rational design of molecules by life cycle engineering as an important approach for green pharmacy and green chemistry. Green Chem 9:899–907
Laskowski R, Bednarska AJ, Kramarz PE, Loureiro S, Scheil V, Kudlek J, Holmstrup M (2010) Interactions between toxic chemicals and natural environmental factors – a meta-analysis and case studies. Sci Total Environ 408:3763–3774
Malaj E, von der Ohe PC, Grote M, Kühne R, Mondy CP, Usseglio-Polatera P, Brack W, Schäfer RB (2014) Organic chemicals jeopardize the health of freshwater ecosystems on the continental scale. Proc Natl Acad Sci USA 111:9549–9554
Maltby L, Forrow DM, Boxall ABA, Calow P, Betton CI (1995) The effects of motorway runoff on freshwater ecosystems: 1. Field study. Environ Toxicol Chem 14:1079–1092
Margot J, Kienle C, Magnet A, Weil M, Rossi L, de Alencastro LF, Abegglen C, Thonney D, Chèvre N, Schärer M, Barry DA (2013) Treatment of micropollutants in municipal wastewater: ozone or powdered activated carbon? Sci Total Environ 461–462:480–498
Mortimer M, Mueller JM, Liess M (2007) Sampling methods in surface waters. In: Nollet LML (ed) Handbook of water analysis. CRC Press/Taylor & Francis Group, Boca Raton, FL, pp 1–45
Pei R, Kim S-C, Carlson KH, Pruden A (2006) Effect of river landscape on the sediment concentrations of antibiotics and corresponding antibiotic resistance genes (ARG). Water Res 40:2427–2435
Pye MC, Vaughan IP, Ormerod SJ (2012) Episodic acidification affects the breakdown and invertebrate colonisation of oak litter. Freshw Biol 57:2318–2329
Randak T, Zlabek V, Pulkrabova J, Kolarova J, Kroupova H, Siroka Z, Velisek J, Svobodova Z, Hajslova J (2009) Effects of pollution on chub in the River Elbe, Czech Republic. Ecotoxicol Environ Saf 72:737–746
Sáyli G, Csaba G, Gaálné-Darin E, Orosz E, Láng M, Majoros G, Kunsági Z, Niklesz C (2000) Effect of the cyanide and heavy metal pollution of the Szamos and Tisza rivers on the aquatic flora and fauna with special attention to fish. Magyar Állatorvosok Lapja 122(8):493–500
Schäfer RB (2014) In response: why we need landscape ecotoxicology and how it could be advanced – an academic perspective. Environ Toxicol Chem 33:1193–1194
Schäfer RB, von der Ohe P, Rasmussen J, Kefford JB, Beketov M, Schulz R, Liess M (2012) Thresholds for the effects of pesticides on invertebrate communities and leaf breakdown in stream ecosystems. Environ Sci Technol 46:5134–5142
Schäfer RB, Kühn B, Malaj E, König A, Gergs R (2016) Contribution of organic toxicants to multiple stress in river ecosystems. Freshw Biol. https://doi.org/10.1111/fwb.12811
Schulz R (2004) Field studies on exposure, effects, and risk mitigation of aquatic nonpoint-source insecticide pollution: a review. J Environ Qual 33:419–448
Schwarzenbach RP, Escher BI, Fenner K, Hofstetter TB, Johnson CA, von Gunten U, Wehrli B (2006) The challenge of micropollutants in aquatic systems. Science 313:1072–1077
Segner H, Schmitt-Jansen M, Sabater S (2014) Assessing the impact of multiple stressors on aquatic biota: the receptor’s side matters. Environ Sci Technol 48:7690–7696
Stampfli NC, Knillmann S, Liess M, Noskov YA, Schäfer RB, Beketov MA (2013) Two stressors and a community – effects of hydrological disturbance and a toxicant on freshwater zooplankton. Aquat Toxicol 127:9–20
Stehle S, Schulz R (2015) Agricultural insecticides threaten surface waters at the global scale. Proc Natl Acad Sci USA 112:5750–5755
Stehle S, Elsaesser D, Gregoire C, Imfeld G, Passeport E, Payraudeau S, Schäfer RB, Tournebize J, Schulz R (2011) Pesticide risk mitigation by vegetated treatment systems: a meta-analysis. J Environ Qual 40:1068–1080
Stehle S, Knabel A, Schulz R (2013) Probabilistic risk assessment of insecticide concentrations in agricultural surface waters: a critical appraisal. Environ Monit Assess 185:6295–6310
Trekels H, Van de Meutter F, Stoks R (2011) Habitat isolation shapes the recovery of aquatic insect communities from a pesticide pulse. J Appl Ecol 48:1480–1489
Vannote RL, Minnshall WG, Cummins KW, Sedell JR, Cushing CE (1980) The river continuum concept. Can J Fish Aquat Sci 37:130–137
Vethaak AD, Lahr J, Schrap SM, Belfroid AC, Rijs GBJ, Gerritsen A, de Boer J, Bulder AS, Grinwis GCM, Kuiper RV, Legler J, Murk TAJ, Peijnenburg W, Verhaar HJM, de Voogt P (2005) An integrated assessment of estrogenic contamination and biological effects in the aquatic environment of the Netherlands. Chemosphere 59:511–524
Vörösmarty CJ, McIntyre PB, Gessner MO, Dudgeon D, Prusevich A, Green P, Glidden S, Bunn SE, Sullivan CA, Liermann CR, Davies PM (2010) Global threats to human water security and river biodiversity. Nature 467:555–561
Vrana B, Allan IJ, Greenwood R, Mills GA, Dominiak E, Svensson K, Knutsson J, Morrison G (2005) Passive sampling techniques for monitoring pollutants in water. TrAC Trends Anal Chem 24:845–868
Wittmer IK, Bader HP, Scheidegger R, Singer H, Luck A, Hanke I, Carlsson C, Stamm C (2010) Significance of urban and agricultural land use for biocide and pesticide dynamics in surface waters. Water Res 44:2850–2862
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made. The images or other third party material in this book are included in the book's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the book's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2018 The Author(s)
About this chapter
Cite this chapter
Schäfer, R.B., Bundschuh, M. (2018). Ecotoxicology. In: Schmutz, S., Sendzimir, J. (eds) Riverine Ecosystem Management. Aquatic Ecology Series, vol 8. Springer, Cham. https://doi.org/10.1007/978-3-319-73250-3_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-73250-3_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-73249-7
Online ISBN: 978-3-319-73250-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)