Abstract
Using modern genome editing tools, scientists are increasingly able to engineer animals and plants for better traits and improved downstream outcomes that benefit humans. As part of the CRISPR-Cas system, guide RNA (gRNA) is used to identify the target sequence, while Cas is an endonuclease that performs the nucleotide cleavage. It is imperative that these two components are delivered to the nucleus of the cell in order to ensure an optimal editing process. As a consequence of differences in the cellular structure and biomolecular composition of the outer membrane, plants are not capable of being cloned genetically in the same manner as animal cells. A more optimized method and pipeline must be developed to improve the efficiency of transformations and genome editing for plants. In this book chapter, we highlight traditional and novel delivery methods used for optimal delivery of plant genome editing components. We discuss the potential and limitations of these methods in the light of recent literature and available experimental validations.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
1 Introduction
CRISPR/Cas systems are a vital component of genome engineering tools for animals and plants due to their ability to analyse gene function and accelerate trait development [1]. Efficient editing, convenient design, cost-effectiveness, and simplicity provide an edge to CRISPR-Cas over other editing tools. The system consists of guide RNA (gRNA), for target sequence recognition, and Cas, a CRISPR-associated protein endonuclease, which exhibits different versions and is sometimes modified and/or linked to other molecules to achieve specific targets. All relevant components must be delivered into the cell nucleus to achieve genome editing or other modifications.
Compared to animal systems, plants have distinctive characteristics which may limit the application of those methods developed for animal cells, thus requiring the development of specific plant-based methods. In this chapter, a number of traditional and novel delivery methods are discussed with reference to their potential and their limitations, with the ultimate aim of increasing efficiency and accuracy, and broadening the application of genotype-independent delivery systems in plants.
2 Biological Delivery Methods
2.1 Bacteria Based Methods
Successful editing outcomes in crops are reliant on the availability of efficient genotype-independent delivery systems which facilitate the introduction of individual genome editing components. Bacterial-mediated delivery of CRISPR/Cas components into plant cells is probably the most popular method in use, and one that incorporates a foreign gene into the plant genome using a binary vector system. The natural ability of Agrobacterium tumefaciens to engineer plant cells in the wild, and whose mechanism has been exploited by plant biotechnologists in the lab, means that this bacterial-based delivery system has been used efficiently for transforming both monocot and eudicot plants, notwithstanding the recalcitrant nature of some plant species to Agrobacterium infection. To address this recalcitrance with some plant species, Raman et al. [2] developed a strategy whereby a type III secretion system from Pseudomonas was expressed in Agrobacterium to allow for the efficient delivery of several effectors to suppress the plant response system and aid transformation. They found an increase of up to 400% in transformation efficiency using this approach. With a unique mode of action, which is reliant on an interplay of host and bacterial functions to effect transformation [3], Agrobacterium retains the title of most popular delivery system in use today. There are many excellent published reviews on what is known to date on the mechanisms of Agrobacterium-mediated delivery to plant cells [4,5,6,7]. Here we will examine the role Agrobacterium plays as an efficient delivery system in the introduction of CRISPR/Cas components.
Cas9 was first discovered in 2005 and the first genome edited plants using CRISPR followed less than a decade later [8]. Since then, plants drawn from over 24 families (including those containing the major food crops) have been edited using CRISPR/Cas9 components [9] in Agrobacterium (both tumefaciens and rhizogenes) where it continues to hold a prominent position as an efficient delivery system including in transient assays where leaf cell agroinfiltration has been used [9]. Agrobacterium-mediated transformation (AMT) relies on the plant being inoculated with the bacteria containing the requisite reagents for gene editing. This means a binary plasmid containing a gene-editing cassette inserted between the border sequences on the T-DNA integrates into the plant genome leading to stable transformation as referenced in earlier reviews [6]. In general, AMT relies on tissue culture for the regeneration of a stable transformant. With this comes the possibility of producing unwelcome somaclonal variants. One way to circumvent this is to use AMT with a Floral Dip procedure. Here, transformed seeds can be obtained outside of the tissue culture process. However, this method has had limited success beyond Arabidopsis thaliana [10]. Another report of successful transformation outside of a tissue culture system is referenced in the same paper by Laforest and Nadakuduti [10]. Here an improved method of gene-editing efficiency is described whereby developmental regulators are co-delivered with CRISPR components and over-expressed leading to improved regeneration and transformation of soil-grown plants using Agrobacterium which had been injected into plants creating de novo meristems. An efficient transformation system using AMT was developed in wheat by Zhang et al. [11]. Earlier transformation studies in wheat were focused on the gene gun which has drawbacks in terms of copy number insertions and gene silencing effects. Generally, with AMT events, single copy insertions are the norm with low levels of gene silencing detected. In the study by Zhang et al. [11], the authors report efficient editing events in wheat. Generally, if DNA-free genome editing is required, alternative methods such as PEG must be employed. One of the drawbacks of Agrobacterium-mediated transformation is the fact that T-DNA is integrated into the genome. And this can be problematic for regulators. A report by Dalla Costa et al. [12] examined two strategies for producing T-DNA free CRISPRed fruit trees using Agrobacterium. The first strategy was based on the site specific recombinase Flp/FRT system. This system was reliant on the recognition of a 34-bp long sequence which was excised along with any undesired sequences from an optimised T-DNA vector system. The second strategy focused on the use of synthetic cleavage target sites (CTS) which were engineered adjacent to the left and right border sequences of the vector. These CTS were recognised by the Cas9 cleavage system and removed. Molina-Risco et al. [13] describe an improved method for the AMT and gene editing of tropical japonica rice where Oryza sativa is seen as a model monocot species.
2.2 Virus Vector-Based Methods
Plant viruses-based vectors are known for being reliable, efficient tools for transient protein expression and VIGS (Virus induced gene silencing) [14, 15]. More recently, plant viruses are being used as a delivery vehicle for genome editing tools, such as CRISPR/Cas9, to introduce specific mutations in the plant genome. These approaches are collectively known as VIGE (Viruses induced genome editing) [16,17,18,19,20,21,22]. VIGE allows DNA integration free methods to study gene function, modify plant traits, and develop novel crop features [23, 24]. The transiently introduced recombinant viral clones replicate within the plant, which then spread and infect the cells, delivering the genome editing machinery to the desired target site. This method has the advantage of being able to reach cells in different tissues and organs of the plant, leading to high efficiency genome editing. The overall choice of virus vector depends on the targeted host plant species and the size of the cargo insert that infectious viral replicon could sustainably carry while replicating in the plant cell. The replicon size threshold also limits the recombinant cargo size (nuclease and sgRNA cassette). The past few years have witnessed development of plants-based VIGE approaches [25, 26].
Several known plant viral vectors have been tested for their functional efficiency in delivering the CRISPR/Cas9 cassette for precise genome editing. These recent studies have optimised critical factors for success in the genome editing process such as choice of host species, infection method and replication of recombinant clones and copy number of sgRNAs. The viruses have better replication and transfection efficiency in their specific hosts, and this limits the utilisation of VIGE vectors in a broad host range. However, if used with the right host the VIGE approach expands beyond gene knockout and recent work proved its application in the precise gene replacement using geminivirus replicon of wheat dwarf virus (WDV) [27]. The WDV replicon could carry Cas9, sgRNA and a donor DNA and the editing efficiency was ~12 fold higher than non-viral methods. Up to that point, viral vectors from two RNA viruses viz. Tobacco etch virus (TEV) and potato virus X (PVX) had been used in tandem to express Cas12a and sgRNA respectively [19, 20]. All reported viral vectors have their own limitations and benefits [26]. Broadly, the use of DNA viruses-based vectors could lead to integration of a viral genome in plants, although the benefit is that they provide more cargo space due to the bigger replicon size. On the other hand, RNA viruses ensure integration-free genome editing but have smaller genomes hence the cargo size is limited. In the following sections, we have discussed some of the most successful VIGE viral vectors across model and crop plants.
2.2.1 Tobacco Rattle Virus (TRV)
Tobacco Rattle Virus (TRV) is a positive single-strand plant virus (Bipartite, RNA1 and RNA2) that infects the roots of tobacco plants and has a broad range of host plants. TRV could systematically infect and replicate in different plant cells and tissues. These attributes make TRV an ideal RNA virus candidate to deliver CRISPR/Cas9 modules. Several recent studies have engineered this virus to express the guide RNA and Cas9 nuclease, which then target specific genes to demonstrate heritable editing. TRV has been successfully tested in Arabidopsis (targets: AtAGL1, AtTT4; [28]) and Tobacco (NbPDS, NbAG and NbPCNA; [17]). These methods provide a rapid and efficient way to introduce genetic modifications in plants and have been applied in various crops, including tobacco, to produce plants with improved traits. TRV has been exclusively used for the delivery of sgRNAs to plants expressing Cas9. The TRV vector system is successfully used in model hosts like N. benthamiana and Arabidopsis. The targeting and editing efficiency were positively impacted by 3′ end sgRNAs tagging with Flowering Locus T (FT) transcript [17].
2.2.2 Barley Stripe Mosaic Virus (BSMV)
Barley Stripe Mosaic Virus (BSMV) vector is a plant virus that has been used as a delivery system for genetic engineering in plants. BSMV is a positive-sense RNA virus with a tripartite genome (alpha, beta and gamma RNA) that infects barley and other cereal crops, causing a mosaic pattern on the leaves. Researchers have utilised the natural replication and movement properties of the virus to deliver transgenes into plant cells, making it an effective tool for plant genetic engineering. BSMV has been used to transiently express sgRNAs and the editing process has been tested successfully in N. benthamiana, wheat and maize plants [21, 29, 30]. For wheat, multiple genes (TaGW2/7, TaUPL3 and TaQ) were targeted by expressing sgRNA scaffold from the gamma chain of BSMV replicon [29]. These engineered viral particles were then used for infection of Cas9 expressing wheat plants. The authors evaluated that sgRNA fusion with mobile RNAs like tRNA, AtFT, and Vern3 did not result in improved editing efficiency. On the contrary, another study on different wheat varieties confirmed the enhanced editing efficiency for BSMV sgRNA fusion with TaFT mRNA [21]. The authors argued that mobile mRNA fusion efficiency could be influenced by choice of wheat genotype and expression level of Cas9 nuclease.
2.2.3 Bean Yellow Dwarf Virus (BeYDV)
Bean yellow dwarf virus (BeYDV) belongs to the plant geminivirus family. It infects legume crops such as beans and peanuts. The virus can replicate and move within the plant along with its satellite replicons, making it an effective tool for delivering bigger transgenes cargos into plant cells. Bigger cargo carrying ability has prompted the use of BeYDV to deliver Cas9 together with sgRNA scaffold. By engineering the virus to contain a specific transgene, the virus can be used to infect the plant, delivering the transgene into the plant genome. These methods have been improvised now to introduce precise genome modifications. So far, BeYDV based CRISPR/Cas9 delivery has been validated in tobacco, potato, and tomato [31,32,33].
3 Physical and Chemical Methods
3.1 Physical Methods
Efficient delivery of the CRISPR/Cas9 complex into the nucleus of the targeted cell is an essential aspect to make it functional. The various forms of delivery approaches are adopted for instance messenger RNA, ribonucleoprotein (RNP-complex of sgRNA and Cas proteins preassembled in vitro), and plasmid DNA (pDNA) [34]. RNPs are usually constituted by a complex of protein (e.g., Cas9, Cas12a) and an RNA, such as the sgRNA-scaffold RNA single strand. Delivery of the complex via RNP avoids drawbacks related to mRNA and pDNA. RNP delivery avoids intracellular transcription and translation and speeds up genome editing. Apart from this, it not only edits efficiently but also decreases the immune response, off-target effects, and insertional mutagenesis, making it a promising method of genome editing [35, 36]. Amongst the options to deliver CRISPR systems into plant cells, biolistic is one which is widely adopted [37, 38].
3.1.1 Gene Gun/Biolistic-Based Delivery
Gene gun based delivery or biolistics is a direct physical delivery method for micro-projectiles carrying foreign DNA into plant cells or tissues at high velocity. DNA is coated onto gold or tungsten microprojectiles before gearing up for cell wall penetration of the target plant. Upon entry into the cell, dissociation from the particles takes place to either integrate stably or express transiently in the host genome [39].
To substantially breach the cell wall barrier, the gene gun method is widely adopted in order to deliver foreign DNA into the plant cell. Previously, RNA-guided Cas9 endonuclease was effectively used to modify the genome of various plants. Regardless of the success, particle bombardment of the plasmids containing the Cas9, gRNA, and marker genes often incorporated in the genome resulting in off-site cutting, gene disruption, and plant mosaicism [40]. Moreover, DNA molecules can also integrate at the double-strand break site hence decreasing the efficacy of gene insertion and gene editing. To address these undesirable effects, one suggested solution was to pre-integrate the Cas9 nuclease in plants to deliver the gRNA in the form of RNA molecules. Though successful the process was laborious and resource-demanding to develop and characterise the pre-integrated lines.
There are several documented reports which demonstrate the effective delivery of CRISPR reagents for genome editing using biolistics. They comprise the in planta genome editing of wheat via SAM and the production of novel variants of the maize ARGOS8 gene. Shi et al. [41] used gene editing approach to develop new allelic variants of ARGOS8 in maize. ARGOS8 encodes a negative regulator of ethylene responses, that is expressed at low levels in most inbred maize lines. The group increased ARGOS8 expression by substituting or knocking in GOS promoter replacing native promoter via HDR to drive ARGOS8 expression. The field evaluation of hybrids exhibited increased yield under stress regimes [41]. In wheat, a group of researchers bombarded 210 plants and found 11 transgenic lines with the mutant TaGASR7 allele, and the mutation was transferred to the next generation of three transgenic lines with no observation of the presence of Cas9 and guide RNA [38, 42]. Recent research in wheat showed some promising results regarding transgene-free genome editing by means of transient expression [43].
RNP complexes were delivered by biolistics into maize embryo cells [44]. Cas9-gRNA RNPs were employed to target four different genes: MS26, MS45 (male fertility genes), acetolactate synthase (ALS2), and liguleless 1 (LIG). The results were comparable to DNA plasmids and ranged from 0.21% to 0.69% in all four immature embryo cells of maize [44]. Similar results have been reported for wheat using the same method where 0.18% was obtained for TaGW2-B1 and 0.21% for TaGW2-D1 [45]. The RNP complex is of similar or greater efficacy as compared to the plasmid-mediated editing method and gives transgene-free plants with reduced off-target frequency along with the ability to directly target the genomic region of interest with the concomitant degradation of the RNP complex within hours [38].
In order to decrease uneven bombardments, a double-barrelled gene gun along with software that counts the cells was applied as a technical improvement [10].
In case of RNP transfections, a single nucleotide mismatch between the sgRNA and the target site greatly reduces the off-targeting of homolog sequences. Besides, RNPs were also found to accommodate the hefty heritable inversion of 75.7 Mb in maize chromosome 2, when constructed with gRNAs flanking the junctions of the required inversion [38, 46]. This highly specific engineering of chromosomes is invaluable to crop breeding.
Apart from all the above-mentioned success stories of RNP transport using biolistic, delivery systems for CRISPR/Cas9 continue to be a considerable hindrance concerning its efficient use. For now, an all-purpose delivery system is still lacking. Each method possesses both merits and demerits. Moreover, options are there for the transport of small cargos as compared to massive protein-nucleic acid complexes. Although the gene gun method is capable of transforming a myriad of cell types and tissues in the absence of a binary vector, some obvious limitations include the laborious work of explant preparation and random incorporation of cargo at various sites in the genome. In the case of RNPs, they offer DNA-free gene editing that instantly alters the target site by sidestepping the transcription and translation machinery of the cell and degrades rapidly. Although there is a possibility that the bombarded explant and resultant phenotypes experience uncertain downstream effects [10]. Additionally, particle bombardment has a high consumable and equipment cost along with complicated integration patterns and gives relatively low throughput [39]. Moreover, the costlier method to deliver Cas9/gRNA RNP lacks the control of bombardment sites like the nucleus, plastid and mitochondria [47].
3.1.2 Electroporation
In electroporation, electrical pulses are used to generate transient pores in the plasma membrane in order to allow nucleic acids to enter the cell [48]. These microscopic pores allow not only the micro-, but also the macromolecules to move either inside or outside the cell. An electroporator device performs the electroporation activity that comprises three basic portions: a power supply for the pulse, electroporation cuvettes, and electrodes. According to the simple mechanism of electroporation, water molecules are the first to pierce the lipid bilayer and make unstable hydrophilic pores. Then reorientation of adjacent lipids with their polar heads towards these water molecules takes place due to the increase in transmembrane voltage that in turn lowers the energy required to form an aqueous aperture leading to the formation of metastable hydrophilic pores [48, 49]. Fortunately, these electro-pores can be recovered and resealed in the case of optimised electric pulses. It has been observed that the duration of the electric field and its intensity are of immense importance concerning the healing of the cells as unsuitable electric currents may lead to cell death [50].
Ensuring uniform electroporation can be difficult to achieve in plant tissue due to the presence of variable cell types and different three-dimensional organisations due to gap junctions. So, this non-homogeneous distribution makes some cells experience a greater degree of electroporation than others. To address this issue, optimization needs to be performed with respect to electrode position, size, shape, and different cells [51].
Some examples of application of electroporation include tobacco protoplasts with cucumber mosaic viral RNA using exponential and square wave electroporation pulses [52]. Similarly, electro-pulsed colt cherry protoplasts demonstrated efficient regeneration capability and a greater number of shoots per callus along with a prolific root system in comparison to non-electro-pulsed ones. Besides, protoplast-derived tissues of Solanum dulcamara L., which is a woody medicinal plant, showed improved morphogenesis when compared to the untreated protoplasts. Apart from this, roots of regenerated shoots were established efficiently with prolific root systems [50]. Recently, electroporation has been used for gene editing with CRISPR technology [53].
3.1.3 Sonication and Pulsed-Laser
Sonication can involve acoustic and ultrasound and has been observed to enhance the growth processes in plants [54]. Moreover, acoustic microstreaming and cavitation are caused by ultrasound and can modify enzyme stability, cell growth, and ultrastructure. It has the potential to discharge DNA from the nucleus, modify the permeability of the cell membrane, cleave the extracellular polymers, enhance cell surface charges, and reduce the stability of the cell. Furthermore, the duration of sound irradiation, frequency, and intensity of sonication are some of the factors to be considered [54]. During the process, the cell wall interacts first with the sound waves and probably experiences the variation followed by the cell membrane [55]. Ultrasound irradiates the bubbles and then leads to the crumpling of those cavities resulting in the release of a large amount of energy. This activity provokes various physical and chemical modifications like microstreaming in the plant cells and cell suspensions enhancing mass transport [54]. However, high-intensity irradiation can spoil the cell structure and inhibit plant growth [56]. The properties of sound waves that influence sonication include duration, intensity, pressure level, and frequency. Apart from these, the distance between the source and the target plant, sensitivity of the cells, genotype, and species are other factors affecting organogenesis and plant growth [57]. Moreover, ribonucleoproteins comprising the sgRNA and Cas9 protein were inserted into the extracellular vesicles by sonication for gene therapy [58]. Plant species modified by ultrasound in vitro include rice, aloe, carrot, commercial squash, gerbera, hazelnut, and red microalga [55, 56, 59]. To further strengthen our understanding of the technique, advanced knowledge about various processes sparked by sonication and more insight into sounds would generate greater yield and better growth [60].
Recently, RNPs have been delivered into tobacco cells via cavitation bubbles generated using a pulsed laser [61]. The resulting shockwave achieves the efficient transfection of walled cells in tissue explants by creating transient membrane pores. Genome-edited plants were produced with an efficiency of 35.2 and 16.5% for phytoene desaturase (PDS) and actin depolymerizing factor (ADF) genes, respectively.
3.1.4 Silicon Carbide Whiskers
Different types of silicon nanoparticles are used for different purposes. Some of them are relatively more difficult to synthesise and process than others and hence their applications are limited. Those that are relatively inexpensive and uncomplicated find more usage and have wider applications. Silicon Carbide (SiC) whiskers/fibres are among those nanoparticles belonging to a wide variety of silicon-based nanoparticles that fit this description. They are needle-like structures attached to a base. They appeared as a tool for the physical delivery of DNA in the 1990s where they were used to create pores in tobacco and maize cells through abrasion allowing the penetration of the exogenous DNA when mixed with the cell suspension [62]. The SiC fibres do not carry the genes themselves, but rather help the foreign DNA slide into the target cells. To date many crops have been transformed using this method. Crops, like wheat and rice that are recalcitrant to the Agrobacterium infection/transfection, have not only shown higher efficacy but more stable transgenic expression with SiC whiskers when compared to the particle bombardment method (cDNA coated microprojectiles) [63]. The transformation has been conducted on cell suspensions, embryos, embryogenic cells, and calli of different plant species such as cotton, maize, tobacco, onion, and rice [64,65,66,67].
Several types of SiC whiskers have been used for research on different plant species. These types differ based on their diameter and length. To achieve optimum results through this method some precautions like the pretreatment of the explant with osmotic agents and proper mixing of cells with SiC whiskers etc., have been adopted [68].
Some of the characteristics of SiC whiskers that make them desirable for DNA transfer into plant cells include compatibility with almost all plant species, the ability to help transform a wide range of cells, enhanced regeneration rate of transformants, ease of setup, and quick and inexpensive. The indirect involvement of SiC whiskers in DNA delivery can also be attributed to their positive/desired character because the amount of DNA available for transformation can be controlled. On the contrary, there are some limitations to this method that include their moderate transformation efficiency, the need for tissue culture and regeneration, and the requirement of a sophisticated protocol for successful transformation.
So far, most of the effort has been directed towards showcasing the effectiveness of this approach through the utilisation of reporter genes like GUS and GFP. Although, SiC-mediated plant transformation has been frequently reported, no studies have been conducted to deliver CRISPR/Cas for genome editing experiments in plants using this technology [69]. However, SiC whiskers have been used to edit the genome of other organisms such as algae and viruses through CRISPR/Cas [70, 71].
3.2 Chemical Methods
3.2.1 PEG-Mediated Delivery
Polyethylene glycol (PEG) is a polyether composed of repeated ethylene glycol units [-(CH2CH2O)n]. PEG is available with different structures (e.g., branched, star) having different molecular weights (MW) and showing different aggregation states: PEGs below MW 700 are liquid, PEGs comprised between MW 1000 and MW 2000 are soft solids, PEG above MW 2000 are hard crystalline solids. Polyethylene oxide (PEO) is a synonym for PEG, however macromolecules with MW below 20,000 are usually referred as PEG while those with MW above 20,000 are called PEO. PEG is biocompatible, highly soluble in water as well as in organic/inorganic solvents therefore being extremely important in solubilization and permeation [72]. In plant science, PEG properties were initially exploited in cell fusion experiments to obtain somatic hybrids (reviewed in [73]), but PEG is also capable of precipitating DNA molecules and stimulating their efficient cellular uptake by endocytosis [74]. Suitable PEG (usually MW 6000) and divalent cations (Ca2+) concentrations were initially identified to achieve genetic transformation [75, 76] with further improvements in efficiencies by Shillito and coauthors [77]. Besides genetic transformation of plant protoplasts, with stable integration in genomic DNA, it became evident that PEG-mediated transfection allowed episomal transient expression of the introduced DNA [78]. These fundamental experiments paved the way for thousands of studies that made large use of PEG-mediated transformation/transfection of isolated plant cells by different types of reagents (i.e., DNA, RNA, protein), with the establishment of consolidated platforms such as the Transient Expression in Arabidopsis Mesophyll Protoplast (TEAMP) system [79]. Typically, the concentration of PEG ranges from 12.5% to 20% (final concentration). The interest in using PEG for transfecting plant protoplasts, revamped in recent years because its application in the site directed mutagenesis protocols of various model species and crops (reviewed in [73, 80]).
Indeed, there are several examples of successful PEG-mediated delivery of RNPs in protoplasts of important crops such as potato, tomato, rice, and others (reviewed in [80]). The use of plant protoplasts requires an established regeneration protocol to obtain mutated adult plants, that additionally may show genetic instability and undesired somaclonal variation [36]. To bypass these issues, in pioneering work, Toda et al. [81] reported the direct delivery of RNPs (Cas9 based) in rice zygotes by PEG+Ca2+ mediated transfection thus achieving somatic mutagenesis with high frequencies (up to 64%).
3.2.2 Lipofection
The delivery of RNPs in plant cells need further development and lipofection could represent a promising delivery method. Briefly, lipofection (i.e., lipid transfection, liposome-based transfection) takes advantage of tiny lipid vesicular structure called liposomes that can be multilamellar or unilamellar and neutral, positively, or negatively charged [74]. Liposomes are easily produced, and they can form lipoplexes by encapsulating DNA, RNA, or proteins; lipoplexes will further release their content into the cells upon endocytosis or fusion with membranes. Lipofection has been extensively used in mammalian cell transfection, while, in plants, following the initial demonstration of exogenous DNA transfer in isolated plant protoplasts by liposomes [82], lipofection by cationic lipids (positively charged liposomes) has been used exclusively for DNA or RNA transfer through the negatively charged plant cell membranes (protoplasts) with limited examples [83]. Recently, sweet orange genome edited plants have been obtained by delivering lipid-based nanostructures, produced with the cationic lipid-based transfection agent Lipofectamine™, in protoplasts. In detail, lipid particles encapsulated the CRISPR/Cas9 DNA construct, protecting it from endosomal and enzymatic degradation [84]. While in a first attempt, Liu et al. [83] tested the delivery of RNPs (Cas9 based) in tobacco protoplasts isolated from BY-2 cells, by using two different reagents (Lipofectamine™ 3000, Lipofectamine™ RNAiMAX) with a transfection efficiency of 66% and a mutation frequency of 6%, but without regenerating plants. RNP delivery in plant cells by lipofection is still in its infancy, but it is considered an easy and inexpensive method for cell transfection that will probably largely benefit in the future by the technological improvements achieved in other research fields.
4 Emerging Technologies
Apart from a few applications, current approaches to precisely modify higher plant genomes rely on de novo regeneration from tissues or cell-derived calli. The lack of genotype-independent protocols and/or the induction of somaclonal variation during the in vitro growth phase call for the development of genotype-independent, simple, and economic in planta procedures. The latter are particularly necessary in view of routinely implementing editing approaches in breeding programs. Some emerging technologies aiming to overcome present limitations of available procedures have recently emerged.
4.1 In planta de novo Induction of Meristems
Two methods to edit higher plant genomes have been recently developed in Nicotiana benthamiana and validated in tomato, potato, and grape [85]. Both rely on the co-delivery of plasmids with the gene of interest and genes encoding Developmental Regulators (Wus2, STM and others) to somatic tissues of germinating seedlings or mature plants grown in vivo. When specific sgRNAs were tested in transgenic Cas9+ plants, edited shoots, able to transmit the induced mutations to the progeny, were regenerated from de novo formed meristems. Nevertheless, to obtain shoot formation, while reducing negative pleiotropic effects on their developments, the method requires a careful combination of different Developmental Regulators in genotypes tested. Further, the co-transfer of the nuclease remains to be demonstrated.
4.2 Editing During Haploid Induction
In several crops, either maternal or paternal haploids by chromosome elimination of one parent after fertilisation can be induced by interspecific crosses, knock-out of specific genes (e.g., MATL in maize or rice) or the manipulation of the gene encoding the centromere-specific histone CENH3 protein [86]. Capitalising on this information, site-directed mutagenesis in maize, Arabidopsis, and wheat has been achieved through the expression of editing reagents in the zygote, prior to elimination of chromosomes derived from the haploid-inducer parent and ploidy doubling in derived plants [86,87,88]. This method is attractive because it allows the production of DNA-free edited plants in just two generations but requires the availability of genotypes able to stably express editing reagents in the gametes and to induce chromosome elimination in the zygotes.
4.3 Editing Through Grafting
A breakthrough approach has been demonstrated recently by Yang and colleagues [89]. The authors produced transgenic Arabidopsis plants expressing a modified version of Cas9 and sgRNAs to which a tRNA sequence had been added. The latter allowed in planta long-distance movement of guide RNAs and nuclease transcripts from rootstock to scion, where translation occurred, producing edits visible both in the mother plant and derived progenies. Interestingly, the same was observed in interspecific graft combinations involving Arabidopsis and Brassica rapa, enlarging the potential scope of the technology to all species where graft compatibility occurs. It is, however, necessary to produce transgenic rootstocks for each editing target, although alternative ways to deliver and express mobile editing reagents in the rootstock could be attempted.
4.4 Nanotechnologies
Innovative nanoparticle-based methods for in vivo delivery of CRISPR/Cas have been recently developed in animal cells [90, 91]. Due to the presence of the cell wall, which shows a Size Exclusion Limit (SEL) of 5–20 nm and other differences with animals (f.i., the absence of a true circulatory system), research for similar alternative methods in plant cells lags behind. Nevertheless, the field is quite dynamic and nanomaterials (e.g., metallic/magnetic, silicon-based, carbon-based, lipid-based, polymeric, DNA nanostructures, peptide-based), showing at least one dimension below 100 nm and adjustable physico-chemical properties, could be adapted to deliver various cargoes precisely and in a controlled way to different plant tissues and without genotype-dependency, as reviewed elsewhere [92,93,94,95,96]. The possibility to directly transfer proteins or multiple biomolecules would allow the exploitation of RNPs without using laborious protoplast or biolistic-based approaches. The nanoparticle-mediated delivery of proteins in plants, however, presents difficulties not strictly related to their size [97].
Magnetic nanoparticles (MNP) have been tested, with results not always reproducible, to deliver plasmid DNA in pollen of cotton and other species using a magnetic field (magnetofection) [98,99,100]. In maize, the pre-treatment of pollen grains to open aperture was critical to obtain positive results. Single-walled carbon nanotubes (SWCNT) show high aspect and surface area-to-volume ratios. Functionalized with polyethyleneimine (PEI), chitosan or imidazolium, they have been assessed to deliver GFP/YFP plasmid DNA in leaves, chloroplasts, and pollen of various species, respectively [101,102,103], obtaining protein expression without gene integration. Intriguingly, in case of chloroplast expression, the delivery system was designed to release the loading DNA based on the stroma pH. Carbon dots (CD) with a 5–10 nm spherical shape showed internalisation and transient plasmid DNA expression in roots, leaves, and embryogenic callus cells [104], while the delivery of Cas9 and gRNA plasmid DNA, leading to somatic editing of SPO11, has been reported by [105] after spraying plasmid coated CDs on wheat leaves. Rosette nanotubes (RNTs) derive from the self-assembly of complementary guanine and cytosine motifs, in solution self-organised in rosettes which eventually form biocompatible hollow nanotubes with an internal and external diameter of 1.1 and 3.4 nm, respectively. They could represent a less toxic alternative to SWCNTs and have been used to express mCherry plasmid DNA in wheat microspores [106].
RNA molecules (siRNA, dsRNA) have also been delivered to leaves and pollen by using SWCNTs, gold nanoparticles of different shape and size, DNA nanostructures with different characteristics, LDH clay nanosheets, inducing gene silencing and virus protection, when a viral gene was targeted [107,108,109,110,111]. Interestingly, with DNA nanostructures as carriers, RNA interference was induced at the transcript or protein level depending on the DNA nanostructure shape and the siRNA attachment locus [108], while, among gold particles, only nanorods entered the cells, but silencing was obtained only with the non-internalised spheres colocalized with the cell wall [109].
The co-delivery of a protein and plasmid DNA has been demonstrated in onion, tobacco and teosinte cells using gold functionalized Mesoporous Silica Nanoparticles (MSN) and the biolistic method [112]. A similar approach allowed the delivery of a functional CRE recombinase in maize embryos, determining the excision of DNA sequences flanked with loxP [113]. Recently, preliminary results of RNP delivery in wheat pollen have been reported by using nanoassemblies (5–10 nm in diameter) with polycation linear homopolymer PDMAEMA [114].
Cell penetrating peptides (CPP) are natural (protein derived) or artificially designed molecules generally between 5 and 30 amino acids, with the ability to be translocated through the cell membrane either directly or via endocytosis. Based on physicochemical properties they are classified as cationic, hydrophobic or amphipathic. They can bind to various kinds of molecular cargoes, either covalently or noncovalently, allowing their transfection in various recipient cells to transiently modify gene expression and metabolic pathways [115, 116]. In relation to genome editing, the feasibility of delivering proteins of varying sizes, including ADH (150 kDa) or GUS (272 kDa), has been demonstrated in triticale microspores as well as in intact Arabidopsis leaves [117, 118]. The transfer of the GUS linear plasmid DNA or of multiple biomolecules at the same time has been also shown [117, 119]. Proof of genome editing of the IPK1 gene, albeit at low frequency, has been obtained in wheat microspores and derived haploid embryos by delivering ZFNs complexed with two different CPPs. Edited plants, however, could not be regenerated [115, 120]. Polyion complex vesicles synthesised by mixing two oligolysine peptides and displaying a CPP (CPP-PICsome) have been recently shown to be able to encapsulate Cas9 RNP complexes [121]. When introduced into Arabidopsis calli by vacuum and compression, editing of the target Phytoene Desaturase gene PDS3 was obtained, although mutation rate was around 0.007%.
With reference to the use of nanotechnologies for genetic engineering and genome editing, quite a lot of research developed in the last years trying to adapt concepts and technologies derived from investigation in the human field to plants, although in most cases only at the proof-of-concept level. Nucleic acids or proteins could be successfully delivered to plant cells, constituting the basis to develop methods for delivering editing reagents. Nevertheless, moving from one system to another, e.g., from small plasmids encoding reporter genes to large constructs encoding Cas encoding genes, is not straightforward, while, concerning DNA binding conditions on Nanoparticless, the trade-off between cell wall trafficking and accessibility of the transcription machinery can be a challenge [122]. The size and other characteristics of Cas molecules generally used are an obstacle for the fast development of plant-based nanotechnological approaches, but the continuous discovery of new nucleases, some of them hypercompact, open new perspectives [123]. The recent results with CPP [121] are also promising. Nevertheless, the possibility to transiently edit in planta the germline, without relying on undifferentiated growth in vitro and de novo regeneration, and passing only the induced mutations to the progeny, remains a desirable objective not yet achieved.
5 Conclusions and Perspectives
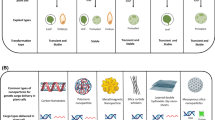
Plant genome editing has been successfully demonstrated and applied to add or delete gene(s) in crop plants for functional genomic studies. The technology has gained momentum recently because of simplicity, efficiency, low cost and ability to target multiple genes. The variation in mutation efficiency in plants is associated with a number of different factors. Most importantly, the mode of delivery of the CRISPR/Cas9 components is of crucial importance for genome modification in plants. Plants have a complex genome structure due to common occurrence of polyploidy and other genomic re-arrangements. Thus, the delivery of CRISPR/Cas components to plant cells is still a challenge for researchers to attain high editing efficiency. There are many CRISPR delivery methods currently in use and development (Fig. 3.1). The various delivery methods such as biological (Agrobacterium-mediated, virus vector based), physical and chemical methods (PEG or biolistic based) are being applied to obtain efficient genome editing efficiency. Each delivery method has advantages and disadvantages. These methods deliver CRISPR systems to cells with the aid of chemicals or devices that make cells more amenable to delivery. Besides that, scientists are developing new ways and means for the delivery of CRISPR components to plant cells with the aim of establishing highly efficient and genotype-independent delivery systems for genome editing (summarised in Table 3.1). We assume the novel delivery methods those provide the opportunity for generating transgene-free genome edited plants will be most preferred in the future. These transgene free methods will boost acceptance of CRISPR/Cas technologies in food and agriculture.
Different delivery methods for components and T-DNA constructs to achieve genome editing in plant tissues. The left part of the scheme represents the delivery methods for in-vitro genome editing. While the right part of the infographic shows the in-planta methods for the delivery of genome editing tools, those potentially could give edits in early generations without integration of T-DNA. Additionally, the cargos for delivery of the different genome editing components are presented at the bottom
References
Makarova, K.S., Wolf, Y.I., Iranzo, J., Shmakov, S.A., Alkhnbashi, O.S., Brouns, S.J.J., Charpentier, E., Cheng, D., Haft, D.H., Horvath, P., Moineau, S., Mojica, F.J.M., Scott, D., Shah, S.A., Siksnys, V., Terns, M.P., Venclovas, Č., White, M.F., Yakunin, A.F., Yan, W., Zhang, F., Garrett, R.A., Backofen, R., van der Oost, J., Barrangou, R., Koonin, E.V.: Evolutionary classification of CRISPR–Cas systems: a burst of class 2 and derived variants. Nat. Rev. Microbiol. 18, 67–83 (2020)
Raman, V., Rojas, C.M., Vasudevan, B., Dunning, K., Kolape, J., Oh, S., Yun, J., Yang, L., Li, G., Pant, B.D., Jiang, Q., Mysore, K.S.: Agrobacterium expressing a type III secretion system delivers Pseudomonas effectors into plant cells to enhance transformation. Nat. Commun. 13, 2581 (2022)
Tiwari, M., Mishra, A.K., Chakrabarty, D.: Agrobacterium-mediated gene transfer: recent advancements and layered immunity in plants. Planta. 256, 37 (2022)
Gelvin, S.B.: Agrobacterium -mediated plant transformation: the biology behind the “gene-jockeying” tool. Microbiol. Mol. Biol. Rev. 67, 16–37 (2003)
Jones, H.D., Doherty, A., Wu, H.: Review of methodologies and a protocol for the agrobacterium-mediated transformation of wheat. Plant Methods. 1, 5 (2005)
Hwang, H.-H., Yu, M., Lai, E.-M.: Agrobacterium -mediated plant transformation: biology and applications. Arabidopsis Book. 15, e0186 (2017)
Thompson, M.G., Moore, W.M., Hummel, N.F.C., Pearson, A.N., Barnum, C.R., Scheller, H.V., Shih, P.M.: Agrobacterium tumefaciens: a bacterium primed for synthetic biology. BioDesign Res. 2020 (2020)
Li, J.-F., Norville, J.E., Aach, J., McCormack, M., Zhang, D., Bush, J., Church, G.M., Sheen, J.: Multiplex and homologous recombination–mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nat. Biotechnol. 31, 688–691 (2013)
Shan, S., Soltis, P.S., Soltis, D.E., Yang, B.: Considerations in adapting CRISPR/Cas9 in nongenetic model plant systems. Appl. Plant Sci. 8, e11314 (2020)
Laforest, L.C., Nadakuduti, S.S.: Advances in delivery mechanisms of CRISPR gene-editing reagents in plants. Front. Genome Editing. 4, 1 (2022)
Zhang, Z., Hua, L., Gupta, A., Tricoli, D., Edwards, K.J., Yang, B., Li, W.: Development of an Agrobacterium-delivered CRISPR/Cas9 system for wheat genome editing. Plant Biotechnol. J. 17, 1623–1635 (2019)
Dalla Costa, L., Piazza, S., Pompili, V., Salvagnin, U., Cestaro, A., Moffa, L., Vittani, L., Moser, C., Malnoy, M.: Strategies to produce T-DNA free CRISPRed fruit trees via Agrobacterium tumefaciens stable gene transfer. Sci. Rep. 10, 20155 (2020)
Molina-Risco, M., Ibarra, O., Faion-Molina, M., Kim, B., Septiningsih, E.M., Thomson, M.J.: Optimizing agrobacterium-mediated transformation and CRISPR-Cas9 gene editing in the tropical japonica Rice variety presidio. Int. J. Mol. Sci. 22, 10909 (2021)
Rössner, C., Lotz, D., Becker, A.: VIGS goes viral: how VIGS transforms our understanding of plant science. Annu. Rev. Plant Biol. 73, 703–728 (2022)
Wang, M., Gao, S., Zeng, W., Yang, Y., Ma, J., Wang, Y.: Plant virology delivers diverse toolsets for biotechnology. Viruses. 12, 1338 (2020)
Ali, Z., Abul-faraj, A., Li, L., Ghosh, N., Piatek, M., Mahjoub, A., Aouida, M., Piatek, A., Baltes, N.J., Voytas, D.F., Dinesh-Kumar, S., Mahfouz, M.M.: Efficient virus-mediated genome editing in plants using the CRISPR/Cas9 system. Mol. Plant. 13, 1–4 (2015)
Ellison, E.E., Nagalakshmi, U., Gamo, M.E., Huang, P.-j., Dinesh-Kumar, S., Voytas, D.F.: Multiplexed heritable gene editing using RNA viruses and mobile single guide RNAs. Nat. Plants. 6, 620–624 (2020)
Luo, Y., Na, R., Nowak, J.S., Qiu, Y., Lu, Q.S., Yang, C., Marsolais, F., Tian, L.: Development of a Csy4-processed guide RNA delivery system with soybean-infecting virus ALSV for genome editing. BMC Plant Biol. 21, 419 (2021)
Uranga, M., Aragonés, V., Selma, S., Vázquez-Vilar, M., Orzáez, D., Daròs, J.: Efficient Cas9 multiplex editing using unspaced sgRNA arrays engineering in a Potato virus X vector. Plant J. 106, 555–565 (2021)
Uranga, M., Vazquez-Vilar, M., Orzáez, D., Daròs, J.-A.: CRISPR-Cas12a genome editing at the whole-plant level using two compatible RNA virus vectors. CRISPR J. 4, 761–769 (2021)
Li, T., Hu, J., Sun, Y., Li, B., Zhang, D., Li, W., Liu, J., Li, D., Gao, C., Zhang, Y., Wang, Y.: Highly efficient heritable genome editing in wheat using an RNA virus and bypassing tissue culture. Mol. Plant. 14, 1787–1798 (2021)
Oh, Y., Kim, H., Kim, S.-G.: Virus-induced plant genome editing. Curr. Opin. Plant Biol. 60, 101992 (2021)
Chen, H., Su, Z., Tian, B., Liu, Y., Pang, Y., Kavetskyi, V., Trick, H.N., Bai, G.: Development and optimization of a barley stripe mosaic virus-mediated gene editing system to improve Fusarium head blight resistance in wheat. Plant Biotechnol. J. 20, 1018–1020 (2022)
Senthil-Kumar, M., Mysore, K.S.: Tobacco rattle virus–based virus-induced gene silencing in Nicotiana benthamiana. Nat. Protoc. 9, 1549–1562 (2014)
Uranga, M., Aragonés, V., Daròs, J.-A., Pasin, F.: Heritable CRISPR-Cas9 editing of plant genomes using RNA virus vectors. STAR Protoc. 4, 102091 (2023)
Zhang, C., Liu, S., Li, X., Zhang, R., Li, J.: Virus-induced gene editing and its applications in plants. Int. J. Mol. Sci. 23, 10202 (2022)
Gil-Humanes, J., Wang, Y., Liang, Z., Shan, Q., Ozuna, C.V., Sánchez-León, S., Baltes, N.J., Starker, C., Barro, F., Gao, C., Voytas, D.F.: High-efficiency gene targeting in hexaploid wheat using DNA replicons and CRISPR/Cas9. Plant J. 89, 1251–1262 (2017)
Ali, Z., Eid, A., Ali, S., Mahfouz, M.M.: Pea early-browning virus-mediated genome editing via the CRISPR/Cas9 system in Nicotiana benthamiana and Arabidopsis. Virus Res. 244, 333–337 (2018)
Wang, W., Yu, Z., He, F., Bai, G., Trick, H.N., Akhunova, A., Akhunov, E.: Multiplexed promoter and gene editing in wheat using a virus-based guide RNA delivery system. Plant Biotechnol. J. 20, 2332–2341 (2022)
Hu, J., Li, S., Li, Z., Li, H., Song, W., Zhao, H., Lai, J., Xia, L., Li, D., Zhang, Y.: A barley stripe mosaic virus-based guide RNA delivery system for targeted mutagenesis in wheat and maize. Mol. Plant Pathol. 20, 1463–1474 (2019)
Baltes, N.J., Gil-Humanes, J., Čermák, T., Atkins, P.A., Voytas, D.F.: DNA replicons for plant genome engineering. Plant Cell. 26, 151–163 (2014)
Butler, N.M., Baltes, N.J., Voytas, D.F., Douches, D.S.: Geminivirus-mediated genome editing in potato (Solanum tuberosum L.) using sequence-specific nucleases. Front. Plant Sci. 7, 1–13 (2016)
Čermák, T., Baltes, N.J., Čegan, R., Zhang, Y., Voytas, D.F.: High-frequency, precise modification of the tomato genome. Genome Biol. 16, 232 (2015)
Wang, M., Glass, Z.A., Xu, Q.: Non-viral delivery of genome-editing nucleases for gene therapy. Gene Ther. 24, 144–150 (2017)
Zhang, S., Shen, J., Li, D., Cheng, Y.: Strategies in the delivery of Cas9 ribonucleoprotein for CRISPR/Cas9 genome editing. Theranostics. 11, 614–648 (2021)
Zhang, Y., Iaffaldano, B., Qi, Y.: CRISPR ribonucleoprotein-mediated genetic engineering in plants. Plant Commun. 2, 100168 (2021)
Miller, K., Eggenberger, A.L., Lee, K., Liu, F., Kang, M., Drent, M., Ruba, A., Kirscht, T., Wang, K., Jiang, S.: An improved biolistic delivery and analysis method for evaluation of DNA and CRISPR-Cas delivery efficacy in plant tissue. Sci. Rep. 11, 7695 (2021)
Ghogare, R., Ludwig, Y., Bueno, G.M., Slamet-Loedin, I.H., Dhingra, A.: Genome editing reagent delivery in plants. Transgenic Res. 30, 321–335 (2021)
Ozyigit, I.I., Yucebilgili Kurtoglu, K.: Particle bombardment technology and its applications in plants. Mol. Biol. Rep. 47, 9831–9847 (2020)
Kanchiswamy, C.N.: DNA-free genome editing methods for targeted crop improvement. Plant Cell Rep. 35, 1469–1474 (2016)
Shi, J., Gao, H., Wang, H., Lafitte, H.R., Archibald, R.L., Yang, M., Hakimi, S.M., Mo, H., Habben, J.E.: ARGOS8 variants generated by CRISPR-Cas9 improve maize grain yield under field drought stress conditions. Plant Biotechnol. J. 15, 207–216 (2017)
Hamada, H., Liu, Y., Nagira, Y., Miki, R., Taoka, N., Imai, R.: Biolistic-delivery-based transient CRISPR/Cas9 expression enables in planta genome editing in wheat. Sci. Rep. 8, 14422 (2018)
Zhang, Y., Liang, Z., Zong, Y., Wang, Y., Liu, J., Chen, K., Qiu, J.-L., Gao, C.: Efficient and transgene-free genome editing in wheat through transient expression of CRISPR/Cas9 DNA or RNA. Nat. Commun. 7, 12617 (2016)
Svitashev, S., Schwartz, C., Lenderts, B., Young, J.K., Mark Cigan, A.: Genome editing in maize directed by CRISPR-Cas9 ribonucleoprotein complexes. Nat. Commun. 7, 13274 (2016)
Liang, Z., Chen, K., Li, T., Zhang, Y., Wang, Y., Zhao, Q., Liu, J., Zhang, H., Liu, C., Ran, Y., Gao, C.: Efficient DNA-free genome editing of bread wheat using CRISPR/Cas9 ribonucleoprotein complexes. Nat. Commun. 8, 14261 (2017)
Schwartz, C., Lenderts, B., Feigenbutz, L., Barone, P., Llaca, V., Fengler, K., Svitashev, S.: CRISPR–Cas9-mediated 75.5-Mb inversion in maize. Nat. Plants. 6, 1427–1431 (2020)
Sandhya, D., Jogam, P., Allini, V.R., Abbagani, S., Alok, A.: The present and potential future methods for delivering CRISPR/Cas9 components in plants. J. Genet. Eng. Biotechnol. 18, 25 (2020)
Kumar, P., Nagarajan, A., Uchil, P.D.: Electroporation. Cold Spring Harb. Protoc. 2019, pdb.top096271 (2019)
Kotnik, T., Rems, L., Tarek, M., Miklavčič, D.: Membrane electroporation and electropermeabilization: mechanisms and models. Annu. Rev. Biophys. 48, 63–91 (2019)
Ozyigit, I.I.: Gene transfer to plants by electroporation: methods and applications. Mol. Biol. Rep. 47, 3195–3210 (2020)
Dymek, K., Rems, L., Zorec, B., Dejmek, P., Galindo, F.G., Miklavčič, D.: Modeling electroporation of the non-treated and vacuum impregnated heterogeneous tissue of spinach leaves. Innov. Food Sci. Emerg. Technol. 29, 55–64 (2015)
Van Wert, S.L., Saunders, J.A.: Electrofusion and electroporation of plants. Plant Physiol. 99, 365–367 (1992)
Lee, M.H., Lee, J., Choi, S.A., Kim, Y.-S., Koo, O., Choi, S.H., Ahn, W.S., Jie, E.Y., Kim, S.W.: Efficient genome editing using CRISPR–Cas9 RNP delivery into cabbage protoplasts via electro-transfection. Plant Biotechnol. Rep. 14, 695–702 (2020)
Rokhina, E.V., Lens, P., Virkutyte, J.: Low-frequency ultrasound in biotechnology: state of the art. Trends Biotechnol. 27, 298–306 (2009)
Chen, B., Huang, J., Wang, J., Huang, L.: Ultrasound effects on the antioxidative defense systems of Porphyridium cruentum. Colloids Surf. B: Biointerfaces. 61, 88–92 (2008)
Liu, Y., Takatsuki, H., Yoshikoshi, A., Wang, B., Sakanishi, A.: Effects of ultrasound on the growth and vacuolar H+-ATPase activity of aloe arborescens callus cells. Colloids Surf. B: Biointerfaces. 32, 105–116 (2003)
George, E.F., Hall, M.A., de Klerk, G.J.: Plant propagation by tissue culture, 3rd edn, p. 1. Springer, Dordrecht (2007)
Horodecka, K., Düchler, M.: CRISPR/Cas9: principle, applications, and delivery through extracellular vesicles. Int. J. Mol. Sci. 22, 6072 (2021)
Safari, M., Ghanati, F., Behmanesh, M., Hajnorouzi, A., Nahidian, B., Mina, G.: Enhancement of antioxidant enzymes activity and expression of CAT and PAL genes in hazel (Corylus avellana L.) cells in response to low-intensity ultrasound. Acta Physiol. Plant. 35, 2847–2855 (2013)
Teixeira da Silva, J.A., Dobránszki, J.: Sonication and ultrasound: impact on plant growth and development. Plant Cell Tissue Organ Cult. 117, 131–143 (2014)
Augustine, S.M., Cherian, A.V., Seiling, K., Di Fiore, S., Raven, N., Commandeur, U., Schillberg, S.: Targeted mutagenesis in Nicotiana tabacum ADF gene using shockwave-mediated ribonucleoprotein delivery increases osmotic stress tolerance. Physiol. Plant. 173, 993–1007 (2021)
Kaeppler, H.F., Gu, W., Somers, D.A., Rines, H.W., Cockburn, A.F.: Silicon carbide fiber-mediated DNA delivery into plant cells. Plant Cell Rep. 9, 415–418 (1990)
Kaeppler, H.F., Somers, D.A., Rines, H.W., Cockburn, A.F.: Silicon carbide fiber-mediated stable transformation of plant cells. Theor. Appl. Genet. 84, 560–566 (1992)
Asad, S., Mukhtar, Z., Nazir, F., Hashmi, J.A., Mansoor, S., Zafar, Y., Arshad, M.: Silicon carbide whisker-mediated embryogenic callus transformation of cotton (Gossypium hirsutum L.) and regeneration of salt tolerant plants. Mol. Biotechnol. 40, 161–169 (2008)
Brisibe, E.A., Gajdosova, A., Olesen, A., Andersen, S.B.: Cytodifferentiation and transformation of embryogenic callus lines derived from anther culture of wheat. J. Exp. Bot. 51, 187–196 (2000)
Matsushita, J., Otani, M., Wakita, Y., Tanaka, O., Shimada, T.: Transgenic plant regeneration through silicon carbide whisker-mediated transformation of Rice (Oryza sativa L.). Breed. Sci. 49, 21–26 (1999)
Petolino, J.F., Hopkins, N.L., Kosegi, B.D., Skokut, M.: Whisker-mediated transformation of embryogenic callus of maize. Plant Cell Rep. 19, 781–786 (2000)
Asad, S., Arshad, M.: Silicon carbide whisker-mediated plant transformation. In: Gerhardt, R. (ed.) Properties and applications of silicon carbide. INTECH (2011)
Mujtaba, M., Wang, D., Carvalho, L.B., Oliveira, J.L., Espirito Santo Pereira, A. do, Sharif, R., Jogaiah, S., Paidi, M.K., Wang, L., Ali, Q., Fraceto, L.F.: Nanocarrier-mediated delivery of miRNA, RNAi, and CRISPR-Cas for plant protection: current trends and future directions. ACS Agric. Sci. Technol. 1, 417–435 (2021)
Chen, J.E., Barbrook, A.C., Cui, G., Howe, C.J., Aranda, M.: The genetic intractability of Symbiodinium microadriaticum to standard algal transformation methods. PLoS One. 14, e0211936 (2019)
Noel, E.A., Weeks, D.P., Van Etten, J.L.: Pursuit of chlorovirus genetic transformation and CRISPR/Cas9-mediated gene editing. PLoS One. 16, e0252696 (2021)
Zarrintaj, P., Saeb, M.R., Jafari, S.H., Mozafari, M.: Application of compatibilized polymer blends in biomedical fields. In: Ajitha, A., Sabu, T. (eds.) Compatibilization of polymer blends. Elsevier, Amsterdam (2020)
Yue, J.-J., Yuan, J.-L., Wu, F.-H., Yuan, Y.-H., Cheng, Q.-W., Hsu, C.-T., Lin, C.-S.: Protoplasts: from isolation to CRISPR/Cas genome editing application. Front. Genome Editing. 3, 19 (2021)
Ortiz-Matamoros, M.F., Villanueva, M.A., Islas-Flores, T.: Genetic transformation of cell-walled plant and algae cells: delivering DNA through the cell wall. Brief Funct. Genomics. 17, 26–33 (2018)
Krens, F.A., Molendijk, L., Wullems, G.J., Schilperoort, R.A.: In vitro transformation of plant protoplasts with Ti-plasmid DNA. Nature. 296, 72–74 (1982)
Paszkowski, J., Shillito, R.D., Saul, M., Mandák, V., Hohn, T., Hohn, B., Potrykus, I.: Direct gene transfer to plants. EMBO J. 3, 2717–2722 (1984)
Shillito, R.D., Saul, M.W., Paszkowski, J., Müller, M., Potrykus, I.: High efficiency direct gene transfer to plants. Nat. Biotechnol. 3, 1099–1103 (1985)
Töpfer, R., Pröls, M., Schell, J., Steinbiß, H.-H.: Transient gene expression in tobacco protoplasts: II. Comparison of the reporter gene systems for CAT, NPT II, and GUS. Plant Cell Rep. 7, 225–228 (1988)
Yoo, S.-D., Cho, Y.-H., Sheen, J.: Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat. Protoc. 2, 1565–1572 (2007)
Rustgi, S., Naveed, S., Windham, J., Zhang, H., Demirer, G.S.: Plant biomacromolecule delivery methods in the 21st century. Front. Genome Editing. 4, 51 (2022)
Toda, E., Koiso, N., Takebayashi, A., Ichikawa, M., Kiba, T., Osakabe, K., Osakabe, Y., Sakakibara, H., Kato, N., Okamoto, T.: An efficient DNA- and selectable-marker-free genome-editing system using zygotes in rice. Nat. Plants. 5, 363–368 (2019)
Lurquin, P.F.: Entrapment of plasmid DNA by liposomes and their interactions with plant protoplasts. Nucleic Acids Res. 6, 3773–3784 (1979)
Liu, W., Rudis, M.R., Cheplick, M.H., Millwood, R.J., Yang, J.-P., Ondzighi-Assoume, C.A., Montgomery, G.A., Burris, K.P., Mazarei, M., Chesnut, J.D., Stewart, C.N.: Lipofection-mediated genome editing using DNA-free delivery of the Cas9/gRNA ribonucleoprotein into plant cells. Plant Cell Rep. 39, 245–257 (2020)
Mahmoud, L.M., Kaur, P., Stanton, D., Grosser, J.W., Dutt, M.: A cationic lipid mediated CRISPR/Cas9 technique for the production of stable genome edited citrus plants. Plant Methods. 18, 33 (2022)
Maher, M.F., Nasti, R.A., Vollbrecht, M., Starker, C.G., Clark, M.D., Voytas, D.F.: Plant gene editing through de novo induction of meristems. Nat. Biotechnol. 38, 84–89 (2020)
Kelliher, T., Starr, D., Su, X., Tang, G., Chen, Z., Carter, J., Wittich, P.E., Dong, S., Green, J., Burch, E., McCuiston, J., Gu, W., Sun, Y., Strebe, T., Roberts, J., Bate, N.J., Que, Q.: One-step genome editing of elite crop germplasm during haploid induction. Nat. Biotechnol. 37, 287–292 (2019)
Wang, B., Zhu, L., Zhao, B., Zhao, Y., Xie, Y., Zheng, Z., Li, Y., Sun, J., Wang, H.: Development of a haploid-inducer mediated genome editing system for accelerating maize breeding. Mol. Plant. 12, 597–602 (2019)
Budhagatapalli, N., Halbach, T., Hiekel, S., Büchner, H., Müller, A.E., Kumlehn, J.: Site-directed mutagenesis in bread and durum wheat via pollination by cas9/guide RNA-transgenic maize used as haploidy inducer. Plant Biotechnol. J. 18, 2376–2378 (2020)
Yang, L., Machin, F., Wang, S., Saplaoura, E., Kragler, F.: Heritable transgene-free genome editing in plants by grafting of wild-type shoots to transgenic donor rootstocks. Nat. Biotechnol. 41, 1–10 (2023)
Duan, L., Ouyang, K., Xu, X., Xu, L., Wen, C., Zhou, X., Qin, Z., Xu, Z., Sun, W., Liang, Y.: Nanoparticle delivery of CRISPR/Cas9 for genome editing. Front. Genet. 12, 788 (2021)
Khirallah, J., Eimbinder, M., Li, Y., Xu, Q.: Clinical progress in genome-editing technology and in vivo delivery techniques. Trends Genet. 39, 208–216 (2023)
Cunningham, F.J., Goh, N.S., Demirer, G.S., Matos, J.L., Landry, M.P.: Nanoparticle-mediated delivery towards advancing plant genetic engineering. Trends Biotechnol. 36, 882–897 (2018)
Wang, P., Zhao, F.J., Kopittke, P.M.: Engineering crops without genome integration using nanotechnology. Trends Plant Sci. 24, 574–577 (2019)
Wang, J.W., Grandio, E.G., Newkirk, G.M., Demirer, G.S., Butrus, S., Giraldo, J.P., Landry, M.P.: Nanoparticle-mediated genetic engineering of plants. Mol. Plant. 12, 1037–1040 (2019)
Demirer, G.S., Silva, T.N., Jackson, C.T., Thomas, J.B., Ehrhardt, D.W., Rhee, S.Y., Mortimer, J.C., Landry, M.P.: Nanotechnology to advance CRISPR–Cas genetic engineering of plants. Nat. Nanotechnol. 16, 243–250 (2021)
Savage, N.: Improving crop resilience with nanoparticles. Nature. 608, S16–S17 (2022)
Wang, J.W., Cunningham, F.J., Goh, N.S., Boozarpour, N.N., Pham, M., Landry, M.P.: Nanoparticles for protein delivery in planta. Curr. Opin. Plant Biol. 60, 102052 (2021)
Zhao, X., Meng, Z., Wang, Y., Chen, W., Sun, C., Cui, B., Cui, J., Yu, M., Zeng, Z., Guo, S., Luo, D., Cheng, J.Q., Zhang, R., Cui, H.: Pollen magnetofection for genetic modification with magnetic nanoparticles as gene carriers. Nat. Plants. 3, 956–964 (2017)
Vejlupkova, Z., Warman, C., Sharma, R., Scheller, H.V., Mortimer, J.C., Fowler, J.E.: No evidence for transient transformation via pollen magnetofection in several monocot species. Nat. Plants. 6, 1323–1324 (2020)
Wang, Z., Zhang, Z., Zheng, D., Zhang, T., Li, X., Zhang, C., Yu, R., Wei, J., Wu, Z.: Efficient and genotype independent maize transformation using pollen transfected by DNA-coated magnetic nanoparticles. J. Integr. Plant Biol. 64, 1145–1156 (2022)
Demirer, G.S., Zhang, H., Matos, J.L., Goh, N.S., Cunningham, F.J., Sung, Y., Chang, R., Aditham, A.J., Chio, L., Cho, M.-J., Staskawicz, B., Landry, M.P.: High aspect ratio nanomaterials enable delivery of functional genetic material without DNA integration in mature plants. Nat. Nanotechnol. 14, 456–464 (2019)
Kwak, S.-Y., Lew, T.T.S., Sweeney, C.J., Koman, V.B., Wong, M.H., Bohmert-Tatarev, K., Snell, K.D., Seo, J.S., Chua, N.-H., Strano, M.S.: Chloroplast-selective gene delivery and expression in planta using chitosan-complexed single-walled carbon nanotube carriers. Nat. Nanotechnol. 14, 447–455 (2019)
Lew, T.T.S., Park, M., Wang, Y., Gordiichuk, P., Yeap, W.-C., Mohd Rais, S.K., Kulaveerasingam, H., Strano, M.S.: Nanocarriers for transgene expression in pollen as a plant biotechnology tool. ACS Mater. Lett. 2, 1057–1066 (2020)
Wang, B., Huang, J., Zhang, M., Wang, Y., Wang, H., Ma, Y., Zhao, X., Wang, X., Liu, C., Huang, H., Liu, Y., Lu, F., Yu, H., Shao, M., Kang, Z.: Carbon dots enable efficient delivery of functional DNA in plants. ACS Appl. Bio. Mater. 3, 8857–8864 (2020)
Doyle, C., Higginbottom, K., Swift, T.A., Winfield, M., Bellas, C., Benito-Alifonso, D., Fletcher, T., Galan, M.C., Edwards, K., Whitney, H.M.: A simple method for spray-on gene editing in planta. bioRxiv, 805036 (2019)
Cho, J.-Y., Bhowmik, P., Polowick, P.L., Dodard, S.G., El-Bakkari, M., Nowak, G., Fenniri, H., Hemraz, U.D.: Cellular delivery of plasmid DNA into wheat microspores using rosette nanotubes. ACS Omega. 5, 24422–24433 (2020)
Mitter, N., Worrall, E.A., Robinson, K.E., Li, P., Jain, R.G., Taochy, C., Fletcher, S.J., Carroll, B.J., Lu, G.Q., Xu, Z.P.: Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat. Plants. 3, 16207 (2017)
Zhang, H., Demirer, G.S., Zhang, H., Ye, T., Goh, N.S., Aditham, A.J., Cunningham, F.J., Fan, C., Landry, M.P.: DNA nanostructures coordinate gene silencing in mature plants. Proc. Natl. Acad. Sci. U. S. A. 116, 7543–7548 (2019)
Zhang, H., Goh, N.S., Wang, J.W., Pinals, R.L., González-Grandío, E., Demirer, G.S., Butrus, S., Fakra, S.C., Del Rio Flores, A., Zhai, R., Zhao, B., Park, S.-J., Landry, M.P.: Nanoparticle cellular internalization is not required for RNA delivery to mature plant leaves. Nat. Nanotechnol. 17, 197–205 (2022)
Demirer, G.S., Zhang, H., Goh, N.S., Pinals, R.L., Chang, R., Landry, M.P.: Carbon nanocarriers deliver siRNA to intact plant cells for efficient gene knockdown. Sci. Adv. 6, eaaz0495 (2020)
Yong, J., Zhang, R., Bi, S., Li, P., Sun, L., Mitter, N., Carroll, B.J., Xu, Z.P.: Sheet-like clay nanoparticles deliver RNA into developing pollen to efficiently silence a target gene. Plant Physiol. 187, 886–899 (2021)
Martin-Ortigosa, S., Valenstein, J.S., Lin, V.S.-Y., Trewyn, B.G., Wang, K.: Gold functionalized mesoporous silica nanoparticle mediated protein and DNA codelivery to plant cells via the biolistic method. Adv. Funct. Mater. 22, 3576–3582 (2012)
Martin-Ortigosa, S., Peterson, D.J., Valenstein, J.S., Lin, V.S.-Y., Trewyn, B.G., Lyznik, L.A., Wang, K.: Mesoporous silica nanoparticle mediated intracellular Cre protein delivery for maize genome editing via loxP sites excision. Plant Physiol. 164, 537 (2013)
Gogoi, N., Kanwal, M., Norman, M., Downs, J., Ahmad, N., Mago, R., Bariana, H., Müllner, M., Bansal, U., Jones, B.: Wheat pollen uptake of CRISPR/Cas9 RNP-PDMAEMA nanoassemblies results in targeted loss of gene function in progeny. bioRxiv 2022.06.02.494465 (2022)
Soliman, A., Laurie, J., Bilichak, A., Ziemienowicz, A.: Cell Penetrating Peptides. Methods in Molecular Biology, vol 2383. Humana, New York, NY (2022)
Wu, H., Zhang, K., Zhang, Z., Wang, J., Jia, P., Cong, L., Li, J., Duan, Y., Ke, F., Zhang, F., Liu, Z., Lu, F., Wang, Y., Li, Z., Chang, M., Zou, J., Zhu, K.: Cell-penetrating peptide: a powerful delivery tool for DNA-free crop genome editing. Plant Sci. 324, 111436 (2022)
Chugh, A., Amundsen, E., Eudes, F.: Translocation of cell-penetrating peptides and delivery of their cargoes in triticale microspores. Plant Cell Rep. 28, 801–810 (2009)
Ng, K.K., Motoda, Y., Watanabe, S., Sofiman Othman, A., Kigawa, T., Kodama, Y., Numata, K.: Intracellular delivery of proteins via fusion peptides in intact plants. PLoS One. 11, e0154081 (2016)
Thagun, C., Motoda, Y., Kigawa, T., Kodama, Y., Numata, K.: Simultaneous introduction of multiple biomacromolecules into plant cells using a cell-penetrating peptide nanocarrier. Nanoscale. 12, 18844–18856 (2020)
Bilichak, A., Sastry-Dent, L., Sriram, S., Simpson, M., Samuel, P., Webb, S., Jiang, F., Eudes, F.: Genome editing in wheat microspores and haploid embryos mediated by delivery of ZFN proteins and cell-penetrating peptide complexes. Plant Biotechnol. J. 18, 1307–1316 (2020)
Odahara, M., Watanabe, K., Kawasaki, R., Tsuchiya, K., Tateishi, A., Motoda, Y., Kigawa, T., Kodama, Y., Numata, K.: Nanoscale Polyion complex vesicles for delivery of cargo proteins and Cas9 ribonucleoprotein complexes to plant cells. ACS Appl. Nano. Mater. 4, 5630–5635 (2021)
Ali, Z., Serag, M.F., Demirer, G.S., Torre, B., Di Fabrizio, E., Landry, M.P., Habuchi, S., Mahfouz, M.: DNA-carbon nanotube binding mode determines the efficiency of carbon nanotube-mediated DNA delivery to intact plants. ACS Appl. Nano. Mater. 5, 4663–4676 (2022)
Liu, S., Sretenovic, S., Fan, T., Cheng, Y., Li, G., Qi, A., Tang, X., Xu, Y., Guo, W., Zhong, Z., He, Y., Liang, Y., Han, Q., Zheng, X., Gu, X., Qi, Y., Zhang, Y.: Hypercompact CRISPR–Cas12j2 (CasΦ) enables genome editing, gene activation, and epigenome editing in plants. Plant Commun. 3, 100453 (2022)
Rastogi, A., Tripathi, D.K., Yadav, S., Chauhan, D.K., Živčák, M., Ghorbanpour, M., El-Sheery, N.I., Brestic, M.: Application of silicon nanoparticles in agriculture. 3 Biotech. 9, 90 (2019)
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2024 The Author(s)
About this chapter
Cite this chapter
Prestwich, B.D., Cardi, T., Bakhsh, A., Nicolia, A., Bhati, K.K. (2024). Novel Delivery Methods for CRISPR-Based Plant Genome Editing. In: Ricroch, A., Eriksson, D., Miladinović, D., Sweet, J., Van Laere, K., Woźniak-Gientka, E. (eds) A Roadmap for Plant Genome Editing . Springer, Cham. https://doi.org/10.1007/978-3-031-46150-7_3
Download citation
DOI: https://doi.org/10.1007/978-3-031-46150-7_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-46149-1
Online ISBN: 978-3-031-46150-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)