Abstract
The effectiveness of biomedical surfaces may be highly affected by the hydrodynamic condition. Surfaces releasing antimicrobial substances when exposed to flow may exhibit shorter lifetimes than at static conditions. Likewise, depending on the fluid flow surrounding the surface, contact-killing surfaces that are adhesive for bacterial cells may be covered by bacterial debris, which decreases their antimicrobial activity. To evaluate the anti-adhesive and antimicrobial performance of novel biomedical materials, a number of flow devices have been designed to recreate in vivo flow conditions. Shear stress and flow rate can be accurately controlled and varied in these in vitro flow systems, which requires prior knowledge of the flow dynamics inside the platform. After limiting their operational range, modified Robbins devices, flow chambers and microfluidic devices are suggested as experimental setups to mimic the flow behavior in urinary catheters and stents.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The complications associated with indwelling ureteral stents, namely bacterial adhesion and biofilm formation, have been the main driving force for the development of new materials or coatings with antimicrobial and anti-adhesive properties. The first approach for testing and optimizing new biomedical surfaces usually consists of evaluating their in vitro efficacy under controlled experimental conditions that reflect the human physiological environment [1]. Consequently, several parameters, including the pathogenic species and their concentration, culture medium, temperature, and hydrodynamic conditions, must be considered when setting an in vitro experiment, hence increasing its predictive value and avoiding, during initial screening, expensive in vivo assays and animal sacrifice [1] without prior evidence of surface effectiveness. Among these parameters, hydrodynamic conditions have a prominent role in the experimental setup as assays performed in static conditions do not mimic the fluid flow that occurs at specific locations of the human body (e.g. urinary tract). Furthermore, it is well known that hydrodynamic conditions affect not only bacterial adhesion to biomedical surfaces [2], but also biofilm growth and architecture [3, 4]. In fact, flow determines the transport rate of planktonic cells to the surface and their subsequent interaction [5], as well as the transport of oxygen and nutrients to the biofilm [6]. Besides, flow influences both bacterial attachment and detachment rates [7].
The effectiveness of biomedical surfaces may also be highly affected by the hydrodynamic conditions [1]. Surfaces releasing antimicrobial substances when exposed to flow may exhibit shorter lifetimes than at static conditions [1]. Likewise, depending on the fluid flow surrounding the surface, contact-killing surfaces that are adhesive for bacterial cells may be covered by bacterial debris, which decreases their antimicrobial activity [1]. Lastly, non-adhesive coatings, such as polymer brush coatings, are generally sensitive to external stimuli, exhibiting higher antifouling performance at quasi-static conditions and more effective fouling release behavior under dynamic conditions [8].
Considering the importance of hydrodynamic conditions and their effects on bacterial adhesion and biofilm formation, a diversity of in vitro flow systems, including the Robbins device (RD) and modifications, the drip flow biofilm reactor, rotary biofilm reactors and flow chambers (FCs), have been developed and optimized to evaluate surfaces effectiveness under physiological conditions [9]. Certain flow systems enable real-time visualization of bacteria adhesion/biofilm development under controlled conditions (e.g. shear stress or shear rate, temperature), allow simultaneous testing of different materials, and can be used as high-throughput platforms [9], while others have some limitations in operating at highly controlled hydrodynamic conditions [1]. Hence, each platform presents advantages and disadvantages that must be considered before use.
In this chapter, the most commonly used platforms for the in vitro assessment of bacterial adhesion and biofilm formation under flow conditions—the modified Robbins device, flow chambers, and microfluidic devices—are introduced, and their main advantages and disadvantages discussed. These three testing platforms have been particularly used to evaluate the anti-adhesive and antibiofilm performance of novel surface materials for urinary tract devices (UTDs), including catheters and stents, due to their ability to control the hydrodynamics (shear stress and flow rate) and recreate in vivo flow conditions.
2 Robbins Device and Modifications
The Robbins device was initially developed by Jim Robbins and Bill McCoy to study biofilm formation in industrial water systems [10]. The RD consists of a pipe with several holes where coupons are mounted on the end of the screws and become in contact with the fluid. Thus, the RD generates submerged biofilms growing in aqueous systems that can be used for the investigation of multispecies communities [10].
Several modifications were later introduced to this design, including the use of a square-channel pipe where coupons are aligned with the inner surface without disturbing flow characteristics [11]. Other designs include a half-pipe geometry that more closely resembles the circular section of a tube [4]. With the modified Robbins devices (MRDs), the flow can be momentarily stopped to allow direct access to the coupons so that time-course experiments are also possible [3].
MRDs have been operated in conditions that mimic the flow in urinary catheters [12, 13] and stents [13, 14]. Tunner et al. [14] were among the first authors to use a continuous flow model based on an MRD to assess encrustation on silicone and polyurethane, the most widely used ureteral stent biomaterials. They revealed that the type and degree of encrustation produced were similar to those found in vivo, recommending this flow system for comparative evaluation of surface candidates for medical devices used in the urinary tract [14]. More recently, in our research group, a MRD (referred to as flow cell system) simulating the hydrodynamic conditions found in urinary catheters (shear rate of 15/s) [15] was used to characterize the microbial physiology of Escherichia coli and Delftia tsuruhatensis individually and in a consortium, in terms of growth kinetics and substrate uptake, when exposed to artificial urine medium (AUM) flow and silicone material [12]. Additionally, we used a custom-made semi-circular flow cell identical to that shown in Fig. 1 to assess the efficacy of different nanocomposite coatings in preventing urinary tract infections (UTIs) [13]. The hydrodynamics of this flow cell was fully characterized by computational fluid dynamics (CFD) [16], and it has been shown that the shear stress field is approximately the same in the curved and flat walls so that coupons can be placed on the flat wall for convenience and still be subjected to the same shear forces acting on the curved wall [17]. Moreover, this flow cell was constructed to have enough inlet length to allow for full flow development and a large surface area on which the hydrodynamic conditions remain constant for a wide range of flow velocities [16]. These dynamic systems are particularly useful for screening purposes as they enable the simultaneous testing of several surfaces [13, 14]. Another advantage of MRDs is that coupons can be removed independently, for instance, at different experimental times [12].
3 Flow Chamber
Despite the many advantages of the MRDs, they are usually not suited for direct analysis of biofilm development [18], and they are not adequate to monitor cell adhesion to a surface. Nowadays, there are several models of flow chambers that can be mounted on a microscope stage and used with video capture systems, enabling real-time observation of microbial adhesion, particularly when used with transparent surfaces [18]. Different custom-made FCs have been used to evaluate the anti-adhesive and antibiofilm properties of novel surfaces for UTDs, namely catheters and stents, in flow conditions that simulate those typically found in these medical devices [2, 15, 19, 20]. Table 1 summarizes several studies found in the literature where flow chamber assays were performed under fully characterized hydrodynamic conditions similar to those of urinary catheters and stents. Most of these studies aimed to monitor the initial adhesion of bacteria associated with UTIs (E. coli, Enterococcus faecalis, Staphylococcus aureus and Pseudomonas aeruginosa) to polymeric surfaces as polydimethylsiloxane (PDMS) [2, 9] and PDMS modified with antimicrobial substances (peptides and carbon nanotubes) [21,22,23] for 30 min to 4 h. In some instances, these systems were also used to investigate bacterial biofilm growth and survival for 24 h on novel surface coatings for UTDs [19, 24, 25].
A custom-made FC system (Fig. 2) was designed by our group to analyse cell adhesion [22, 26] and biofilm formation [19, 24]. This system includes a parallel-plate flow chamber (PPFC) coupled to a jacketed tank and connected to centrifugal pumps and a valve by a silicone tubing system. The valve allows the bacterial suspension to circulate through the system at a controlled flow rate, and the recirculating water bath is connected to the tank jacket to enable temperature control. To illustrate the type of data that can be obtained with this platform, biofilm formation experiments with E. coli were carried out for 24 h using PDMS as the test surface [27] and AUM recirculated through the FC system at 4 mL/s to mimic the urine flow behavior in ureteral stents (shear rate of 15/s). After 24 h, the system was stopped, and the biofilm formed on the PDMS surface was stained with a fluorescent dye and analysed by confocal laser scanning microscopy (CLSM) (Fig. 3 and Table 2).
3-D projection of biofilms formed on PDMS at a flow rate of 4 mL/s mimicking ureteral stents in the described PPFC system. Shown is an E. coli biofilm stained with SYTO 61 (633 nm laser line, LEICA HCX PL APO 10 ×/0.40 CS). This representative image was obtained using the “Easy 3D” tool of IMARIS 8.4.1 software (Bitplane, Switzerland) from a confocal z stack, and presents an aerial view of the biofilm structure with the shadow projection on the right
CLSM is an optical imaging technique used to obtain high-resolution images of biofilms at various depths in their naturally hydrated form and to generate three-dimensional (3-D) reconstructions of the samples [28]. It is particularly well suited for monitoring 3-D structure formation in flow chamber-grown biofilms due to its non-invasive and non-destructive character [29, 30]. Early research investigating the use of CLSM in biofilm studies was more descriptive, using qualitative metrics to evaluate biofilm architecture [31]. The development of imaging software packages, specifically for biofilm samples, has enhanced the quantitative output from CLSM images of biofilms [32]. Among these, the COMSTAT ImageJ plugin [32] used in the present work (Table 2) or the PHLIP Matlab toolbox [33, 34] represent a set of reference tools that are efficient and reliable to characterize biofilms in terms of biomass, thickness distribution, surface coverage, roughness coefficient, or porosity.
4 Microfluidic Devices
Microfluidic platforms have demonstrated high potential and versatility for the study of bacterial adhesion and biofilm formation under different growth conditions. These platforms allow the testing of different channel architectures and types of materials or surfaces at highly controlled flow conditions through a rapid and precise analysis [5]. For these reasons, microfluidic platforms have been used to explore the combined effect of several factors on the development of clinically relevant biofilms [35,36,37]. Table 3 lists several studies using microfluidic devices for the evaluation of bacterial adhesion and biofilm formation under flow conditions that represent relevant hydrodynamic regions of ureteral stents.
Although microfluidic devices can be constructed by different methodologies and from a diversity of materials, PDMS has been the material of choice for the construction of these devices, with most of the PDMS-based microfluidic devices being designed for a specific purpose. Several studies have investigated the initial bacterial adhesion on different materials using microfluidic platforms [5, 7, 38,39,40,41]. In general, the bacterial residence time and surface coverage increased linearly up to 3.5 Pa [7] and 20/s [40], respectively, and the adhesion rates were higher in locations with a sudden increase in shear forces [39]. For the particular case of ureteral stents, De Garcia et al. [5] demonstrated that unobstructed devices (wall shear stress ≤ 0.0875 Pa) showed no short-term bacterial adhesion, while in obstructed devices, the cavity region and nearby proximal side-hole (wall shear stress of 0.131–0.175 Pa) exhibited higher levels of bacterial attachment compared to other regions of the model. Although channel architecture and geometry affect bacterial adhesion [41], these findings indicate that flow influences both attachment and detachment rates [7].
PDMS-based microfluidic devices have also been applied to explore how bacterial colonization, competition, and dispersal occur at flow conditions. Indeed, flow can confer growth advantages to pathogens by allowing the bacteria upstream movement [42]. Similarly, the study of biofilm development is also possible using these microfluidic platforms [35, 43,44,45,46,47,48]. Several authors revealed that flow alone was able to induce the formation of polysaccharide intracellular adhesins [46] and was the major modulator of the biofilm structures [45]. Additionally, Lee et al. [35] demonstrated that the morphology of Staphylococcus epidermidis biofilm formation was influenced by local hydrodynamic conditions. While higher wall shear stress limited vertical biofilm growth, resulting in a monolayer structure, cells growing in stagnant areas were able to proliferate rapidly, resulting in the formation of a large multilayer structure [35]. Likewise, biofilm thickness was also affected by flow after 48 h, increasing significantly at 0.010 Pa (36 ± 9 μm) and slightly at 0.0035 Pa (20 ± 4 μm). Contrarily, no increase was detected for higher shear stresses [44]. Accordingly, Kim et al. [48] revealed that quorum sensing-mediated communication during biofilm formation was generally repressed by flow, impairing biofilm growth. The comprehensive analysis of gene expression during S. aureus biofilm formation was successfully conducted by Moormeier et al. [49, 50] using a different microfluidic device, the BioFlux system (Fluxion Systems, South San Francisco, CA), and compared with static conditions. The BioFlux system was presented as the most prominent commercial microfluidic platform that overcomes the limitations of static well plates and conventional laminar flow chambers. In this system, biofilm formation can be followed by light microscopy in microfluidic wells, allowing rapid screening of the effects of several compounds on the viability of biofilms under hydrodynamic conditions [51]. One of the early studies performed on this platform evaluated the effect of several antimicrobials on 8 h-developed P. aeruginosa biofilms under controlled hydrodynamic conditions at 37 °C. Results suggested that biofilm viability measured with the plate reader agreed with those determined using plate counts and with the results of fluorescence microscope image analysis. Since then, the BioFlux system has been considered a high-throughput methodology for the study of biofilm development under defined hydrodynamic conditions [36, 49, 50, 52,53,54].
Although only 1 of 21 analysed studies had the specific objective of evaluating bacterial adhesion in urinary stents, all provided a comprehensive analysis of adhesion and biofilm formation at flow conditions representative of relevant hydrodynamic regions of ureteral stents [55] and should be considered when testing a new surface or coating for these medical settings.
5 Operating Conditions
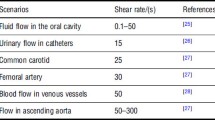
As previously shown, MRDs, flow chambers and microfluidic devices have been used to study bacterial adhesion and biofilm formation under hydrodynamic conditions that simulate the UTDs. Because the flow rate by itself provides little information about shear without taking into account the geometry of the in vitro flow system, it is crucial to mimic the flow conditions in a catheter or stent by using either the wall shear stress or the shear rate [1]. The wall shear rate (σ, with unit/s) is a measure of change of the fluid velocity near the wall of the tube in the radial direction toward the center of the tube. In laminar conditions, the shear rate is related to the force which the fluid flow exerts on the wall, expressed as shear stress (τ, with unit Pa), through τ = μ × σ, where μ is the dynamic viscosity of the fluid (10−3 Pa s for water). In the flow systems under study, the flow rate should be adjusted to approach an average shear rate of around 15/s as an estimate of the intraluminal urine flow, based on predictable daily urine production and internal catheter diameter [15]. Nevertheless, urinary output values are highly variable and may reach more than 10 times the mean value [56], yielding a proportional increase in wall shear rate. Some authors performed FC tests at a shear rate of 33/s, which is higher than mean values but still within the range of shear rates found in urinary catheters [25].
Regarding the flow chamber system described in this work (Fig. 2), the numerical simulations indicated that the shear rate of 15/s reported for urinary flow in catheters can be attained at a flow rate of 2 mL/s [2, 9]. On the other hand, the average shear stress in problematic zones of ureteral stents that are prone to encrustation (0.024 Pa) [55] can be obtained by operating the PPFC system at a flow rate of 4 mL/s [21]. In the case of MRDs used by our research group, the recirculation flow rates can range from 5 [12] to 53 mL/s [13] to mimic the shear forces on urinary catheters, depending on the geometry of the flow cell.
PDMS-based microfluidic devices are usually designed for a particular application, having their own architecture and geometry with specific operating conditions. In the case of the commercially available BioFlux system, numerical simulations revealed that the average shear stress value of 0.02 Pa reported for ureteral stents [55] can be reached at a flow rate of 66 μL/h [52].
6 Strengths and Limitations of Flow Platforms
Among the advantages of flow systems are the ability to compare, for instance, the effect that different substrates, media and hydrodynamic conditions exert on a biofilm at different developmental stages. These dynamic models may also provide an evaluation of the effect that transiently occurring molecules, such as antibiotics or adherence inhibitors, have on biofilms. However, the technical disadvantages of flow reactors include increased experimental complexity as well as possible formation/trapping of air bubbles in the setup tubing (particularly severe in microfluidic systems), as this can affect flow and biofilm architecture [57].
Choosing the experimental platform for flow experiments determines what kind of data can be extracted, and care must be taken to ensure that the selected reactor fulfills the objectives of the experiments. The three platforms covered in this chapter (modified Robbins device, flow chamber and microfluidics-based device) have benefits and limitations, which are summarized in Table 4.
7 Conclusions
To evaluate the anti-adhesive and antimicrobial performance of novel biomedical materials, a number of flow devices have been designed to recreate in vivo flow conditions. Shear stress and flow rate can be accurately controlled and varied in these in vitro flow systems, which requires prior knowledge of the flow dynamics inside the platform. After limiting their operational range, modified Robbins devices, flow chambers and microfluidic devices are suggested as experimental setups to mimic the flow behavior in urinary catheters and stents.
Abbreviations
- 3-D:
-
Three-dimensional
- AUM:
-
Artificial urine medium
- CFD:
-
Computational fluid dynamics
- CLSM:
-
Confocal laser scanning microscopy
- FC:
-
Flow chamber
- MRD:
-
Modified Robbins device
- PDMS:
-
Polydimethylsiloxane
- PPFC:
-
Parallel-plate flow chamber
- RD:
-
Robbins device
- UTD :
-
Urinary tract device
- UTI:
-
Urinary tract infection
References
Ramstedt M, Ribeiro IAC, Bujdakova H, Mergulhão FJ, Jordao L, Thomsen P, et al. Evaluating efficacy of antimicrobial and antifouling materials for urinary tract medical devices: challenges and recommendations. Macromol Biosci. 2019;19(5):e1800384.
Moreira JMR, Araújo JDP, Miranda JM, Simões M, Melo LF, Mergulhão FJ. The effects of surface properties on Escherichia coli adhesion are modulated by shear stress. Colloids Surf B Biointerfaces. 2014;123:1–7.
Teodósio JS, Simões M, Melo LF, Mergulhão FJ. Flow cell hydrodynamics and their effects on E. coli biofilm formation under different nutrient conditions and turbulent flow. Biofouling. 2011;27(1):1–11.
Pereira MO, Kuehn M, Wuertz S, Neu T, Melo LF. Effect of flow regime on the architecture of a Pseudomonas fluorescens biofilm. Biotechnol Bioeng. 2002;78(2):164–71.
De Grazia A, LuTheryn G, Meghdadi A, Mosayyebi A, Espinosa-Ortiz EJ, Gerlach R, et al. A microfluidic-based investigation of bacterial attachment in ureteral stents. Micromachines. 2020;11(4):408.
Pousti M, Zarabadi MP, Abbaszadeh Amirdehi M, Paquet-Mercier F, Greener J. Microfluidic bioanalytical flow cells for biofilm studies: a review. Analyst. 2019;144(1):68–86.
Lecuyer S, Rusconi R, Shen Y, Forsyth A, Vlamakis H, Kolter R, et al. Shear stress increases the residence time of adhesion of Pseudomonas aeruginosa. Biophys J. 2011;100(2):341–50.
Yang W, Zhou F. Polymer brushes for antibiofouling and lubrication. Biosurf Biotribol. 2017;3(3):97–114.
Moreira JMR, Ponmozhi J, Campos JBLM, Miranda JM, Mergulhão FJ. Micro- and macro-flow systems to study Escherichia coli adhesion to biomedical materials. Chem Eng Sci. 2015;126:440–5.
McCoy WF, Bryers JD, Robbins J, Costerton JW. Observations of fouling biofilm formation. Can J Microbiol. 1981;27(9):910–7.
Stoodley P, Warwood BK. Use of flow cells an annular reactors to study biofilms. In: Lens P, O’Flaherty V, Moran AP, Stoodley P, Mahony T, editors. Biofilms in medicine, industry and environmental biotechnology: characteristics, analysis and control. 1st ed. Cornwall: IWA Publishing; 2003. p. 197–213.
Azevedo AS, Almeida C, Gomes LC, Ferreira C, Mergulhão FJ, Melo LF, et al. An in vitro model of catheter-associated urinary tract infections to investigate the role of uncommon bacteria on the Escherichia coli microbial consortium. Biochem Eng J. 2017;118:64–9.
Vladkova T, Angelov O, Stoyanova D, Gospodinova D, Gomes LC, Soares A, et al. Magnetron co-sputtered TiO2/SiO2/Ag nanocomposite thin coatings inhibiting bacterial adhesion and biofilm formation. Surf Coat Technol. 2020;384:125322.
Tunney MM, Keane PF, Gorman SP. Assessment of urinary tract biomaterial encrustation using a modified Robbins device continuous flow model. J Biomed Mater Res. 1997;38(2):87–93.
Velraeds MMC, Van Der Mei HC, Reid G, Busscher HJ. Inhibition of initial adhesion of uropathogenic Enterococcus faecalis to solid substrata by an adsorbed biosurfactant layer from Lactobacillus acidophilus. Urology. 1997;49(5):790–4.
Teodósio JS, Simões M, Alves MA, Melo LF, Mergulhão FJ. Setup and validation of flow cell systems for biofouling simulation in industrial settings. Sci World J. 2012;2012:361496.
Teodósio JS, Silva FC, Moreira JMR, Simões M, Melo LF, Alves MA, et al. Flow cells as quasi-ideal systems for biofouling simulation of industrial piping systems. Biofouling. 2013;29(8):953–66.
Azeredo J, Azevedo NF, Briandet R, Cerca N, Coenye T, Costa AR, et al. Critical review on biofilm methods. Crit Rev Microbiol. 2017;43(3):313–51.
Alves P, Gomes LC, Vorobii M, Rodriguez-Emmenegger C, Mergulhão FJ. The potential advantages of using a poly(HPMA) brush in urinary catheters: effects on biofilm cells and architecture. Colloids Surf B Biointerfaces. 2020;191:110976.
Habash MB, Mei HCV, Busscher HJ, Reid G. The effect of water, ascorbic acid, and cranberry derived supplementation on human urine and uropathogen adhesion to silicone rubber. Can J Microbiol. 1999;45(8):691–4.
Dolid A, Gomes LC, Mergulhão FJ, Reches M. Combining chemistry and topography to fight biofilm formation: fabrication of micropatterned surfaces with a peptide-based coating. Colloids Surf B Biointerfaces. 2020;196:111365.
Vagos MR, Gomes M, Moreira JMR, Soares OSGP, Pereira MFR, Mergulhão FJ. Carbon nanotube/poly(dimethylsiloxane) composite materials to reduce bacterial adhesion. Antibiotics. 2020;9(8):434.
Vagos MR, Moreira JMR, Soares OSGP, Pereira MFR, Mergulhão FJ. Incorporation of carbon nanotubes in polydimethylsiloxane to control Escherichia coli adhesion. Polym Compos. 2019;40(S2):E1697–E704.
Alves P, Gomes LC, Rodríguez-Emmenegger C, Mergulhão FJ. Efficacy of a poly(MeOEGMA) brush on the prevention of Escherichia coli biofilm formation and susceptibility. Antibiotics. 2020;9(5):216.
Andersen TE, Kingshott P, Palarasah Y, Benter M, Alei M, Kolmos HJ. A flow chamber assay for quantitative evaluation of bacterial surface colonization used to investigate the influence of temperature and surface hydrophilicity on the biofilm forming capacity of uropathogenic Escherichia coli. J Microbiol Methods. 2010;81(2):135–40.
Alves P, Nir S, Reches M, Mergulhão FJ. The effects of fluid composition and shear conditions on bacterial adhesion to an antifouling peptide-coated surface. MRS Commun. 2018;8(3):938–46.
Gomes M, Gomes LC, Teixeira-Santos R, Mergulhão FJ. PDMS in urinary tract devices: applications, problems and potential solutions. In: Carlsen PN, editor. Polydimethylsiloxane: structure and applications. New York: Nova Science Publishers; 2020. p. 95–144.
Cattò C, Cappitelli F. Testing anti-biofilm polymeric surfaces: where to start? Int J Mol Sci. 2019;20(15):3794.
Tolker-Nielsen T, Sternberg C. Growing and analyzing biofilms in flow chambers. Curr Protoc Microbiol. 2011;21(1):1B.2.1–B.2.17.
Reichhardt C, Parsek MR. Confocal laser scanning microscopy for analysis of Pseudomonas aeruginosa biofilm architecture and matrix localization. Front Microbiol. 2019;10(677):677.
Thomas RN. In situ cell and glycoconjugate distribution in river snow studied by confocal laser scanning microscopy. Aquat Microb Ecol. 2000;21(1):85–95.
Heydorn A, Nielsen AT, Hentzer M, Sternberg C, Givskov M, Ersbøll BK, et al. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology. 2000;146(Pt10):2395–407.
Mueller LN, de Brouwer JFC, Almeida JS, Stal LJ, Xavier JB. Analysis of a marine phototrophic biofilm by confocal laser scanning microscopy using the new image quantification software PHLIP. BMC Ecol. 2006;6(1):1.
Gomes LC, Deschamps J, Briandet R, Mergulhão FJ. Impact of modified diamond-like carbon coatings on the spatial organization and disinfection of mixed-biofilms composed of Escherichia coli and Pantoea agglomerans industrial isolates. Int J Food Microbiol. 2018;277:74–82.
Lee JH, Kaplan JB, Lee WY. Microfluidic devices for studying growth and detachment of Staphylococcus epidermidis biofilms. Biomed Microdevices. 2008;10(4):489–98.
Tremblay YD, Vogeleer P, Jacques M, Harel J. High-throughput microfluidic method to study biofilm formation and host–pathogen interactions in pathogenic Escherichia coli. Appl Environ Microbiol. 2015;81(8):2827–40.
Shields RC, Burne RA. Growth of Streptococcus mutans in biofilms alters peptide signaling at the sub-population level. Front Microbiol. 2016;7:1075.
Ponmozhi J, Moreira JMR, Mergulhão FJ, Campos JBLM, Miranda JM. Fabrication and hydrodynamic characterization of a microfluidic device for cell adhesion tests in polymeric surfaces. Micromachines. 2019;10(5):303.
Neves SF, Ponmozhi J, Mergulhão FJ, Campos JBLM, Miranda JM. Cell adhesion in microchannel multiple constrictions—evidence of mass transport limitations. Colloids Surf B Biointerfaces. 2020;198:111490.
Rusconi R, Guasto JS, Stocker R. Bacterial transport suppressed by fluid shear. Nat Phys. 2014;10(3):212–7.
Secchi E, Vitale A, Miño GL, Kantsler V, Eberl L, Rusconi R, et al. The effect of flow on swimming bacteria controls the initial colonization of curved surfaces. Nat Commun. 2020;11(1):2851.
Siryaporn A, Kim MK, Shen Y, Stone HA, Gitai Z. Colonization, competition, and dispersal of pathogens in fluid flow networks. Curr Biol. 2015;25(9):1201–7.
Zhang XY, Sun K, Abulimiti A, Xu PP, Li ZY. Microfluidic system for observation of bacterial culture and effects on biofilm formation at microscale. Micromachines. 2019;10(9):606.
Janakiraman V, Englert D, Jayaraman A, Baskaran H. Modeling growth and quorum sensing in biofilms grown in microfluidic chambers. Ann Biomed Eng. 2009;37(6):1206–16.
Kim MK, Drescher K, Pak OS, Bassler BL, Stone HA. Filaments in curved streamlines: rapid formation of Staphylococcus aureus biofilm streamers. New J Phys. 2014;16(6):065024.
Weaver WM, Milisavljevic V, Miller JF, Di Carlo D. Fluid flow induces biofilm formation in Staphylococcus epidermidis polysaccharide intracellular adhesin-positive clinical isolates. Appl Environ Microbiol. 2012;78(16):5890–6.
Zarabadi MP, Paquet-Mercier F, Charette SJ, Greener J. Hydrodynamic effects on biofilms at the biointerface using a microfluidic electrochemical cell: case study of Pseudomonas sp. Langmuir. 2017;33(8):2041–9.
Kim MK, Ingremeau F, Zhao A, Bassler BL, Stone HA. Local and global consequences of flow on bacterial quorum sensing. Nat Microbiol. 2016;1:15005.
Moormeier DE, Endres JL, Mann EE, Sadykov MR, Horswill AR, Rice KC, et al. Use of microfluidic technology to analyze gene expression during Staphylococcus aureus biofilm formation reveals distinct physiological niches. Appl Environ Microbiol. 2013;79(11):3413–24.
Lehman MK, Bose JL, Sharma-Kuinkel BK, Moormeier DE, Endres JL, Sadykov MR, et al. Identification of the amino acids essential for LytSR-mediated signal transduction in Staphylococcus aureus and their roles in biofilm-specific gene expression. Mol Microbiol. 2015;95(4):723–37.
Benoit MR, Conant CG, Ionescu-Zanetti C, Schwartz M, Matin A. New device for high-throughput viability screening of flow biofilms. Appl Environ Microbiol. 2010;76(13):4136–42.
Feng SH, Stojadinovic A, Izadjoo M. Distinctive stages and strain variations of A. baumannii biofilm development under shear flow. J Wound Care. 2013;22(4):173–4.
Vanhommerig E, Moons P, Pirici D, Lammens C, Hernalsteens J-P, De Greve H, et al. Comparison of biofilm formation between major clonal lineages of methicillin resistant Staphylococcus aureus. PLoS One. 2014;9(8):e104561.
Goetz C, Tremblay YDN, Lamarche D, Blondeau A, Gaudreau AM, Labrie J, et al. Coagulase-negative staphylococci species affect biofilm formation of other coagulase-negative and coagulase-positive staphylococci. J Dairy Sci. 2017;100(8):6454–64.
Mosayyebi A, Yue QY, Somani BK, Zhang X, Manes C, Carugo D. Particle accumulation in ureteral stents is governed by fluid dynamics: in vitro study using a “stent-on-chip” model. J Endourol. 2018;32(7):639–46.
Fallis WM. Indwelling Foley catheters: is the current design a source of erroneous measurement of urine output? Crit Care Nurse. 2005;25(2):44–6.
Magana M, Sereti C, Ioannidis A, Mitchell CA, Ball AR, Magiorkinis E, et al. Options and limitations in clinical investigation of bacterial biofilms. Clin Microbiol Rev. 2018;31(3):e00084–16.
Lopez-Mila B, Alves P, Riedel T, Dittrich B, Mergulhão FJ, Rodriguez-Emmenegger C. Effect of shear stress on the reduction of bacterial adhesion to antifouling polymers. Bioinspir Biomim. 2018;13(6):065001.
Fundeanu I, van der Mei HC, Schouten AJ, Busscher HJ. Polyacrylamide brush coatings preventing microbial adhesion to silicone rubber. Colloids Surf B Biointerfaces. 2008;64(2):297–301.
Cringus-Fundeanu I, Luijten J, van der Mei HC, Busscher HJ, Schouten AJ. Synthesis and characterization of surface-grafted polyacrylamide brushes and their inhibition of microbial adhesion. Langmuir. 2007;23(9):5120–6.
Roosjen A, Kaper HJ, van der Mei HC, Norde W, Busscher HJ. Inhibition of adhesion of yeasts and bacteria by poly(ethylene oxide)-brushes on glass in a parallel plate flow chamber. Microbiology. 2003;149(11):3239–46.
Millsap K, Reid G, van der Mei HC, Busscher HJ. Displacement of Enterococcus faecalis from hydrophobic and hydrophilic substrata by Lactobacillus and Streptococcus spp. as studied in a parallel plate flow chamber. Appl Environ Microbiol. 1994;60(6):1867–74.
Gabi M, Hefermehl L, Lukic D, Zahn R, Vörös J, Eberli D. Electrical microcurrent to prevent conditioning film and bacterial adhesion to urological stents. Urol Res. 2011;39(2):81–8.
Acknowledgements
This work was financially supported by: LA/P/0045/2020 (ALiCE), UIDB/00511/2020, and UIDP/00511/2020 (LEPABE), funded by national funds through the FCT/MCTES (PIDDAC); Project PTDC/CTMCOM/4844/2020 funded by the Portuguese Foundation for Science and Technology (FCT); and by Project 2SMART (NORTE-01-0145-FEDER-000054), supported by Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF). L. C. Gomes and M. J. Romeu acknowledge FCT for the financial support of her work contract through the Scientific Employment Stimulus—Individual Call—[CEECIND/01700/2017] and for a PhD grant (SFRH/BD/140080/2018), respectively. R. Teixeira-Santos acknowledges the receipt of a junior researcher fellowship from the Project PTDC/BII-BIO/29589/2017—POCI-01-0145-FEDER-029589. Support from the EU COST Action ENIUS (CA16217) is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this chapter
Cite this chapter
Gomes, L.C., Teixeira-Santos, R., Romeu, M.J., Mergulhão, F.J. (2022). Bacterial Adhesion and Biofilm Formation: Hydrodynamics Effects. In: Soria, F., Rako, D., de Graaf, P. (eds) Urinary Stents. Springer, Cham. https://doi.org/10.1007/978-3-031-04484-7_19
Download citation
DOI: https://doi.org/10.1007/978-3-031-04484-7_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-04483-0
Online ISBN: 978-3-031-04484-7
eBook Packages: MedicineMedicine (R0)