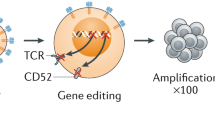
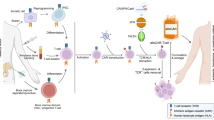
Abstract
CAR-T cell manufacturing starts from a collection of mononuclear cells (MNCs, although specifically only T lymphocytes will be used for the preparation) from the patient using apheresis. Although several initiatives are working on the development of allogeneic CAR-T cells, currently only CAR-T therapies of autologous origin are approved in the European Union. The present chapter only discusses already or soon-to-be marketed autologous CAR-T cells and excludes investigational CAR-T cells or rare CAR-T cells approved in the context of hospital exemption, such as the ARI-001 product (Ortiz-Maldonado et al. 2021); on this topic, please refer to Chapter 3c.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Introduction
CAR-T cell manufacturing starts from a collection of mononuclear cells (MNCs, although specifically only T lymphocytes will be used for the preparation) from the patient using apheresis. Although several initiatives are working on the development of allogeneic CAR-T cells, currently only CAR-T therapies of autologous origin are approved in the European Union. The present chapter only discusses already or soon-to-be marketed autologous CAR-T cells and excludes investigational CAR-T cells or rare CAR-T cells approved in the context of hospital exemption, such as the ARI-001 product (Ortiz-Maldonado et al. 2021); on this topic, please refer to Chap. 3.
Institutions aspiring to be CAR-T centres must generate sufficient apheresis capacity to ensure immediate access to apheresis slots; apheresis capacity must grow in synchrony with the CAR-T program (Tables 6.1, 6.2, 6.3, 6.4, and 6.5).
Key Points
-
Prepare for apheresis by assessing the clinical and biological condition of the patient.
-
Discontinue treatments that can lower immune effector cell numbers and functions.
-
Tailor apheresis parameters to the patient condition.
-
Tailor apheresis parameters to suit the manufacturer’s needs and requirements.
-
Tightly coordinate with the Cell Processing Facility to ensure smooth shipment to the manufacturing site, in compliance with the manufacturer’s needs and requirements.
References
Cid J, Carbasse G, Alba C, Perea D, Lozano M. Leukocytapheresis in nonmobilized donors for cellular therapy protocols: evaluation of factors affecting collection efficiency of cells. J Clin Apher. 2019;34:672–9.
Constantinou VC, Bouinta A, Karponi G, et al. Poor stem cell harvest may not always be related to poor mobilization: lessons gained from a mobilization study in patients with beta-thalassemia major. Transfusion. 2017;57:1031–9.
Gopalasingam N, Thomsen AE, Folkersen L, Juhl-Olsen P, Sloth E. A successful model to learn and implement ultrasound-guided venous catheterization in apheresis. J Clin Apher. 2017;32:437–43.
Jarisch A, Rettinger E, Sorensen J, et al. Unstimulated apheresis for chimeric antigen receptor manufacturing in pediatric/adolescent acute lymphoblastic leukemia patients. J Clin Apher. 2020;35(5):398–405. https://doi.org/10.1002/jca.21812.
Ortiz-Maldonado V, Rives S, Castella M, et al. CART19-BE-01: a multicenter trial of ARI-0001 cell therapy in patients with CD19(+) relapsed/refractory malignancies. Mol Ther. 2021;29:636–44.
Sörensen J, Jarisch A, Smorta C, et al. Pediatric apheresis with a novel apheresis device with electronic interface control. Transfusion. 2013;53:761–5.
Stenzinger M, Bonig H. Risks of leukapheresis and how to manage them—a non-systematic review. Transfus Apher Sci. 2018;57:628–34.
Yakoub-Agha I, Chabannon C, Bader P, et al. Management of adults and children undergoing CAR-T cell therapy: best practice recommendations of the EBMT and JACIE. Haematologica. 2020;105:297–316.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this chapter
Cite this chapter
Bonig, H., Chabannon, C., Lozano, M. (2022). Providing the Starting Material to the Manufacturer of an Approved and Commercially Available Autologous CAR-T Cell Treatment. In: Kröger, N., Gribben, J., Chabannon, C., Yakoub-Agha, I., Einsele, H. (eds) The EBMT/EHA CAR-T Cell Handbook. Springer, Cham. https://doi.org/10.1007/978-3-030-94353-0_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-94353-0_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-94352-3
Online ISBN: 978-3-030-94353-0
eBook Packages: MedicineMedicine (R0)