Abstract
The carbon atoms deposited in tree rings originate from the CO2 in the atmosphere to which the tree’s canopy is exposed. Thus, the first control on the stable carbon-isotope composition of tree rings is by δ13C of atmospheric CO2. There has been an inter-annual trend of decreasing δ13C of atmospheric CO2 over the past two centuries as a result of combustion of fossil fuels and land-use change. Atmospheric CO2 is, for the most part, well mixed, but the sub-canopy air space can become depleted in 13C due to inputs from soil and plant respiration when turbulent exchange with the troposphere is hindered, for example by a high leaf area index at night. This is less likely to occur during daytime when turbulence is higher and photosynthesis takes place. Discrimination against 13C (∆13C) occurs upon assimilation of atmospheric CO2 by C3 photosynthesis. Trees using the C3 photosynthetic pathway comprise the overwhelming majority of all trees. The primary control on the extent of discrimination during C3 photosynthesis is the drawdown in CO2 concentration from the air outside the leaf to the site of carboxylation in the chloroplast. Part of this drawdown is captured by ci/ca, that is, the ratio of intercellular to ambient CO2 concentrations. The ci/ca represents the balance between the CO2 supply by stomata and its demand by photosynthesis. It can be related to water-use efficiency, the amount of CO2 taken up by photosynthesis for a given amount of water loss to the atmosphere, assuming a given evaporative demand. To predict time-averaged ci/ca from wood ∆13C, a simplified, linear model can be employed. In this linear model, the slope is determined by \(\overline{b }\), the effective enzymatic discrimination. The value of \(\overline{b }\) can be estimated by comparing wood ∆13C to representative measurements of ci/ca. The \(\overline{b }\) was originally estimated from observations of leaf tissue to have a value of 27‰. We compiled data for woody stem tissue here, and our analysis suggests that a lower \(\overline{b }\) should be used in the simplified model for wood (\(\overline{b }\) = 25.5‰) than for leaves (\(\overline{b }\) = 27‰). This is also consistent with widespread observations that woody tissues are enriched in 13C compared to leaves.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
1 Introduction
The abundance of the heavier stable carbon isotope, 13C, in plant material is modulated both by its environment and by plant metabolism. The ratio 13C/12C is typically expressed as δ13C, which is the relative deviation of the ratio in the sample of interest from that of an internationally accepted standard, Vienna Pee Dee Belemnite (Craig 1957; Coplen 2011). With respect to plant metabolism, stable isotopes have the unique feature of integrating plant responses over time and space. Thus, they offer a powerful tool to investigate photosynthetic processes and responses to environmental change from the leaf to the ecosystem. In this chapter, we focus on δ13C in tree rings and how it is related to assimilation of CO2 by the tree’s canopy.
Tree rings have the potential to provide a time-structured archive of information related to a tree’s growth environment and its physiological responses to changes in that environment (Fritts and Swetnam 1989; Briffa et al. 2004). Tree ring analyses have provided an indispensable tool in efforts to understand how the terrestrial biosphere is responding to the accelerating impacts of the Anthropocene (Saurer et al. 2004; Peñuelas et al. 2011; Frank et al. 2015; van der Sleen et al. 2015). One of the more tractable analyses that can be conducted on tree rings is to measure the δ13C of the wood that comprises the individual rings or sequences of rings. This represents an integration of carbon laid down over a period of time. For annual rings, this is taken as the course of a growing season (Chap. 14), or the full year in the case of tropical trees without distinct non-growing seasons (Chap. 22). In the latter case, there may also be a lack of clear annual rings. It is assumed that the majority of carbon will have originated from canopy photosynthesis in that same time period, although there can also be a contribution from stored carbon produced in previous years (Monserud and Marshall 2001; Drew et al. 2009; Belmecheri et al. 2018).
The carbon isotope ratio of the photosynthate produced by a tree’s canopy is determined primarily by two factors: the δ13C of the atmospheric CO2, which provides the substrate for photosynthesis, and the discrimination against 13C (∆13C) which takes place during conversion of gaseous CO2 into carbohydrates through the process of photosynthesis. When these two factors are sufficiently understood, one can use the measured δ13C in a tree ring to make inferences about how the process of 13C discrimination responded to climatic and other environmental changes. Also, because the ∆13C is responsive to climate, there exists the possibility to reconstruct climate from measured changes in ∆13C once a calibration relating the two has been developed (McCarroll and Loader 2004; Hartl-Meier et al. 2015). Perhaps the most widespread use of tree ring analyses of δ13C has been to reconstruct changes in intrinsic water-use efficiency, the ratio of photosynthesis to stomatal conductance to water vapour, over the course of a tree’s adult life in response to climatic change, mainly rising atmospheric CO2 concentration (Francey and Farquhar 1982; Saurer et al. 2004; Peñuelas et al. 2011; Frank et al. 2015; van der Sleen et al. 2015). This is possible because there is a reliable relationship between ∆13C, as recorded in plant biomass, and the ratio of intercellular to ambient CO2 concentrations, ci/ca, which in turn is related to the intrinsic water-use efficiency (Farquhar et al. 1982a, b; Farquhar and Richards 1984). The latter represents an index of the amount of carbon that a tree took up by photosynthesis relative to its potential for releasing water to the atmosphere through transpiration (see Chap. 17). If the atmospheric vapour pressure deficit is known, then the intrinsic water use efficiency can be converted to an actual water use efficiency in terms of molar or mass units of water exchanged for carbon. More detailed physiological conclusions can be drawn when the δ13C values are combined with δ18O from the same sample (e.g. Chap. 16).
2 The δ13C of Atmospheric CO2
Prior to the industrial revolution, the δ13C of atmospheric CO2 fluctuated between about −7.5 and −6.2‰ for the previous 160,000 years (Fig. 9.1). These data are based on analysis of air that was trapped in ice cores. The onset of industrial activity saw increasing combustion of fossil fuels, made up of plant carbon deposited in geological reservoirs millions of years ago. This fossil carbon carries a δ13C signature reflecting photosynthetic discrimination against 13C, and has δ13C roughly similar to C3 plants of today, with global emissions having a weighted mean δ13C of ~−28‰ in recent decades (Andres et al. 1996, 2000). The CO2 released from combustion of fossil fuels associated with the industrial revolution began to accumulate in the atmosphere after the mid-18th Century, and the atmospheric CO2 concentration increased from a pre-industrial value of ~280 µmol mol−1 to ~407 µmol mol−1 in 2018. Associated with this, the δ13C of atmospheric CO2 began to decline (Fig. 9.1).From about 1960 onwards, it declined at a steeper rate, reaching −8.5‰ in 2018 (Table 9.1). This was associated with an acceleration in the rate of fossil fuel emissions around this time (Andres et al. 2012). The depletion in 13C of atmospheric CO2 caused by the addition of CO2 from combustion of fossil fuels during the industrial period is referred to as the 13C Suess Effect (Keeling 1979), by analogy to the decrease in 14C of CO2 discovered by Hans Suess (1955).
The stable isotope composition (δ13C, panels a to c) and CO2 concentration ([CO2], panels d to f) of atmospheric CO2 over the last ~160,000 years. Time is in years before present (yrs BP), where zero corresponds to the year 1950 of the current era (CE). In panels c and f both yrs BP and CE scales are presented. Data are from studies reporting both δ13C and [CO2] in either ice cores or atmospheric air samples. For each data series the information presented next corresponds to the number in the legend—symbol—time span (Kyrs BP or CE)—sample origin (ice core drilling location or atmospheric station)—reference. Data series: 1—White circles—156.3 to 104.3 Kyrs BP—European Project for IceCoring in Antarctica (EPICA) Dome C (EDC) and Talos Dome—Schneider et al. (2013); 2—Black triangles—151.7 to 125.2 Kyrs BP—EDC—Lourantou et al. (2010); 3—Red circles—149.5 to 1.5 Kyrs BP – EDC, Talos Dome and EPICA Dronning Maud Land (EDML) – Eggleston et al. (2016); 4—Blue triangles—46.4 to 10.9 Kyrs BP—Taylor Dome—Bauska et al. (2016, 2018); 5—Black circles—24.4 to 0.5 Kyrs BP—EDC and Talos Dome—Schmitt et al. (2012); 6—White triangles—22.0 to 8.8 Kyrs BP—EDC—Lourantou et al. (2010); 7—Blue circles—27.1 to 1.3 Kyrs BP—Taylor Dome—Indermuhle et al. (1999); Smith et al. (1999); 8—Red triangles—1.8 to −0.04 Kyrs BP—Law Dome—Rubino et al. (2019); 9—White squares—1.2 to −0.01 Kyrs BP—WAIS Divide—Bauska et al. (2015); 10 – Green horizontal lines – air samples at Mauna Loa and South Pole – 1960 to 2018 CE—Keeling et al. (2001, 2017), Table S3 in Supplemental Materials). Series 10 shows seasonally detrended monthly records while Series 11 (Green line in the inserts of panels c and f) show the seasonal trends for δ13C (Keeling et al. 2001) and [CO2] (NOAA ESRL-Global Monitoring Division) in air samples from Mauna Loa. The Blue line (12) is the Monte Carlo spline fitted to the δ13C data in series 1, 3 and 5 by Eggleston et al. (2016). The Grey lines (13) are the splines fitted to Law Dome ice core records of δ13C and [CO2] by Rubino et al. (2019). Online resources: 1. https://doi.pangaea.de/10.1594/PANGAEA.817041 2. ftp://ftp.ncdc.noaa.gov/pub/data/paleo/icecore/antarctica/epica_domec/edc2010d13co2-t2.txt 3. https://doi.org/10.1594/PANGAEA.859209, https://doi.org/10.1594/PANGAEA.859179 4. https://www1.ncdc.noaa.gov/pub/data/paleo/icecore/antarctica/taylor/taylor2018d13co2.txt 5. https://doi.org/10.1594/PANGAEA.772713 6. ftp://ftp.ncdc.noaa.gov/pub/data/paleo/icecore/antarctica/epica_domec/edc2010d13co2.txt 7. ftp://ftp.ncdc.noaa.gov/pub/data/paleo/icecore/antarctica/taylor/taylor_co2-latequat.txt 8 and 13. https://doi.org/10.25919/5bfe29ff807fb 9. ftp://ftp.ncdc.noaa.gov/pub/data/paleo/icecore/antarctica/wais2015d13co2.txt and ftp://ftp.ncdc.noaa.gov/pub/data/paleo/icecore/antarctica/wais2015co2.txt 10. https://scrippsco2.ucsd.edu 11. https://scrippsco2.ucsd.edu/assets/data/atmospheric/stations/flask_isotopic/daily/daily_flask_c13_mlo.csv and ftp://aftp.cmdl.noaa.gov/products/trends/co2/co2_mm_mlo.txt 12. http://www1.ncdc.noaa.gov/pub/data/paleo/icecore/antarctica/eggleston2016d13co2.txt. The age chronologies are: AICC2012 (Bazin et al. 2013) for series 1 and 3, EDC3_gas_a according to the 4th scenario (Loulergue et al. 2007) for series 2 and 6, LDC 2010 (Lemieux-Dudon et al. 2010) for series 5, Baggenstos et al. (2017) for series 4, and st9810 (Steig et al. 1998) for series 7
Both the atmospheric CO2 concentration and its δ13C show an intra-annual, or seasonal, cycle associated with photosynthesis in summer months and respiration in winter months in the northern hemisphere (Fig. 9.1). This seasonal cycle is most pronounced at high latitudes in the northern hemisphere, less pronounced at tropical latitudes, and essentially absent at high latitudes of the southern hemisphere, where there is very little land mass and therefore little terrestrial productivity (Keeling et al. 2005). In addition to this latitudinal and hemispheric gradient in the seasonal cycle of CO2 concentration and isotopic composition, there is also an interhemispheric gradient in seasonally adjusted values for these variables; that is, their values when the seasonal cycle has been statistically removed. The interhemispheric gradient is such that the atmospheric CO2 concentration is higher in the northern than in the southern hemisphere, and this concentration difference has been increasing since direct atmospheric measurements commenced around 1960 (Keeling et al. 2011). It is accompanied by a difference in seasonally adjusted atmospheric δ13C of CO2 on the order of 0.1‰, in which δ13C of CO2 in the northern hemisphere is more negative than that in the southern hemisphere. These interhemispheric gradients largely reflect the greater intensity of fossil fuel emissions in the northern hemisphere compared to the southern hemisphere. However, there is also a natural gradient that can be seen if fossil fuel emissions are statistically removed; this appears to be related to oceanic transport processes (Keeling et al. 2011).
Despite these complexities, it is still true from a broader perspective that in the troposphere, the concentration of CO2 and its δ13C are generally well mixed. For example, the interhemispheric gradient in δ13C of CO2 of ~0.1‰ is of the same order of magnitude as the measurement uncertainly for δ13C in wood samples. Thus, it is probably not relevant for tree ring studies. However, at the land surface, in ecosystems where vegetation canopies are dense and fluxes of carbon into and out of vegetation and soils are large, the air CO2 concentration and δ13C can become partly uncoupled from the free troposphere above. This uncoupling should be most pronounced where carbon cycling is vigorous and leaf area indices are high, such as in tropical rainforests. An example of the air CO2 concentration and its δ13C for a tropical rainforest in French Guiana is shown in Fig. 9.2. There is a notable build-up of CO2 beneath the canopy at night, with the highest values near the forest floor fed by respiration from soils that are relatively warm and moist, and have large root biomass. The build-up of CO2 shifts the δ13C toward that of C3 plants, because the additional CO2 comes from respiration fuelled by carbohydrates captured in photosynthesis and decomposition of dead plant material. As a result, the δ13C of atmospheric CO2 in the forest understory can be as low as −12‰ (Buchmann et al. 1997; Pataki et al. 2003). However, such pronounced build-up of respired CO2 is generally limited to night time conditions when there is little atmospheric turbulence and therefore less effective mixing of air beneath the canopy with that above.
The CO2 concentration (a) and its δ13C (b) measured in a tropical rainforest in early morning, before the onset of turbulent mixing, and at midday, when the canopy air space is typically well mixed. The more negative δ13C of CO2 in the understory is also reflected in the δ13C of leaf dry matter (c), explaining part of the gradient in leaf dry matter δ13C from top of canopy to the understory. Comparison of panels b and c shows that other factors in addition to δ13C of CO2 must be driving the reduction in δ13Cplant from canopy top to understory, with reduction in light likely the most important of these. The figure is redrawn from Buchmann et al. (1997), using data they presented for the dry season
Under photosynthetic conditions, when the sun shines, the land surface heats causing turbulence, and atmospheric mixing is therefore more effective. Buchmann et al. (1997) estimated that at 2 m height in a tropical rainforest the daytime δ13C of CO2 weighted by the top of canopy photosynthetically active radiation was only about 1‰ more negative than the free tropospheric value. In contrast to this relatively modest daytime shift in δ13C of atmospheric CO2 with canopy depth, the gradient in δ13C of leaf biomass (δ13Cp) from upper canopy to understory can be up to 5‰ (Fig. 9.2c). The much steeper gradient in leaf δ13Cp compared to that in daytime δ13C of CO2 suggests that physiological effects predominate in driving the changes in leaf biomass δ13C (Le Roux et al. 2001; Buchmann et al. 2002; Duursma and Marshall 2006; Ubierna and Marshall 2011). These physiological effects are likely driven by the reduction in light with canopy depth. The amount of photosynthetically active radiation in the understory of a forest with leaf area index of 8, for example, can be as little as 1% of that above the canopy (Duursma and Mäkelä 2007). Such strong gradients in light result in lower chloroplastic CO2 concentrations at top of the canopy than at depth, and therefore lower photosynthetic 13C discrimination in sun than in shade foliage.
For trees that grow with their crowns in the forest canopy or in communities with lower leaf area indices, the δ13C of atmospheric CO2 that forms the source for photosynthesis can be assumed similar to that of the free troposphere (Buchmann et al. 2002). For trees with their crowns near the forest floor in communities with dense canopies, Buchmann et al. (2002) provide a relatively simple, empirical approach to estimating the daytime depletion of δ13C of atmospheric CO2 as a function of canopy height. This is most relevant to the lowermost 2 m of the canopy air space near the forest floor.
Typically for tree ring studies, an annually averaged value for the δ13C of CO2 in the troposphere is needed. This can be compiled for years prior to 1980 based on ice core data, and for years after 1980 from flask measurements of atmospheric CO2 that can be accessed online (https://scrippsco2.ucsd.edu/data/atmospheric_co2/sampling_stations.html), with details of the measurements described in Keeling et al. (2001). In Table 9.1, we compile annually averaged values which are updated since the values given by McCarroll and Loader (2004). The ice core δ13C record was revised recently (Rubino et al. 2019), such that the value in 1850 is best estimated as ~−6.7‰, rather than about ~−6.4‰ at the time that McCarroll and Loader (2004) compiled their table. In Table 9.1, we list the spline fitted data from Rubino et al. (2019) for the years 1850 to 1979, because the directly measured ice core data are not annually resolved. For years from 1980 to 2018, we list the average of flask measurements from Mauna Loa and South Pole (Graven et al. 2017; Keeling et al. 2017). The interhemispheric gradient in δ13C of CO2 between Mauna Loa and South Pole is small, less than 0.1‰ for most years, and the continuity with the ice core record at the changeover point from 1979 to 1980 is good (Table 9.1). An alternative to using spline fitted data is to use separate regression equations for prior to 1960 and following 1960, an approach favoured by McCarroll and Loader (2004). For comparison to their values, we provide such regression equations in Fig. 9.3. Note, however, that the annual values listed in Table 9.1 are not from these regression equations, but rather from the sources described above. During preparation of this book chapter, Belmecheri and Lavergne (2020) also published a new up-to-date compilation of atmospheric CO2 concentrations and δ13C for use in tree ring studies. Although they used different datasets for their compilation than we did for Table 9.1, the two compilations agree to within 1.6 ppm for [CO2] and 0.1‰ for δ13C for individual years, with mean differences of 0.5 ppm and 0.03‰ for [CO2] and δ13C, respectively.
Alternatively to values given in Table 9.1, annual δ13C and CO2 concentration can be estimated from fitted functions. In this case, two time periods are considered as distinct (McCarroll and Loader, 2004): from 1850 to 1960 (Period 1), and from 1961 to 2018 (Period 2). Data used for Period 1 are the original ice core records from Rubino et al. (2019) (black circles in panels a and b), which differ from the spline fitted values displayed in Table 9.1. Data used for Period 2 are a combination of ice cores (clear circles, from 1961 to 1979, Rubino et al. 2019 original values) and atmospheric CO2 records (clear triangles, from 1980 to 2018, Keeling et al. 2001). The grey shaded areas around each fitted line represent the 95% prediction limits. Functions for δ13C are: Period 1) δ13C = (0.3217 ± 0.4487) − (−0.0038 ± 0.0002)*Year, R2 = 0.83, P < 0.0001, df = 51; Period 2) δ13C = (39.7336 ± 0.7971) – (0.0239 ± 0.0004)*Year, R2 = 0.99, P < 0.0001, df = 51. Functions for [CO2] are: Period 1) [CO2] = (-299.7999 ± 9.8124) – (0.3140 ± 0.0051)*Year, R2 = 0.96, P < 0.0001, df = 158; Period 2) [CO2] = (43,217 ± 2120.2725) – (44.6794 ± 2.1335)*Year + (0.0116 ± 0.0005)*Year2, R2 = 1, P < 0.0001, df = 68
3 Photosynthetic Discrimination Against 13C
Once an estimate for the δ13C of the air that a plant was exposed to (δ13Ca) has been obtained, and the δ13C of plant tissue measured (δ13Cp), the 13C discrimination of the plant tissue (∆13Cp) can be calculated (Farquhar et al. 1989),
The delta values are typically expressed in per mil, which means that they will have been multiplied by 1000. When Eq. (9.1a) is scaled to per mil, the left side of the equation and the terms in the numerator of the right side will be multiplied by the factor 1000. Therefore, if the δ13Ca and δ13Cp are already expressed in per mil, Eq. (9.1a) will be written as,
Thus, the 13C discrimination essentially expresses the difference between the δ13C of atmospheric CO2 and that of plant tissue, with the denominator on the right side of the equation typically increasing that value by a factor of 1.02 to 1.03.
For C3 plants, which include the vast majority of all tree species, the ∆13Cp can then be related to ci/ca according to the theoretical model of Farquhar et al. (1982b). In its simplest form, this model can be expressed as,
Here, as is the 13C/12C fractionation that takes place during diffusion of CO2 through static air, such as in the stomatal pore. The as has a theoretical value of 4.4‰. The term \(\overline{b }\) represents discrimination against 13CO2 by carboxylating enzymes, mainly Rubisco. In this simplified form of the model, the term \(\overline{b }\) also encompasses some other known sources of variation in δ13Cp, such as the diffusion resistance from the intercellular air spaces to the sites of carboxylation in the chloroplasts (Ubierna and Farquhar 2014). The value that is commonly assumed for \(\overline{b }\) is 27‰. This estimate was first based on comparison of instantaneous measurements of ci/ca from leaf gas exchange with ∆13Cp measured in leaf tissue (Farquhar et al. 1982a). Subsequent measurements of instantaneous gas exchange and leaf tissue δ13Cp have also generally supported a value for \(\overline{b }\) of 27‰ with respect to leaf dry matter (Farquhar et al. 1989; Cernusak et al. 2013; Cernusak 2020).
The objective for tree ring studies is often to retrieve an estimate of ci/ca from measurements of ∆13Cp. For this, Eq. (9.2) can be rearranged,
Finally, the intrinsic water use efficiency, A/gs, where A is net photosynthesis and gs is stomatal conductance to water vapour, can be calculated as,
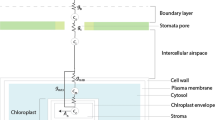
The factor of 1.6 in the denominator represents the ratio between the stomatal conductance to water vapour and that to CO2. Note that Eq. (9.4) ignores both boundary layer resistance and ternary effects, and is thus a reasonable simplification of a more precise treatment (von Caemmerer and Farquhar 1981).
As noted above, Eq. (9.2) represents a simplified version of a more elaborate model for ∆13C during C3 photosynthesis (Farquhar et al. 1982b; Farquhar and Cernusak 2012; Busch et al. 2020),
Here, ab is the 13C/12C fractionation during diffusion of CO2 through the boundary layer (2.9‰), and am is that for dissolution and diffusion from the intercellular air spaces to the sites of carboxylation in the chloroplasts (1.8‰). The term b represents fractionation by Rubisco (~29‰), e is fractionation during day respiration, and f is fractionation during photorespiration. The fractionation factor assigned for e should take into account both respiratory fractionation, estimated at between 0 and 5‰ (Tcherkez et al. 2010, 2011) and any offset between δ13C of respiratory substrate and the substrate currently being produced by photosynthesis (Wingate et al. 2007; Busch et al. 2020). Estimates of fractionation for photorespiration, f, range from 8 to 16‰ (Gillon and Griffiths 1997; Lanigan et al. 2008; Evans and von Caemmerer 2013). The Rd is the rate of day respiration, and Γ* is the CO2 compensation point in the absence of day respiration. The terms cs and cc represent the CO2 concentrations at the leaf surface and at the sites of carboxylation, respectively. The term t is a ternary correction factor, defined approximately as t≈E/2gc, where E is transpiration rate and gc is stomatal conductance to CO2 (Farquhar and Cernusak 2012). For further description of the terms in Eq. (9.5), the reader is referred to Ubierna et al. (2018).
The reader will notice that the value taken for b, discrimination by Rubisco, in the more complete model, Eq. (9.5), is typically 29‰, whereas the value taken for \(\overline{b }\) in the simple model, Eq. (9.2), for leaf tissue is smaller at 27‰. Below we discuss an even smaller value that should be used in the simple model for woody tissue. The difference arises because \(\overline{b }\) becomes something of a catch all for several less important terms that are in Eq. (9.5), but neglected from Eq. (9.2). A hierarchical approach to removing these terms was provided by Ubierna and Farquhar (2014), from which the impacts can be explored. Interestingly, such a bottom up approach suggested that the expected value for \(\overline{b }\) is actually less than 27‰, and the estimate of 27‰ likely includes developmental effects in leaf tissue δ13C (Cernusak et al. 2009a; Vogado et al. 2020) and possibly other post-photoysnthetic processes (Ubierna and Farquhar 2014). The largest impact on the difference between b and \(\overline{b }\) comes from the drawdown in CO2 concentration between the intercellular air spaces and the sites of carboxylation in the chloroplasts. This is the effect of a finite mesophyll conductance to diffusion of CO2. An additional term that could be of interest in tree ring studies is the photorespiratory fractionation, f(Γ*/ca). Over large changes in atmospheric CO2 concentration, there is a discernible impact on ∆13C from changes in Γ*/ca, independent of impacts caused by changes in ci/ca (Schubert and Jahren 2012, 2018; Porter et al. 2019).
Equation (9.5) is thought to include all processes that impact upon discrimination against 13C in photosynthetic CO2 uptake by C3 photosynthesis. Even so, there are further modifications that could take place depending on the arrangement of mitochondria with respect to chloroplasts (Tholen et al. 2012; Ubierna et al. 2019), and the model does not address allocation of the products of photosynthesis, for example to starch versus export from the chloroplast (Tcherkez et al. 2004). Post photosynthetic fractionation is discussed further in Chap. 13. Equation (9.5) requires several additional parameters compared to Eq. (9.2) which are difficult to estimate retrospectively, as would be required for application to tree rings. Therefore, Eq. (9.2) represents a good compromise between mechanistic representation and tractability with respect to parameterisation. For situations where other parameters can also be measured or where accompanying datasets are available, application of the more complete model to tree rings could yield more subtle, but important, insights about past climate, leaf gas exchange, and carbon allocation dynamics within trees (Ogee et al. 2009). However, there remain challenges in understanding time integration and post-photosynthetic fractionation with respect to the δ13C signal in tree rings, and these create additional complexities for knowing how and when Eq. (9.5) can be applied effectively.
4 Relating the δ13C of Wood to Leaf Gas Exchange
As mentioned above, the value originally estimated for \(\overline{b }\) in Eq. (9.2) of 27‰ was based on comparison of instantaneous measurements of ci/ca by leaf gas exchange with δ13Cp measured in leaf tissue (Farquhar et al. 1982a). At the same time, it has long been recognized that δ13Cp of wood is typically less negative than that of the leaf tissue which supplies it with photosynthate (Craig 1953; Leavitt and Long 1982; Francey et al. 1985; Leavitt and Long 1986; Badeck et al. 2005; Cernusak et al. 2009a). Differences are typically such that δ13Cp of leaves is more negative than that of stem or branch wood by about 1 to 3‰. A number of hypotheses have been suggested to account for this difference, none of which are mutually exclusive (Cernusak et al. 2009a). Part of the explanation involves a depletion in leaf δ13Cp that takes place during leaf expansion, such that when leaves mature, they export carbon less negative in δ13C compared to their structural carbon (Evans 1983; Francey et al. 1985; Cernusak et al. 2009a; Vogado et al. 2020). There are likely additional processes during the transfer of photosynthate from chloroplasts to newly differentiating woody tissue that could contribute (Offermann et al. 2011; Gessler et al. 2014; Bögelein et al. 2019), with further discussion in Chap. 13.
Although it is difficult to define the exact processes involved, it would nevertheless seem reasonable that the value of \(\overline{b }\) assigned for woody tissue in Eq. (9.2) could be less than that which would be assigned for leaf tissue. In Fig. 9.4, we compile data for 33 woody plant species in which ci/ca was measured by leaf gas exchange and the δ13Cp was measured in both leaves and woody stem tissue. We present the data for individual plants, rather than as species averages, because in many cases treatments were imposed within a species that resulted in a within species range of ci/ca and δ13Cp. The full dataset is available in a Dryad Digital Repository (https://doi.org/10.5061/dryad.jm63xsjct). The fitted value for \(\overline{b }\) for the leaf tissue dataset, with as fixed at 4.4‰, was 26.9 ± 0.1‰ (coefficient ± SE; R2 = 0.52, n = 451). This estimate is consistent with previous estimates of \(\overline{b }\) = 27‰ for leaf tissue. On the other hand, the estimate of \(\overline{b }\) for stem tissue was 25.5 ± 0.1‰ (R2 = 0.63, n = 449), consistent with the idea that wood is less negative in δ13Cp than leaves of the same plant. Therefore, we recommend that if one aims to reconstruct ci/ca from leaf δ13Cp, a value for \(\overline{b }\) of 27‰ should be used in Eq. (9.3), as is typically done. On the other hand, if one aims to reconstruct ci/ca from woody tissue, as is the case for tree rings, one should use a value for \(\overline{b }\) of 25.5‰. The difference in ci/ca estimates will vary depending on the measured δ13Cp, but will be on the order of 0.05 in ci/ca. Thus, the difference is not large, but at the same time it will better align estimates of ci/ca from leaf and woody tissue with each other. Also, when carried through to the calculation of intrinsic water-use efficiency, the proportional change is larger, about 17% decrease in estimated A/gs when ci/ca shifts from 0.7 to 0.75, for example. Note that if some parameters from Eq. (9.5) are brought in to Eq. (9.2), but Eq. (9.5) is not adopted in its entirety, then \(\overline{b }\) will need to be adjusted. This would create a challenge in merging the empirically determined value of \(\overline{b }\) from organic material analyses with parameters drawn from other contexts, and should be approached with caution (Vogado et al. 2020).
Carbon isotope discrimination (∆13C) measured in leaf biomass a and in stem biomass b plotted against the ratio of intercellular to ambient CO2 concentrations (ci/ca) measured by instantaneous gas exchange in 33 woody plant species. Further details of the measurements can be found in the original publications (Cernusak et al. 2007, 2008, 2009b, 2011; Garrish et al. 2010; Cernusak 2020). The data are available in a Dryad Digital Repository (https://doi.org/10.5061/dryad.jm63xsjct). Dashed lines show regression lines fitted with the intercepts fixed at 4.4‰. The inset equations show the regression slopes applied to the simplified model of Farquhar et al. (1982b)
In order to test for species specificity in the value of \(\overline{b }\) for woody tissues, we constructed a mixed effects model for wood ∆13C as a function of ci/ca with a fixed intercept of 4.4‰; species × ci/ca was additionally taken as a random effect. The model thus allowed us to test for different slopes among species (indicating different \(\overline{b }\) among species). The random effect was shown to be significant, with 14 out of 33 species having a slope significantly different than the overall mean slope, suggesting that \(\overline{b }\) can indeed vary among species. Thus, the value for \(\overline{b }\) for woody stems of 25.5‰ is a cross-species average value. However, the situation is entirely analogous to taking \(\overline{b }\) = 27‰ based on the average estimate for leaf tissue, as this is also a cross-species average and varies by species, as shown in Fig. 9.4a. Thus, we are suggesting moving the average from 27 to 25.5‰ for woody tissues, to correct for the overall average difference between leaf and wood ∆13C, but this does not address the variance around this average due to species or environment. It is an incremental step, but nonetheless seems an easy and appropriate one to take.
Often for tree-ring studies, investigators prefer to extract cellulose prior to isotopic analysis, which has both advantages and disadvantages (McCarroll and Loader 2004). The δ13C of cellulose is typically less negative than that of whole wood by about 1‰ (Leavitt and Long 1982; Loader et al. 2003; Harlow et al. 2006). We recommend that if cellulose is analysed for δ13C, that an approximation of the offset between this and whole wood δ13C be subtracted from the cellulose δ13C before application of Eq. (9.3) with \(\overline{b }\) = 25.5‰, since this value of \(\overline{b }\) was determined for whole wood.
5 Conclusions
In this chapter, we have reviewed the primary influences on the δ13C of carbon captured by photosynthesis in C3 plants. The first control is the δ13C of atmospheric CO2 that the plant canopy was exposed to. The δ13C of atmospheric CO2 has decreased since the onset of the industrial revolution due to release of carbon from geological reservoirs. The δ13C of atmospheric CO2 inferred from ice cores was recently revised down slightly, so that the value in 1850 is now estimated at ~−6.7‰. The mean value for 2018 was −8.5‰. The δ13C of CO2 beneath forest canopies can be more negative than that in the free troposphere above due to the influence of soil and plant respired CO2. This decrease in δ13C occurs mainly at night, and is most pronounced in forests with high leaf area indices. During daytime when photosynthesis takes place, turbulent exchange of canopy air with that in the troposphere diminishes this effect. Thus, forest respiration can reduce the δ13C of the CO2 that forms the source for photosynthesis of understory plants and small statured trees, but the decrease is probably not more than about 1‰ under photosynthetic conditions. A simplified model provides a means of calculating ci/ca, the ratio of intercellular to ambient CO2 concentrations, from measurements of δ13C of plant tissues and an inference of the δ13C of atmospheric CO2 at the time when the plant tissue was formed. The long standing observation that woody tissues are less negative in δ13C than the leaves that supply them with photosynthate suggests that the coefficient relating ci/ca to δ13C should differ for the two tissue types (see also Chap. 13). We used a dataset comprising measurements in 33 woody plant species to estimate that the coefficient \(\overline{b }\) should be taken as 27‰ in the simplified model for leaf tissue, and as 25.5‰ for woody tissue, including tree rings. While the difference in estimated ci/ca using the two coefficients is not large, the revision will aid in aligning ci/ca inferred from tree rings with that which would be measured by instantaneous gas exchange.
References
Andres RJ, Marland G, Boden T, Bischof S (2000) Carbon dioxide emissions from fossil fuel consumption and cement manufacture, 1751–1991, and an estimate of their isotopic composition and latitudinal distribution. In: Schimel DS, Wigley TML (eds) The carbon cycle. Cambridge University Press, Cambridge, pp 53–62
Andres RJ, Boden TA, Breon FM, Ciais P, Davis S, Erickson D, Gregg JS, Jacobson A, Marland G, Miller J, Oda T, Olivier JGJ, Raupach MR, Rayner P, Treanton K (2012) A synthesis of carbon dioxide emissions from fossil-fuel combustion. Biogeosciences 9:1845–1871
Andres RJ, Boden TA, Marland G. 1996. Annual fossil-fuel CO2 emissions: global stable carbon isotopic signature. Environmental System Science Data Infrastructure for a Virtual Ecosystem, Carbon Dioxide Information Analysis Center (CDIAC), Oak Ridge National Laboratory (ORNL), Oak Ridge, TN (United States)
Badeck FW, Tcherkez G, Nogues S, Piel C, Ghashghaie J (2005) Post-photosynthetic fractionation of stable carbon isotopes between plant organs-a widespread phenomenon. Rapid Commun Mass Spectrom 19:1381–1391
Baggenstos D, Bauska TK, Severinghaus JP, Lee JE, Schaefer H, Buizert C, Brook EJ, Shackleton S, Petrenko VV (2017) Atmospheric gas records from Taylor Glacier, Antarctica, reveal ancient ice with ages spanning the entire last glacial cycle. Clim Past 13:943–958
Bauska TK, Joos F, Mix AC, Roth R, Ahn J, Brook EJ (2015) Links between atmospheric carbon dioxide, the land carbon reservoir and climate over the past millennium. Nat Geosci 8:383–387
Bauska TK, Baggenstos D, Brook EJ, Mix AC, Marcott SA, Petrenko VV, Schaefer H, Severinghaus JP, Lee JE (2016) Carbon isotopes characterize rapid changes in atmospheric carbon dioxide during the last deglaciation. Proc Natl Acad Sci 113:3465–3470
Bauska TK, Brook EJ, Marcott SA, Baggenstos D, Shackleton S, Severinghaus JP, Petrenko VV (2018) Controls on millennial-scale atmospheric CO2 variability during the last glacial period Geophys Res Lett 45:7731–7740
Bazin L, Landais A, Lemieux-Dudon B, Kele HTM, Veres D, Parrenin F, Martinerie P, Ritz C, Capron E, Lipenkov V, Loutre M-F, Raynaud D, Vinther B, Svensson A, Rasmussen SO, Severi M, Blunier T, Leuenberger M, Fischer H, Masson-Delmotte V, Chappellaz J, Wolff E (2013) An optimized multi-proxy, multi-site Antarctic ice and gas orbital chronology (AICC2012): 120–800 ka. Clim Past 9:1715–1731
Belmecheri S, Wright WE, Szejner P, Morino KA, Monson RK (2018) Carbon and oxygen isotope fractionations in tree rings reveal interactions between cambial phenology and seasonal climate. Plant Cell Environ 41:2758–2772
Belmecheri S, Lavergne A (2020) Compiled records of atmospheric CO2 concentrations and stable carbon isotopes to reconstruct climate and derive plant ecophysiological indices from tree rings. Dendrochronologia 63: 125748
Bögelein R, Lehmann MM, Thomas FM (2019) Differences in carbon isotope leaf-to-phloem fractionation and mixing patterns along a vertical gradient in mature European beech and Douglas fir. New Phytol 222:1803–1815
Briffa KR, Osborn TJ, Schweingruber FH (2004) Large-scale temperature inferences from tree rings: a review. Global Planet Change 40:11–26
Buchmann N, Guehl JM, Barigah TS, Ehleringer JR (1997) Interseasonal comparison of CO2 concentrations, isotopic composition, and carbon dynamics in an Amazonian rainforest (French Guiana). Oecologia 110:120–131
Buchmann N, Brooks JR, Ehleringer JR (2002) Predicting daytime carbon isotope ratios of atmospheric CO2 within forest canopies. Funct Ecol 16:49–57
Busch FA, Holloway-Phillips M, Stuart-Williams H, Farquhar GD (2020) Revisiting carbon isotope discrimination in C3 plants shows respiration rules when photosynthesis is low. Nat Plants 6:245–258
Cernusak LA (2020) Gas exchange and water-use efficiency in plant canopies. Plant Biol 22(Suppl. 1):52–67
Cernusak LA, Winter K, Aranda J, Turner BL, Marshall JD (2007) Transpiration efficiency of a tropical pioneer tree (Ficus insipida) in relation to soil fertility. J Exp Bot 58:3549–3566
Cernusak LA, Winter K, Aranda J, Turner BL (2008) Conifers, angiosperm trees, and lianas: growth, whole-plant water and nitrogen use efficiency, and stable isotope composition (δ13C and δ18O) of seedlings grown in a tropical environment. Plant Physiol 148:642–659
Cernusak LA, Winter K, Turner BL (2009a) Physiological and isotopic (δ13C and δ18O) responses of three tropical tree species to water and nutrient availability. Plant Cell Environ 32:1441–1455
Cernusak LA, Tcherkez G, Keitel C, Cornwell WK, Santiago LS, Knohl A, Barbour MM, Williams DG, Reich PB, Ellsworth DS, Dawson TE, Griffiths H, Farquhar GD, Wright IJ (2009b) Why are non-photosynthetic tissues generally 13C enriched compared to leaves in C3 plants? Review and synthesis of current hypotheses. Funct Plant Biol 36:199–213
Cernusak LA, Winter K, Martinez C, Correa E, Aranda J, Garcia M, Jaramillo C, Turner BL (2011) Responses of legume versus nonlegume tropical tree seedlings to elevated CO2 concentration. Plant Physiol 157:372–385
Cernusak LA, Ubierna N, Winter K, Holtum JAM, Marshall JD, Farquhar GD (2013) Environmental and physiological determinants of carbon isotope discrimination in terrestrial plants. New Phytol 200:950–965
Coplen TB (2011) Guidelines and recommended terms for expression of stable-isotope-ratio and gas-ratio measurement results. Rapid Commun Mass Spectrom 25:2538–2560
Craig H (1953) The geochemistry of the stable carbon isotopes. Geochim Cosmochim Acta 3:53–92
Craig H (1957) Isotopic standards for carbon and oxygen and correction factors for mass-spectrometric analysis of carbon dioxide. Geochim Cosmochim Acta 12:133–149
Drew DM, Schulze ED, Downes GM (2009) Temporal variation in δ13C, wood density and microfibril angle in variously irrigated Eucalyptus nitens. Funct Plant Biol 36:1–10
Duursma RA, Mäkelä A (2007) Summary models for light interception and light-use efficiency of non-homogeneous canopies. Tree Physiol 27:859–870
Duursma RA, Marshall JD (2006) Vertical canopy gradients in δ13C correspond with leaf nitrogen content in a mixed-species conifer forest. Trees Struct Funct 20:496–506
Eggleston S, Schmitt J, Bereiter B, Schneider R, Fischer H (2016) Evolution of the stable carbon isotope composition of atmospheric CO2 over the last glacial cycle. Paleoceanography 31:434–452
Evans JR, von Caemmerer S (2013) Temperature response of carbon isotope discrimination and mesophyll conductance in tobacco. Plant Cell Environ 36:745–756
Evans JR (1983) Photosynthesis and nitrogen partitioning in leaves of Triticum aestivum L. and related species. PhD thesis, Australian National University, Canberra
Farquhar GD, Cernusak LA (2012) Ternary effects on the gas exchange of isotopologues of carbon dioxide. Plant Cell Environ 35:1221–1231
Farquhar GD, Richards RA (1984) Isotopic composition of plant carbon correlates with water-use efficiency in wheat genotypes. Aust J Plant Physiol 11:539–552
Farquhar GD, O’Leary MH, Berry JA (1982a) On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Aust J Plant Physiol 9:121–137
Farquhar GD, Ball MC, von Caemmerer S, Roksandic Z (1982b) Effect of salinity and humidity on δ13C value of halophytes- evidence for diffusional isotope fractionation determined by the ratio of intercellular/atmospheric partial pressure of CO2 under different environmental conditions. Oecologia 52:121–124
Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40:503–537
Francey RJ, Farquhar GD (1982) An explanation of 13C/12C variations in tree rings. Nature 297:28–31
Francey RJ, Gifford RM, Sharkey TD, Weir B (1985) Physiological influences on carbon isotope discrimination in huon pine (Lagarostrobos franklinii). Oecologia 66:211–218
Frank DC, Poulter B, Saurer M, Esper J, Huntingford C, Helle G, Treydte K, Zimmermann NE, Schleser GH, Ahlstrom A, Ciais P, Friedlingstein P, Levis S, Lomas M, Sitch S, Viovy N, Andreu-Hayles L, Bednarz Z, Berninger F, Boettger T, D’Alessandro CM, Daux V, Filot M, Grabner M, Gutierrez E, Haupt M, Hilasvuori E, Jungner H, Kalela-Brundin M, Krapiec M, Leuenberger M, Loader NJ, Marah H, Masson-Delmotte V, Pazdur A, Pawelczyk S, Pierre M, Planells O, Pukiene R, Reynolds-Henne CE, Rinne KT, Saracino A, Sonninen E, Stievenard M, Switsur VR, Szczepanek M, Szychowska-Krapiec E, Todaro L, Waterhouse JS, Weigl M (2015) Water-use efficiency and transpiration across European forests during the Anthropocene. Nat Clim Change 5:579–583
Fritts HC, Swetnam TW (1989) Dendroecology: a tool for evaluating variations in past and present forest environments. In: Begon M, Fitter AH, Ford ED, MacFadyen A (eds) Academic Press, Advances in Ecological Research, pp 111–188
Garrish V, Cernusak LA, Winter K, Turner BL (2010) Nitrogen to phosphorus ratio of plant biomass versus soil solution in a tropical pioneer tree, Ficus insipida. J Exp Bot 61:3735–3748
Gessler A, Ferrio JP, Hommel R, Treydte K, Werner RA, Monson RK (2014) Stable isotopes in tree rings: towards a mechanistic understanding of isotope fractionation and mixing processes from the leaves to the wood. Tree Physiol 34:796–818
Gillon JS, Griffiths H (1997) The influence of (photo)respiration on carbon isotope discrimination in plants. Plant Cell Environ 20:1217–1230
Graven H, Allison CE, Etheridge DM, Hammer S, Keeling RF, Levin I, Meijer HAJ, Rubino M, Tans PP, Trudinger CM, Vaughn BH, White JWC (2017) Compiled records of carbon isotopes in atmospheric CO2 for historical simulations in CMIP6. Geosci Model Dev 10:4405–4417
Harlow BA, Marshall JD, Robinson AP (2006) A multi-species comparison of δ13C from whole wood, extractive-free wood and holocellulose. Tree Physiol 26:767–774
Hartl-Meier C, Zang C, Buntgen U, Esper J, Rothe A, Gottlein A, Dirnback T, Treydte K (2015) Uniform climate sensitivity in tree-ring stable isotopes across species and sites in a mid-latitude temperate forest. Tree Physiol 35:4–15
Indermuhle A, Stocker TF, Joos F, Fischer H, Smith HJ, Wahlen M, Deck B, Mastroianni D, Tschumi J, Blunier T, Meyer R, Stauffer B (1999) Holocene carbon-cycle dynamics based on CO2 trapped in ice at Taylor Dome, Antarctica. Nature 398:121–126
Keeling CD (1979) The Suess effect: 13Carbon-14Carbon interrelations. Environ Int 2:229–300
Keeling CD, Piper SC, Whorf TP, Keeling RF (2011) Evolution of natural and anthropogenic fluxes of atmospheric CO2 from 1957 to 2003. Tellus B 63:1–22
Keeling RF, Graven HD, Welp LR, Resplandy L, Bi J, Piper SC, Sun Y, Bollenbacher A, Meijer HAJ (2017) Atmospheric evidence for a global secular increase in carbon isotopic discrimination of land photosynthesis. Proc Natl Acad Sci USA 114:10361–10366
Keeling CD, Piper SC, Bacastow RB, Wahlen M, Whorf TP, Heimann M, Meijer HA (2001) Exchanges of atmospheric CO2 and 13CO2 with the terrestrial biosphere and oceans from 1978 to 2000. I. Global aspects, SIO Reference Series, No. 01-06: Scripps Institution of Oceanography, San Diego
Keeling CD, Piper SC, Bacastow RB, Wahlen M, Whorf TP, Heimann M, Meijer HA (2005) Atmospheric CO2 and 13CO2 exchange with the terrestrial biosphere and oceans from 1978 to 2000: observations and carbon cycle implications. In: Baldwin IT, Caldwell MM, Heldmaier G, Jackson RB, Lange OL, Mooney HA, Schulze ED, Sommer U, Ehleringer JR, Denise Dearing M, Cerling TE (eds) A history of atmospheric CO2 and its effects on plants, animals, and ecosystems. Springer, New York, pp 83–113
Lanigan GJ, Betson N, Griffiths H, Seibt U (2008) Carbon isotope fractionation during photorespiration and carboxylation in Senecio. Plant Physiol 148:2013–2020
Le Roux X, Bariac T, Sinoquet H, Genty B, Piel C, Mariotti A, Girardin C, Richard P (2001) Spatial distribution of leaf water-use efficiency and carbon isotope discrimination within an isolated tree crown. Plant Cell Environ 24:1021–1032
Leavitt SW, Long A (1982) Evidence for 13C/12C fractionation between tree leaves and wood. Nature 298:742–744
Leavitt SW, Long A (1986) Stable-carbon isotope variability in tree foliage and wood. Ecology 67:1002–1010
Lemieux-Dudon B, Blayo E, Petit J-R, Waelbroeck C, Svensson A, Ritz C, Barnola J-M, Narcisi BM, Parrenin F (2010) Consistent dating for Antarctic and Greenland ice cores. Quatern Sci Rev 29:8–20
Loader NJ, Robertson I, McCarroll D (2003) Comparison of stable carbon isotope ratios in the whole wood, cellulose and lignin of oak tree-rings. Palaeogeogr Palaeoclimatol Palaeoecol 196:395–407
Loulergue L, Parrenin F, Blunier T, Barnola J-M, Spahni R, Schilt A, Raisbeck G, Chappellaz J (2007) New constraints on the gas age-ice age difference along the EPICA ice cores, 0–50 kyr. Clim Past 3:527–540
Lourantou A, Chappellaz J, Barnola JM, Masson-Delmotte V, Raynaud D (2010) Changes in atmospheric CO2 and its carbon isotopic ratio during the penultimate deglaciation. Quatern Sci Rev 29:1983–1992
McCarroll D, Loader NJ (2004) Stable isotopes in tree rings. Quatern Sci Rev 23:771–801
Monserud RA, Marshall JD (2001) Time-series analysis of δ13C from tree rings 1: time trends and autocorrelations. Tree Physiol 21:1087–1102
Offermann C, Ferrio JP, Holst J, Grote R, Siegwolf R, Kayler Z, Gessler A (2011) The long way down-are carbon and oxygen isotope signals in the tree ring uncoupled from canopy physiological processes? Tree Physiol 31:1088–1102
Ogee J, Barbour MM, Wingate L, Bert D, Bosc A, Stievenard M, Lambrot C, Pierre M, Bariac T, Loustau D, Dewar RC (2009) A single-substrate model to interpret intra-annual stable isotope signals in tree-ring cellulose. Plant Cell Environ 32:1071–1090
Pataki DE, Ehleringer JR, Flanagan LB, Yakir D, Bowling DR, Still CJ, Buchmann N, Kaplan JO, Berry JA (2003) The application and interpretation of Keeling plots in terrestrial carbon cycle research. Global Biogeochem Cycles 17:15
Peñuelas J, Canadell JG, Ogaya R (2011) Increased water-use efficiency during the 20th century did not translate into enhanced tree growth. Glob Ecol Biogeogr 20:597–608
Porter AS, Gerald CEF, Yiotis C, Montanez IP, McElwain JC (2019) Testing the accuracy of new paleoatmospheric CO2 proxies based on plant stable carbon isotopic composition and stomatal traits in a range of simulated paleoatmospheric O2:CO2 ratios. Geochim Cosmochim Acta 259:69–90
Rubino M, Etheridge DM, Thornton DP, Howden R, Allison CE, Francey RJ, Langenfelds RL, Steele LP, Trudinger CM, Spencer DA, Curran MAJ, van Ommen TD, Smith AM (2019) Revised records of atmospheric trace gases CO2, CH4, N2O, and δ13C-CO2 over the last 2000 years from Law Dome, Antarctica. Earth Syst Sci Data 11:473–492
Saurer M, Siegwolf RTW, Schweingruber FH (2004) Carbon isotope discrimination indicates improving water-use efficiency of trees in northern Eurasia over the last 100 years. Glob Change Biol 10:2109–2120
Schmitt J, Schneider R, Elsig J, Leuenberger D, Lourantou A, Chappellaz J, Köhler P, Joos F, Stocker TF, Leuenberger M, Fischer H (2012) Carbon isotope constraints on the deglacial CO2 rise from ice cores. Science 336:711–714
Schneider R, Schmitt J, Köhler P, Joos F, Fischer H (2013) A reconstruction of atmospheric carbon dioxide and its stable carbon isotopic composition from the penultimate glacial maximum to the last glacial inception. Clim Past 9:2507–2523
Schubert BA, Jahren AH (2012) The effect of atmospheric CO2 concentration on carbon isotope fractionation in C3 land plants. Geochim Cosmochim Acta 96:29–43
Schubert BA, Jahren AH (2018) Incorporating the effects of photorespiration into terrestrial paleoclimate reconstruction. Earth Sci Rev 177:637–642
Smith HJ, Fischer H, Mastroianni D, Deck B, Wahlen M (1999) Dual modes of the carbon cycle since the Last Glacial Maximum. Nature 400:248–250
Steig EJ, Brook EJ, White JWC, Sucher CM, Bender ML, Lehman SJ, Morse DL, Waddington ED, Clow GD (1998) Synchronous climate changes in Antarctica and the North Atlantic. Science 282:92–95
Suess HE (1955) Radiocarbon concentration in modern wood. Science 122:415
Tcherkez G, Farquhar G, Badeck F, Ghashghaie J (2004) Theoretical considerations about carbon isotope distribution in glucose of C3 plants. Funct Plant Biol 31:857–877
Tcherkez G, Schaufele R, Nogues S, Piel C, Boom A, Lanigan G, Barbaroux C, Mata C, Elhani S, Hemming D, Maguas C, Yakir D, Badeck FW, Griffiths H, Schnyder H, Ghashghaie J (2010) On the 13C/12C isotopic signal of day and night respiration at the mesocosm level. Plant Cell Environ 33:900–913
Tcherkez G, Mauve C, Lamothe M, Le Bras C, Grapin A (2011) The 13C/12C isotopic signal of day-respired CO2 in variegated leaves of Pelargonium x hortorum. Plant Cell Environ 34:270–283
Tholen D, Ethier G, Genty B, Pepin S, Zhu XG (2012) Variable mesophyll conductance revisited: theoretical background and experimental implications. Plant Cell Environ 35:2087–2103
Ubierna N, Farquhar GD (2014) Advances in measurements and models of photosynthetic carbon isotope discrimination in C3 plants. Plant Cell Environ 37:1494–1498
Ubierna N, Marshall JD (2011) Vertical and seasonal variation in the δ13C of leaf-respired CO2 in a mixed conifer forest. Tree Physiol 31:414–427
Ubierna N, Cernusak LA, Holloway-Phillips M, Busch FA, Cousins AB, Farquhar GD (2019) Critical review: incorporating the arrangement of mitochondria and chloroplasts into models of photosynthesis and carbon isotope discrimination. Photosynth Res 141:5–31
Ubierna N, Holloway-Phillips MM, Farquhar GD (2018) Using stable carbon isotopes to study C3 and C4 photosynthesis: models and calculations. In: Covshoff S (ed) Photosynthesis: methods and protocols. Springer, New York, pp 155–196
van der Sleen P, Groenendijk P, Vlam M, Anten NPR, Boom A, Bongers F, Pons TL, Terburg G, Zuidema PA (2015) No growth stimulation of tropical trees by 150 years of CO2 fertilization but water-use efciency increased. Nat Geosci 8:24–28
Vogado NO, Winter K, Ubierna N, Farquhar GD, Cernusak LA (2020) Directional change in leaf dry matter δ13C during leaf development is widespread in C3 plants. Annals Botany 126:981–990
von Caemmerer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153:376–387
Wingate L, Seibt U, Moncrieff JB, Jarvis PG, Lloyd J (2007) Variations in 13C discrimination during CO2 exchange by Picea sitchensis branches in the field. Plant Cell Environ 30:600–616
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 © The Author(s)
About this chapter
Cite this chapter
Cernusak, L.A., Ubierna, N. (2022). Carbon Isotope Effects in Relation to CO2 Assimilation by Tree Canopies. In: Siegwolf, R.T.W., Brooks, J.R., Roden, J., Saurer, M. (eds) Stable Isotopes in Tree Rings. Tree Physiology, vol 8. Springer, Cham. https://doi.org/10.1007/978-3-030-92698-4_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-92698-4_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-92697-7
Online ISBN: 978-3-030-92698-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)