Abstract
The clumped isotope composition (Δ47, the anomaly of the mass 47 isotopologue relative to the abundance expected from a random isotope distribution) of CO2 has been suggested as an additional tracer for gross CO2 fluxes. However, the effect of photosynthetic gas exchange on Δ47 has not been directly determined and two indirect/conceptual studies reported contradicting results. In this study, we quantify the effect of photosynthetic gas exchange on Δ47 of CO2 using leaf cuvette experiments with one C4 and two C3 plants. The experimental results are supported by calculations with a leaf cuvette model. Our results demonstrate the important roles of the Δ47 value of CO2 entering the leaf, kinetic fractionation as CO2 diffuses into, and out of the leaf and CO2–H2O isotope exchange with leaf water. We experimentally confirm the previously suggested dependence of Δ47 of CO2 in the air surrounding a leaf on the stomatal conductance and back-diffusion flux. Gas exchange can enrich or deplete the Δ47 of CO2 depending on the Δ47 of CO2 entering the leaf and the fraction of CO2 exchanged with leaf water and diffused back to the atmosphere, but under typical ambient conditions, it will lead to a decrease in Δ47.
Similar content being viewed by others
Introduction
The carbon dioxide (CO2) concentration in the atmosphere is controlled by various large exchange fluxes with the bio-, hydro- and geosphere, and by anthropogenic emissions. Important tools for quantifying the different terms of the budget are measurement of the mole fraction and the isotopic composition of CO21. The stable carbon isotope composition allows to distinguish between the CO2 uptake by the ocean and by plants2, and the stable oxygen isotope composition allows to determine the magnitude of the large gross carbon fluxes between the atmosphere and biosphere3,4,5,6. Recent advancements in precise measurement techniques of 17O-excess7 enable the 17O-excess, Δ17O, of tropospheric CO2 to be used as tracer of terrestrial gross primary production8,9,10,11,12,13 or stratospheric influx14,15,16. In addition, the analysis of radiocarbon has been used to quantify the amount of anthropogenic CO2 emissions17,18. Despite these four independent isotopic tracers, the CO2 budget still remains uncertain. It has been suggested that the abundance of the isotopologue 18O13C16O in the atmosphere might be a promising new tracer to complement the existing isotope tracers19,20,21,22,23.
The abundance of the double substituted (i.e., contain two rare isotopes) isotopologue 13C18O16O, compared to its abundance at stochastic isotope distribution for a given bulk composition, is also referred to as the clumped isotopic composition of CO2 (Δ47, see Sect. “Theory” for definition). One of the main reasons for its applicability as a tracer to constrain CO2 fluxes is that it is mainly sensitive to the temperature at which the CO2 is formed or exchanges isotopes with water19. A disadvantage of using Δ47 is that signals are very small: For typical ambient surface temperatures of 5 to 30 °C, the thermodynamic equilibrium value of Δ47 ranges between 0.90 to 1.04 ‰19,24,25, so high precision measurements are required. Thermodynamic equilibrium values of Δ47 decrease towards 0 at increasingly higher temperatures, and it is possible to distinguish high temperature (e.g. combustion) from low temperature process (e.g. respiration) using Δ4719,20,22,26.
It is well established that oxygen isotope exchange between CO2 and water in the biosphere and hydrosphere is the dominant process controlling the oxygen isotope composition of atmospheric CO227,28. The CO2–H2O exchange in soils and leaf water is catalyzed by the enzyme carbonic anhydrase (CA)27,29,30,31. In the leaf, CO2–H2O exchange takes place in the mesophyll, and the mole fraction of the CO2 at the CO2–H2O exchange site is expressed as cm (see Sects. “CO2 exchange fluxes during photosynthesis” and “Mesophyll conductance”). Laboratory studies have shown that the rate of exchange between CO2 and water is the same for δ18O and Δ4732,33. This finding suggests that the equilibration between CO2 and leaf water, soil water or open surface water should affect Δ47 with similar kinetics as δ18O, but in contrast to δ18O, Δ47 does not depend on the isotopic composition of the different water pools. The rapid isotope exchange of CO2 with leaf and surface waters is thought to drive the Δ47 of atmospheric CO2 towards the thermodynamic equilibrium value1,19.
Measurements of Δ47 in CO2 from air samples show that the clumped isotopic composition in the atmosphere is not in thermodynamic equilibrium at the global mean air temperature. For instance, background CO2 from remote air observatories shows Δ47 values of 0.92 ‰21, which is 0.06 ‰ lower than the expected value of 0.98 ‰ for a global mean air temperature of 15°C34. In urban and suburban air, Δ47 values are generally even lower and the variability of the reported Δ47 values was higher, possibly due to input from anthropogenic CO2 formed in high temperature combustion. In Pasadena, Affek and Eiler20, Affek, et al.26, found Δ47 values between 0.73 and 1.01 ‰, and Eiler and Schauble19 reported even lower values between 0.62 and 0.93 ‰ from a similar location. Laskar and Liang22 reported Δ47 values between 0.75 and 0.93 ‰ for urban and suburban air in Taiwan.
Eiler and Schauble19 developed a conceptual model to mathematically describe the effect of air-leaf interaction on the Δ47 signature of atmospheric CO2. The main assumption is that the carbonic anhydrase catalyzed exchange between CO2 and H2O within the mesophyll will imprint a Δ47 value that reflects the effect of leaf temperature on the CO2 that diffuses back out of the stomata to the atmosphere. In addition, kinetic fractionation during the diffusion into and out of the leaf through the stomata affects Δ47 during photosynthetic gas exchange. Eiler and Schauble19 proposed that this kinetic isotope fractionation is significant especially for plant species that show a low carbonic anhydrase activity. In this case, the diffusive component during photosynthesis might lead to a depletion in Δ47 of atmospheric CO2 of about 0.1 ‰ relative to the thermodynamic equilibrium value of CO2–H2O exchange alone.
Surprisingly, a recent study, Laskar and Liang22 reported Δ47 measurements of CO2 sampled in a greenhouse that show enrichment by up to 0.08 ‰ in Δ47 relative to the thermodynamic equilibrium value. The authors attributed this deviation to kinetic effects associated with the photosynthetic exchange, but as described in Eiler and Schauble19, such a kinetic effect should lead to lower, not higher Δ47 values relative to the thermodynamic equilibrium. Furthermore Laskar and Liang22 concluded that photosynthetic gas exchange would decouple Δ47 and δ18O in contradiction to the simple CO2–H2O exchange model of Eiler and Schauble19. This discrepancy calls for controlled air-leaf gas exchange experiments to characterize the effect of photosynthesis on Δ47 in detail.
Here, we report results from photosynthetic gas exchange experiments under controlled conditions to quantify the effect of gas exchange on the isotopic composition of CO2. We investigated the effect on Δ47 of the residual CO2 (i) for different photosynthetic pathways (C3 vs. C4 plants), (ii) for two different values of leaf conductance in C3 plants, and (iii) for variations in light intensities for one C3 plant. This choice of plant species and gas exchange conditions enable us to directly test the proposed hypothesis proposed by Eiler and Schauble19 that CO2–H2O exchange and kinetic fractionation with back-diffusion of CO2 to the atmosphere are the main drivers controlling the Δ47 fractionation effect of photosynthetic gas exchange.
Materials and methods
Theory
Background
Δ47 describes the deviation of the abundance of 13C18O16O (the dominant isotopologue with nominal mass 47) from the random distribution of all isotopes across all isotopologues in a CO2 sample with the same bulk isotopic composition24. There are three isotopologues of CO2 with nominal mass of 47, 13C18O16O (abundance = 46 × 10–6), 17O12C18O (abundance = 1.6 × 10–6) and 17O13C17O (abundance = 1.6 × 10–9)19. The existing isotope ratio mass spectrometer instruments do not have sufficient mass resolving power to separate these isotopologues. Thus, the measured isotope ratio for nominal mass of 47 is a combination of these three isotopologues. Nevertheless, approximately 97% of the CO2 with nominal mass 47 is 13C16O18O and the Δ47 value is mostly referred to as the value of 13C16O18O isotopologue. Δ47 is calculated as19,35:
where R stands for the measured ratio of a rare isotopologue of the indicated mass to the most abundant isotopologue, and R* is the isotopologue abundance ratio assuming that the heavy isotopes are distributed stochastically over all isotopologues19,24. In this case, 47R* can be calculated from the isotopologues of mass 44 and 47 as 47R* = [47]*/[44]* where [44]* = [12C][16O][16O] and 47 = 2[13C][16O][18O] + 2[12C][17O][18O] + [13C][17O][17O]. Note that the factor 2 is a symmetry number. This leads to
Measurements of both 13R and 18R (ratios 13C/12C and 18O/16O) require solving Eq. 3.
where K is 17Rstd/(18Rstd) λ36 and λ is the three isotope exponent.
13R and 18R can be calculated from the corresponding δ values as (\(^{{13}} R = \left( {\delta ^{{13}} {\text{C}}_{{{\text{VPDB}}}} + 1} \right) \times 0.011180\), and \(^{{18}} R = \left( {\delta ^{{18}} {\text{O}}_{{{\text{VSMOW}}}} + 1} \right) \times 0.0020052\)). It is impossible to measure 17R of CO2 directly with traditional gas source isotope ratio mass spectrometry due to the isobaric interference of 13C16O16O on 12C17O16O. Variations in the isotope ratios 18R and 17R in a sample are closely linked in most common processes via the mass dependent fractionation equation [18R/18Rstd]λ = 17R/17Rstd where std stands for standard36. In this study, a value of 0.528 is used as recommended by37,38. 17R was calculated as (\({}_{{}}^{{17}} R = \left( {{}_{{\text{~}}}^{{18}} {\text{R}}/0.0020052} \right)^{{0.528}} \times 0.0003799\))35,37,38. Recently, we have shown that 17R can be measured independent of 13C interference on O fragment ions7.
δ13C and δ18O of the sample are calculated from δ45 and δ46 (i.e. δ13CVPDB ≅ δ45sample + 2 × (17R/13R)VPDB-CO2 × ( δ45sample − λ × δ45sample) and δ18OVPDB-CO2 ≅ [ δ46sample − 0.0021 × δ13CVPDB]/0.99904), where 17R/13R is 0.0351637. The δ value is calculated as follow:
where x can be 13, 18, 45, 46 and 47 (for 13C, 18O, 13C16O16O, 12C16O18O and 13C16O18O, respectively).
CO2 exchange fluxes during photosynthesis
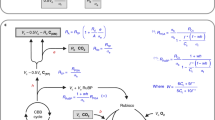
The CO2 uptake by C3 plants is schematically illustrated in Fig. 1. Net photosynthetic CO2 uptake in a leaf generates a concentration gradient from the atmosphere (ca) to the boundary layer (cs), intercellular airspace (ci), the mesophyll cell (cm) and the chloroplast (cc), where CO2 is fixed (in C3 plants) (see Fig. 1)39,40,41,42. In the chloroplast, the enzyme RubisCO catalyzes the conversion of carbon dioxide to the three-carbon acid 3-phosphoglyceric acid (3PGA). cc determines the availability of CO2 for carboxylation, which is the rate-limiting substrate. The concentration gradient between \(c_{{\text{a}}}\) and \(c_{\text{c}}\) drives the diffusion of CO2 into the leaf. The diffusion process can be described mathematically following Fick’s law of diffusion as:
where gL is the total conductance (inverse of resistance, 1/rL) of the leaf for CO2 diffusion. Total gL is conveniently subdivided into three parts that act in series. The boundary layer conductance (gb) represents the conductance through a thin layer of near-stagnant air surrounding the leaf; it is a function of air turbulence and leaf area. The stomatal conductance (gs) varies with the opening and frequency of the stomata. The mesophyll conductance (gm) quantifies the conductance for transport of CO2 from the intercellular air space in the leaf to the site of CO2–H2O exchange in the mesophyll or the carboxylation in the chloroplast. The latter is expressed as gm13 and the former is denoted as gm18, which refers to estimation using δ13C and δ18O respectively (see Sect. “Mesophyll conductance"). A small part of this transport occurs in the gas phase through a residual part of the intercellular air space. The major transport pathway is in the liquid phase, through the wall of the mesophyll cell, the plasmalemma and further into the cell to the site of oxygen exchange between CO2 and H2O that is catalyzed by carbonic anhydrase (CA). CO2 diffuses further through the chloroplast envelope and into the chloroplast where carboxylation occurs39,40,43.
Schematic illustration of CO2 exchange fluxes, concentrations and conductivities during photosynthetic gas exchange. Net photosynthetic CO2 uptake in a leaf generates a concentration gradient over the leaf where ca is the CO2 concentration of the air, cs the CO2 concentration at the leaf surface, ci is the CO2 concentration in the intercellular air space, cm is the CO2 concentration in the mesophyll, i.e., the site of CO2-H2O exchange and cc the CO2 concentration in the chloroplasts, the site of CO2 uptake. The gb is the boundary layer conductance of CO2 from the atmosphere to the leaf surface, the stomatal conductance gs quantifies the gas exchange through the stomatal opening, gm13 is the mesophyll conductance to the site of carbon uptake and gm18 is the mesophyll conductance to the site of CO2-H2O exchange.
Mesophyll conductance
For C3 plants, we used the discrimination against 13C (ΔA13C) (Eq. 6) to estimate the mesophyll conductance (\(g_{{m13}}\)) from the intercellular airspace to the carboxylation site (Eq. 7) as described in44. The overall isotope fractionation associated with photosynthetic gas exchange is referred to as discrimination, which quantifies the enrichment or depletion of the isotope composition of CO2 in the surrounding atmosphere relative to the CO2 assimilated, see Eq. (6)42,45,46. Experimentally, the discrimination (ΔA) is calculated from the isotopic composition and mole fraction of CO2 entering and leaving the cuvette47,48,49, as shown in Eq. (6).
where x is either 13, 18 or 47 (for 13C, 18O and 13C18O16O isotope composition, respectively), \(\zeta = c_{e} /\left( {c_{e} - c_{a} } \right)\) and c is the mole fraction of the CO2 entering (index e) and leaving (index a) the cuvette.
\(g_{{m13}}\) is calculated from the difference between the observed 13C discrimination (ΔA13Cobs) and the discrimination at infinite gm (ci = cc) as:
where P is the ambient pressure, t13 is a ternary correction factor for 13CO2, b the fractionation due to uptake by Rubisco, and am the combination of the fractionations associated with 13CO2 dissolution and diffusion through water, respectively. e’, RD, \(\alpha _{e}\), and \(\alpha _{b}\) are the fractionation factor for mitochondrial respiration including the apparent fractionation, the day respiration rate (mitochondrial respiration in the light) the fractionation factor for day respiration with respect to net assimilation and the fractionation factor for C3 carboxylation, respectively. \(\alpha _{R}\) = 1 + (RD/An) × (e’/\(\alpha _{e}\)). A detailed description of the equations, used parameters and definitions of fractionation factors is provided in Table S1 of the supplementary material.
In C4 plants, the CO2 is converted to bicarbonate and fixed to a four-carbon acid catalyzed by phosphoenol pyruvate carboxylase (PEPC) in the mesophyll. Unlike for C3 plants, it is impossible to estimate mesophyll conductance from ΔA13C for C4 plants due to the low photosynthetic fractionation50,51. However, the apparent discrimination against 18O (ΔA18O) can be used to estimate the mesophyll conductance from the intercellular air space to the site of CO2–H2O isotope equilibration, \(g_{{m18}}\), as described in41,47,52,53,54, for both C3 and C4 plants. The analytical expression for estimating \(g_{{m18}}\) (Eq. 8) assumes that the degree of equilibration between CO2 and H2O is 100%. Some studies have reported that the degree of equilibration can be lower than 100%, especially for C4 plants, which have a lower CA activity29,31,47,54. Detailed information is provided in Table S1 of the supplementary material.
δ18Oi is δ18O of CO2 in the intercellular airspace, \(\alpha _{{18w}}\) is the fractionation factor for 12C18O16O during diffusion and dissolution in water, \(a_{{18w}}\) is the discrimination against 12C18O16O during diffusion and dissolution in water, \(a_{{18w}} = \alpha _{{18w}} - 1\), δ18OA is δ18O of the assimilated CO2 and δ18Om is the δ18O of CO2 in equilibrium with leaf water at the CO2–H2O exchange site. In previous studies, the difference between δ18O of bulk leaf water and the water at the evaporation site was about 1 to 2 ‰ higher13,54, but larger differences have also been reported55. In this study, we assumed that the δ18O of leaf water at the CO2–H2O exchange site is enriched by 2 ‰ compared to bulk leaf water and the degree of equilibration between CO2 and leaf water is 100%.
Photosynthetic Δ47 discrimination
The effect of photosynthetic gas exchange on Δ47 depends on the assimilation rate, the various conductances mentioned before and the fraction of CO2 that diffuses back to the atmosphere after isotope exchange with leaf water. For a laminar boundary layer, diffusion through the boundary layer decreases the Δ47 value of residual CO2 by an amount equal to the boundary layer diffusive fractionation (+ 0.2 ‰) multiplied by the fraction of CO2 enters leaves and is fixed (~ 1/3), i.e., 0.07 ‰. In this study, we used a fan (see Sect. “Leaf cuvette set-up”), which creates a turbulent boundary layer with an estimated conductance of 5 mol m−2 s−1. The boundary layer conductance for the leaf cuvette was determined using wet filter paper from measurements of relative humidity and temperature of air in the cuvette as described in Parkinson 56. As a result, the fractionation in Δ47 due to diffusion through the boundary layer is negligible compared to the precision of the measurement (ca. ~ 0.002 ‰). Figure 2 illustrates the effect of photosynthetic gas exchange on Δ47 for two extreme scenarios. The first “uptake dominated” scenario assumes that all the CO2 entering stomata gets assimilated, leading to cm/ca ~ 0 whereas in the second “exchange dominated” scenario all CO2 diffuses back to the atmosphere after exchange with the leaf water leading to cm/ca ~ 1. In the uptake dominated scenario, the resulting Δ47 signal is mainly affected by diffusion, in the exchange dominated scenario the Δ47 signal is dominated by the CO2–H2O exchange. A similar scheme for δ18O is shown in Figure S2 of the supplementary material.
Schematic illustration of the clumped isotope fractionation during air-leaf gas exchange. Δ47 of the CO2 in the air surrounding the leaf is mainly controlled by CO2-H2O exchange and kinetic fractionation during diffusion into and out of the leaf stomata. In the uptake dominated case (cm/ca ~ 0), − 0.3 ‰ comes from the fact that diffusion mediated leaf air interaction will reduce the Δ47 value by 0.3 ‰, neglecting other effects. Diffusion only will reduce the Δ47 value by 0.3 ‰ multiplied by the fraction of CO2 entering the leaf that is assimilated 19. The boundary layer conductance is large and we have omitted it here.
The fractionation of Δ47 associated with photosynthetic gas exchange (ΔAΔ47obs) was calculated in a similar way as \(\Delta _{{{\text{Aobs}}}}^{{x}}\) in Eq. (6) from the difference in CO2 concentration and isotopic composition between the air entering and leaving the leaf cuvette (Eq. 9). For mixing of two different populations of CO2, Δ47 is not a conserved quantity and the error introduced by adding or subtracting Δ47 values linearly depends on the relative difference in the δ18O and δ13C of the two gases19,20,57,58. In our study, the maximum difference between the δ18O and δ13C value of the CO2 entering and leaving the cuvette is 14 ‰ and 4 ‰, respectively. For this rather small range, the error introduced due to linear addition and subtraction of Δ47 values of the CO2 entering and leaving the cuvette is not significant (< 0.01 ‰).
Leaf cuvette model
To explore the effects of conductance, assimilation rate and back-diffusion of CO2 to the atmosphere on the Δ47 of ambient CO2 in detail we used a leaf cuvette model [https://git.wur.nl/leaf-model]59 that has been used for the interpretation of Δ17O measurements in leaf exchange experiments recently13. In the model we assumed that CO2–H2O exchange in the mesophyll is rapid enough to constantly set Δ47 to thermodynamic equilibrium with the water, i.e., Δ47 = 0.95 ‰ at 20 °C25. Thus, any change in bulk isotopic composition of CO2 due to assimilation does not affect Δ4719. Furthermore, CO2 diffusion into and out of the intercellular air space through the stomata is associated with a fractionation constant of + 0.3 ‰ for Δ4719 whereas CO2 diffusion through the boundary layer has a fractionation constant of + 0.2 ‰ for Δ47.
The steady state model considers five compartments: (i) atmosphere (air in the leaf surrounding), (ii) the leaf surface, (iii) the intercellular airspace of the leaf, (iv) the mesophyll cell of the leaf, and (v) the chloroplast (see Fig. 1). The air enters the leaf cuvette at a flow rate Fe and a CO2 concentration ce with a well-defined isotopic composition δe (where δ can stand for δ13C, δ18O, δ47 and Δ47, see Sect. “Background” for definition). The leaf inside the cuvette takes up a portion of the CO2 and this uptake is associated with an isotope fractionation. The air flowing out of the cuvette has flow rate Fa, CO2 concentration ca and isotopic composition δa. The photosynthetic uptake in the chloroplasts leads to a concentration gradient between the air surrounding the leaf and the chloroplasts so that there is a net flow of CO2 from the cuvette into the intercellular airspace, to the mesophyll cell, and finally to the chloroplasts. The corresponding CO2 concentrations decrease accordingly in the order ca, cs, ci, cm and cc. Diffusion, isotopic equilibration with H2O, CO2 uptake and mixing between the model reservoirs lead to a change in isotopic composition (δa, δs, δi, δm and δc). The magnitude of the exchange fluxes between the compartments is defined by the boundary layer conductance gb, stomatal conductance gs and the mesophyll conductances gm18 and gm13. In the leaf cuvette model, the boundary layer conductance gb is assumed 5 mol m−2 s−1, similar to the value determined in the experiment.
Figure 3 shows how the Δ47 value changes between incoming and outgoing CO2 in the leaf cuvette model for cm/ca ratios ranging from 0.3 to 0.9 and Δ47 values of the entering CO2 between 0.0 ‰ and 1.0 ‰. It is evident that the relative changes are small when Δ47 of the incoming CO2 is close to the equilibrium value (0.9—1.0 ‰) at ambient temperatures. The changes are much larger if the Δ47 of the incoming CO2 is close to a random distribution (Δ47 = 0.0 ‰). This motivated us to carry out the gas exchange experiments with isotopically ‘scrambled' (i.e., Δ47 close to zero) CO2 (see below).
Difference between Δ47 of out- and inflowing CO2 as function of the fraction of CO2 diffusing back to the atmosphere (cm/ca) calculated with the leaf cuvette model. The black dashed line shows the cm/ca ratio where the Δ47 relative difference between the ingoing and outgoing CO2 becomes zero for the corresponding Δ47 of CO2 entering the cuvette.
Plant material and growth conditions
Three plant species were used for the experiments, belonging to different functional groups: Sunflower (Helianthus annuus L. cv, Giganteus), Atlantic ivy [Hedera hybernica (Kirchner) Bean (syn. Hedera helix var. hibernica)], and maize (Zea mays L. cv, Torres). The fast-growing annual C3 species Helianthus is characterized by short-lived leaves with high photosynthetic capacity and conductance for CO2 diffusion. The other C3 species, the evergreen Hedera, has long-lived leaves with a lower photosynthetic capacity and conductance. The third species, Zea, has C4 metabolism, a high photosynthetic capacity and low conductance.
Helianthus and Zea were grown from seed in a growth room at 20 °C, a relative humidity of 70% and a photosynthetic photon flux density (PPFD) of 250 µmol m−2 s−1 for a day length of 16 h. The first pair of two leaves of Helianthus was used for the experiments when fully grown at three to four weeks after planting. Younger leaves that shaded them were removed. For Zea mays, a section of the fifth or sixth leaf at about two thirds of total leaf length was used at a plant age of around 7 weeks. These leaves were about 5 cm wide, giving a sufficiently large leaf area in the cuvette. Hedera plants were obtained from a grower, pruned to reduce self-shading and further grown in the experimental garden in full daylight. They were used for the experiments in early November 2015 when outside average maximum day temperature during the preceding month was 14 °C. Mature leaves that could be accommodated intact in the cuvette were used for the experiments. For all the experiments, leaves remained attached to the plants during the experiments. Both Helianthus and Zea are watered from tap water whereas Hedera received largely rain water.
Leaf cuvette set-up
The isotopic effect of CO2 exchange during photosynthesis was investigated with an open gas exchange measurement system similar to the one described by13,60 (Fig. 4). A controlled flow of air entered and left the leaf cuvette, which had a 7 × 7 cm transparent window on top that limited the maximum width of the leaves that could be accommodated. A fan inside the leaf cuvette increased boundary layer conductance to around 5 mol m−2 s−1 and mixed the air thoroughly so that the air leaving the cuvette was a representative sample of the air inside. The chamber was illuminated from above by a halogen lamp that allowed control of the PPFD incident on the leaf. Leaf temperature was measured with thermocouples appressed to the abaxial side of the leaf. Water at 20 °C was circulated through the double wall of the cuvette, which stabilized leaf temperature up to 3 °C higher depending on PPFD and transpiration rate (Table 1).
Leaf chamber set-up, modified from Adnew et al. (2020)13. A single leaf was placed in the leaf cuvette and the light intensity could be regulated to manipulate the assimilation rate. A gas-mixing unit was used to mix dry synthetic air with scrambled CO2 with humidified CO2-free air to obtain an overall CO2 concentration of the ingoing air of 500 µmol mol−1. The flow rate was adjusted to the photosynthetic activity of the leaf to obtain a CO2 concentration of about 400 µmol mol−1 at the outlet (0.6 to 1.5 L min−1). CO2 and H2O concentrations were monitored with an infrared gas analyzer (IRGA). Once steady state was reached, the outgoing air was sampled in one 2 L and one 1 L glass flasks, in series. The bulk isotope composition (δ13C and δ18O) of the normal CO2 and scrambled CO2 are identical.
A gas-mixing unit made from ¼ inch stainless-steel tubing was attached to the inlet port of the cuvette. Synthetic dry air with a CO2 concentration of about 1500 µmol mol−1 was mixed with CO2-free air of controlled humidity to set the CO2 concentration of inlet air to 500 µmol mol−1. Air flows were controlled with flow controllers. Two of these mixing units were used, one with untreated CO2 and the other one with the same CO2 that was previously isotopically scrambled at 1000 °C (target Δ47 ~ 0.0 ‰, see below for discussion of deviations). The air flow rate was adjusted so that the CO2 concentration at the outlet (and thus also in the cuvette) was about 400 µmol mol−1. Thus, the decrease in CO2 concentration in the cuvette as a result of uptake by the leaf was about 100 µmol mol−1. In typical experiments the air flow rate was between 0.6 and 1.5 L min−1 depending on the CO2 uptake rate of the leaf. It generally took 1 h to reach steady state gas exchange conditions. Gas exchange was measured before and after sampling with an infrared gas analyzer (IRGA) (LI-6262; Li-Cor, Lincoln, NE, USA) operated in the absolute mode. CO2 and H2O concentrations of inlet and outlet air were measured subsequently. The IRGA used for CO2 mole fraction measurements, was calibrated every day with compressed air (dry) which has a known CO2 mole fraction, and the reference cell was flushed with CO2-free N2 gas. For the water mole fraction, the IRGA was calibrated using a dew point mirror, and the reference cell was flushed with CO2-free N2 gas. Gas exchange variables were calculated according to von Caemmerer and Farquhar 61 (see supplementary material Table S1).
The measurements before and after sampling were used to check whether leaves remained sufficiently in steady state over the sampling period, if not, the samples were discarded. The mean value of the two gas exchange measurements (before and after collecting an air sample) were used to calculate the gas exchange parameters. Preparing scrambled CO2 is labor intensive, as a result we used normal CO2 during the gas exchange experiment until reaching steady state. After the steady state was reached (i.e. constant CO2 and H2O mole fractions) the CO2 source was switched to the scrambled CO2 supply (Fig. 4) and we waited 15 min to re-establish steady state conditions. Sampling of air was done by attaching a Mg(ClO4)2 dryer (Sigma Aldrich, USA) and 6-L of glass flasks to the outlet of the cuvette. The duration of completely flushing and filling the flasks depended on the flow rate and varied between 20 and 50 min.
We regularly determined the isotopic composition of the entering CO2 by flushing it through an empty leaf cuvette and sampling the air at the outlet as a blank experiment. We used two different synthetic air cylinders spiked with scrambled CO2: The first cylinder had a δ13C value of − 2.50 ± 0.02 ‰, a δ18O value of 25.3 ± 0.1 ‰ and a Δ47 value of 0.24 ± 0.02 ‰; the second cylinder had a δ13C value of − 2.43 ± 0.03 ‰, a δ18O value of 25.5 ± 0.2 ‰ and a Δ47 value of 0.07 ± 0.03 ‰ (Table S1, supplementary material). If not indicated differently, these and all further errors reported in this manuscript are 1 σ standard deviation, determined from repeated analysis of samples.
Automated CO2 extraction from air
We used an automated CO2 extraction and purification system to prepare the sample CO2 for 13C18O16O analysis. The system was mainly manufactured from stainless steel parts and consisted of four main units: (i) an air inlet system, (ii) chemical and cryogenic CO2 drying units, (iii) a cryogenic CO2 trap and (iv) a GC column for purification (Fig. 5). The outlet of the extraction line was directly connected to the sample bellows of the mass spectrometer. The design of the air inlet system and the cryogenic water and CO2 traps were based on an automated CO2 extraction system dedicated to conventional mass spectrometric isotope analysis of atmospheric CO262.
The air inlet system allowed connecting several air samples via an 8-port Valco multiport valve: One position of the multiport valve was capped off and was used as a ‘closed’ position, two positions were generally used for working gas cylinders and the remaining five positions could be used to connect the sample flasks. About 2.5 L of air were required for one analysis. For a few samples we measured duplicates. Prior to an extraction sequence, the analytical system was evacuated up to the flask valves. The outlet of the multiport valve was connected to a mass flow controller (10–100 mL min−1; MKS Instruments) to regulate the gas flow during the extraction system. The flow rate was routinely set to 50 mL min−1 for extracting the CO2 from air.
In order to minimize exchange with water the gas was thoroughly dried at three different positions in the automated extraction system: (i) before the mass flow controller with a magnesium perchlorate unit, (ii) after the mass flow controller with two cryogenic water traps, and (iii) after the CO2 trap with a phosphorus pentoxide (P2O5) unit. The magnesium perchlorate was filled in a ca. 10 cm long open-end glass tube with 10 mm inner diameter in the central part and ¼” connectors. The drying agent was held in place with glass wool. The cryogenic water traps were made from 25 cm long stainless steel tubes with a diameter of ¼ ” and inner tubes with a diameter of 1/8 ”63. The outer tubes were welded tight at the bottom and electropolished afterwards to reobtain a smooth surface. The inner tubes ended about 0.5 cm above the bottom of the outer tubes to force the air through the entire length of the water trap. To enhance the cold transfer between the outer and inner tube, the inner tubes were slightly bent to touch the outer tubes. The cryogenic water traps were cooled with a mixture of dry ice and ethanol to − 72 °C. The position of the Dewar with the cooling bath was controlled with a hydraulic lift. Due to the relatively large size of the Dewar, the temperature of the cooling bath stayed constant overnight. The (P2O5) drying unit consisted of a 10 cm long and 6 mm o.d. wide glass finger containing phosphorous pentoxide (P2O5), which was connected via a 1/8″ stainless steel tube to the CO2 trap. It was immersed in liquid nitrogen using a hydraulic lifter.
The chemical drying units were added to the system at a later stage in an attempt to reduce isotopic re-equilibration during the extraction process (see below). However, also these additional measures did not prevent partial re-equilibration in the extraction line so that we had to correct all Δ47 data for this partial re-equilibration. The size of the corresponding correction was established by frequently analyzing heated and non-heated CO2 in air standards (see Sect. "Isotopic analysis of CO2 (δ13C, δ18O and Δ47").
Carbon dioxide (and nitrous oxide) was trapped by immersing the CO2 trap in liquid nitrogen. The design of the carbon dioxide trap and cooling unit was the same as the cryogenic water trap described above. During extraction, the air was processed through the system with a fore vacuum pump. A mechanical flow restriction (crimped 1/16″ stainless steel tubing) after the CO2 trap slowed down the flow rate in the CO2 trap. The crimping was adjusted such that the pressure in the water and CO2 trap was about 180 mbar at a flow rate of 50 mL min−1.
After trapping the CO2 in the liquid nitrogen trap, the trap was warmed up to room temperature and the sample CO2 was frozen to the P2O5 trap. Then, the valve on top of the P2O5 glass finger was closed and the CO2 was brought to room temperature for final drying for about 10 min. Afterwards, the CO2 was transferred to a 1/16″ focusing trap in front of the gas chromatographic column, by immersing this focusing trap in liquid nitrogen. The CO2 was then released into a helium stream at a flow rate of approx. 25 mL min−1 through the gas chromatographic (GC) column packed with Porapak Q. The GC column was cooled to 20 °C during purification with cold nitrogen gas provided from a liquid nitrogen Dewar, to hold back possible hydrocarbon impurities20 and it was heated up to 250 °C in-between extractions. After passing through the GC column, the CO2 was trapped in a second 1/16″ trap and the helium was evacuated using the fore and high vacuum pump of the isotope ratio mass spectrometer (IRMS). Finally, the pure CO2 was warmed to room temperature and injected into the mass spectrometer.
The whole CO2 extraction line (including the liquid nitrogen cooling) was controlled via LabVIEW (Version 15.0). The LabVIEW software also gave a signal to the IRMS software Isodat (Version 2.0) to start a measurement. Extraction and purification of one sample took about 4.5 h and we generally analyzed two working standards and three samples per day.
Isotopic analysis of CO2 (δ13C, δ18O and Δ47)
Mass spectrometric analysis of the isotopic composition of the extracted and purified CO2 was carried out in dual inlet mode on a MAT 253 mass spectrometer (Thermo Fisher Scientific, Germany) with a modified collector unit that allowed simultaneous analysis of the mass-to-charge ratios (m/z) 44, 45, 46, 47, 48 and 49. The overall reproducibility for Δ47 analysis was about 0.04 ‰, determined from replicate measurements of the compressed air cylinder.
The mass spectrometric Δ47 measurements were calibrated relative to CO2 heated to 1000 °C and CO2 equilibrated with water at 28°C25,64. We analyzed heated and water equilibrated CO2 with a different bulk isotopic composition and determined a slope of 0.0024 for the heated and water-equilibrated gas line (Figure S3, supplementary material). This slope was used to correct for small, negative background effects on m/z 47. The bulk isotopic composition of our in-house reference CO2 gas was δ13CVPDB = − 2.82 ‰ and δ18OVSMOW = − 26.11 ‰ and the maximum δ47 difference between the sample and the reference CO2 was 17 ‰ resulting in a maximum Δ47 bulk isotope composition effect − 0.04 ‰ which is corrected based on heated and equilibration gas slope.
In order to calibrate our δ13C, δ18O and Δ47 measurements of CO2 in air, we prepared two cylinders of CO2 in synthetic air as working reference cylinders (Table S2). The first synthetic air cylinder was spiked with CO2 resulting in a concentration of about 500 µmol mol−1, δ13CVPDB = − 2.76 ‰, δ18OVSMOW = 25.65 ‰ and Δ47 = 0.82 ‰. For the second synthetic air cylinder we used scrambled CO2 with a similar bulk isotopic composition, i.e. δ13CVPDB = − 2.73 ‰, δ18OVSMOW = − 25.83 ‰ but a low clumped isotope signature of Δ47 = 0.11 ‰ and a CO2 concentration of 400 µmol mol−1.
The clumped isotopic composition of the first air cylinder was determined by analyzing the pure CO2 directly in the dual inlet system of the IRMS versus the heated and water equilibrated CO2 samples and versus CO2 obtained by acid digestion at 70 °C from a set of clumped isotope carbonate standards (ETH1, ETH2, ETH3 and ETH4). Both procedures gave consistent results of Δ47 = 0.82 ± 0.04 ‰. The same CO2 was then mixed in reference air cylinder 1 with synthetic air. After extracting and purifying the CO2, we found an average Δ47 value of 0.86 ± 0.04 ‰. The reason for this offset of 0.04 ± 0.04 ‰ might be partial re-equilibration within the extraction line, where full equilibration at room temperature would result in Δ47 = 0.93 ‰.
For preparing the second air cylinder, we heated the pure CO2 to 1000 °C for more than 2 hours64 and then mixed it into synthetic, CO2-free air. After extracting and purifying the CO2 from the second cylinder, we determined an average Δ47 value of 0.42 ± 0.04 ‰. The reason for this significant deviation from the expected Δ47 value of 0.03 ‰ is most likely twofold: (i) partial re-equilibration during mixing of scrambled CO2 with synthetic air, and (ii) partial re-equilibration in the automated CO2 extraction line. Subsequently, we prepared several other mixtures of heated CO2 with an expected clumped isotope signature of Δ47 = 0.03 ‰ in synthetic air cylinders. For all cylinders that were prepared this way we found Δ47 values that were at least + 0.26 ‰ enriched in Δ47 relative to the expected value. It is not straightforward to decide whether the clumped signal of a CO2-in-air standard was altered during the preparation of the air standard or during the CO2 extraction step. Comparison between the Δ47 of the pure CO2 and Δ47 value after processing the CO2-air mixtures through the CO2 extraction line reveals a Δ47 scale contraction of 24%, i.e. we measure only 76% of the true difference between samples when determining the clumped isotopic composition of CO2 in air. This apparent scale contraction was highly reproducible over the course of the experiments reported here and we corrected all Δ47 values accordingly. This illustrates that referencing Δ47 measurements for atmospheric CO2 samples is challenging because there is no Δ47 standard for CO2-in-air studies available.
The leaf gas exchange samples contained up to about 0.3 µmol mol−1 N2O. All CO2 isotope measurements of δ13C, δ18O and Δ47 were corrected for the mass interference from the N2O isotopologues because N2O was not separated from CO2 during the purification step. The amount of N2O was inferred from the intensity of the N fragment at m/z 14 relative to the intensity of m/z 4420. For typical N2O mole fractions of 0.3 µmol mol−1, the N2O correction was approximately + 0.17 ‰ for δ13C, + 0.45 ‰ for δ18O and − 0.13 ‰ for Δ47.
Considering all correction procedures, most notable the 24% Δ47 scale contraction, we obtain an overall reproducibility for repeated extraction of CO2 from air and subsequent isotopic analysis of 0.08 ‰ for δ13C, 0.3 ‰ for δ18O and 0.045 ‰ for Δ47.
Leaf water extraction and δ18O analysis
Immediately after sampling of air for Δ47 measurements, the leaf was placed between plastic sheets, its area was measured, and it was enclosed in a vial and frozen. Leaf water was extracted from the leaves by cryogenic vacuum distillation, i.e. the leaf sample was heated in vacuum to 60 °C and the evaporated water was directly frozen in a vial cooled to liquid nitrogen temperature13. The distillation process was carried out for at least 4 h to ensure quantitative extraction (West et al., 2006). The δ18O value of the leaf water was determined by equilibrating CO2 and water in a GasBench II (Thermo Scientific), and subsequent analysis of the oxygen isotope composition of the equilibrated CO2 with a Delta V mass spectrometer (Thermo Scientific, Germany). The oxygen isotope composition was calibrated versus VSMOW and SLAP.
Results
Gas exchange data and isotopic composition of leaf water
Gas exchange of Helianthus was characterized by high stomatal conductance relative to the other two species (Table 1). The net assimilation An was 12 µmol m−2 s−1 at a PPFD of 200 µmol m−2 s−1 and increased to 22 µmol m−2 s−1 at 700 µmol m−2 s−1. The net assimilation increased only little further when measured at a PPFD of 1800 µmol m−2 s−1 (25 µmol m−2 s−1). ci/ca decreased with increasing irradiance, from 0.82 (at PPFD of 200 µmol m−2 s−1) to 0.75 (at 1800 µmol m−2 s−1).
Hedera was measured at a PPFD of 700 µmol m−2 s−1 only where An was lower than that of the other two species (15 µmol m−2 s−1). Compared to Helianthus at the same PPFD, the stomatal conductance gs was much lower (0.08 mol m−2 s−1), causing a clearly lower ci/ca ratio (0.41) (Table 1). The C4 species Zea was also measured at 700 µmol m−2 s−1 only where An was the highest of the three (28 µmol m−2 s−1) but gs was rather low (0.13 mol m−2 s−1) causing the lowest ci/ca ratio of the three species, (0.32) (Table 1).
For Helianthus, mesophyll conductance calculated using ΔA13C (gm13) increased with light intensity whereas the mesophyll conductance measured using ΔA18O (gm18) did not show a clear correlation with the light intensity (Table 1 and Figure S2). Our estimates of gm18 had relatively larger errors compared to gm13 (Table 1) and the values were larger (Table 1). For Helianthus, the gm18 estimates were 1.3 to 2.5 times larger and for Hedera 1.5 times.
The δ18O value of the bulk leaf water of Helianthus varied between 4.3 and 6.9 ‰ with an average value of 6 ± 1 ‰. For Hedera, the bulk leaf water isotopic composition was δ18O = 3.1 ± 1.6 ‰. The relative difference in the δ18O value of the bulk leaf water between the Helianthus and Hedera is due to the difference in the δ18O of source water. For Zea, the bulk leaf water isotopic composition of the leaf part inserted in the cuvette was δ18O = 20.5 ± 1.3 ‰. We used a section of the Zea leaves at about 1/3 from the tip for gas exchange experiments and such high enrichments in δ18O of leaf water compared to the source water are typical for sections towards the tip of elongated leaves (see, 65) and at higher vapor pressure deficit66.
Effect of photosynthetic gas exchange on the isotopic composition of CO2
For Helianthus Δ47 increased from 0.24 ‰ in the incoming air to 0.50 ‰ to 0.61 ‰ in the outgoing air, at a cm/ca ratio of 0.66 to 0.78 (Table 1 and 2). For Hedera, at a cm/ca ratio of 0.28, the change in Δ47 between incoming and outgoing air was more variable than for the other species at similar light intensity. The average change of all the experiments under similar conditions is insignificant (from 0.24 ‰ to 0.22 ‰). For Zea, at lower cm/ca ratio (0.20), results were more consistent and no statistically significant decrease in Δ47 between incoming and outgoing air was observed (Table 1 and 2).
When these changes are converted to discrimination (ΔAΔ47), for Helianthus, we observed an average ΔAΔ47 of 1.7 ± 0.4 ‰. Slightly negative but non-significant discriminations were observed for Hedera (− 0.07 ± 0.4 ‰), and Zea (− 0.07 ± 0.06 ‰) (Fig. 6a, Table 2). The ΔAΔ47 correlates strongly with the cm/ca ratio, higher ΔAΔ47 are observed at higher cm/ca ratio. The change in Δ47 between CO2 entering and leaving the cuvette correlates strongly with the change in δ18O of CO2 entering and leaving the cuvette, with an R2 value of 0.864 (Fig. 6c). The positive correlation between Δ47 and δ18O of CO2 indicates that photosynthetic gas exchange affects Δ47 and δ18O similarly (Fig. 6a).
Effect of photosynthetic gas exchange on Δ47 and δ18O of CO2. ΔAΔ47 (a) and ΔA18O (b) during photosynthetic gas exchange experiments as a function of the cm/ca ratio. (LL = low light: PPFD = 200 μmol m− 2 s−1, ML = medium light: PPFD = 700 μmol m−2 s−1, HL = high light: PPFD = 1800 μmol m−2 s−1). a) discrimination against 13C18O16O (ΔAΔ47). (b) discrimination against 12C18O16O (ΔA18O). (c) Relative difference between Δ47 the CO2 entering and leaving the cuvette as a function of the difference between δ18O of CO2 entering and leaving the cuvette. The solid line is a linear regression fit with a function of (Δ47a− Δ47a) = (0.041 ± 0.004) × (δ18Oa–δ18Oe)—0.151 ± 0.040. For the leaf cuvette model, we assumed δ18O = 10 ‰ for the leaf water and a mesophyll conductance of 0.5 mol m−2 s−1 bar−1.
In addition to the ΔAΔ47, we also measured ΔA13C and ΔA18O (Table 2). The average net carbon isotope discrimination was ΔA13C = 21.6 ± 1.2 ‰ for Helianthus, 10.1 ± 0.7 ‰ for Hedera and 3.6 ± 0.3 ‰ for Zea. The magnitude of ΔA13C also correlates with the cc/ca ratio, in agreement with previous studies13,42,48. As cc/ca depends on light intensity, the gas exchange experiments with Helianthus show a slightly higher ΔA13C at low light intensity (22.8 ± 0.6 ‰) compared to mid and high light conditions (21.1 ± 0.9 ‰). The average apparent oxygen isotope discrimination ΔA18O was 62 ± 12 ‰ for Helianthus, 18 ± 3 ‰ for Hedera and 21 ± 9 ‰ for Zea. Similar to ΔA13C, ΔA18O is higher at low light intensities (78 ± 6 ‰) compared to mid and high light conditions (55 ± 4 ‰) (Table 2, Fig. 6b).
Discussion
The Δ47 value of CO2 has been suggested as a possible tracer for gross primary production, however two previous studies presented contradicting conclusions on the effect of photosynthesis on the Δ47 value of CO2 19,22. In this study, using a leaf cuvette experiment under controlled conditions (light, CO2, temperature and humidity) and a leaf cuvette model, we showed that photosynthetic gas exchange can in principle increase or decrease the Δ47 value of CO2 depending on the Δ47 value of the CO2 entering the leaf, the CO2–H2O exchange temperature and the back-diffusion flux (quantified as cm/ca ratio). However, under conditions similar for the current atmosphere, photosynthesis depletes the Δ47 value of atmospheric CO2.
The photosynthetic effect on Δ47 of the residual CO2 for the C3 species Helianthus and Hedera correlated with the CO2 concentration gradient over the leaf, i.e. cm/ca, and the discrimination in Δ47 showed a similar pattern to ΔA18O (Fig. 6). The main driver for the discrimination against δ18O and Δ47 values of CO2 is isotope exchange with leaf water, and the fractionation associated with the initial fixation by the enzyme RuBisCO (Ribulose-1,5-bisphosphate carboxylase-oxygenase) or PEP (Phosphoenolpyruvate) has no/negligible effect on the δ18O and Δ47 value of CO2. Δ47 value is independent of fractionations in the bulk isotope composition (i.e., variations in δ18O value due to isotope exchange with leaf water and changes in δ13C due to metabolic carbon fixation). The lower discrimination in Δ47 by C4 plant Zea is due to the lower back-diffusion flux (lower conductance and higher assimilation rate) and the fractionation is dominated by diffusion in agreement with the hypothesis of Eiler and Schauble19. Gas phase diffusion causes a decrease in Δ47 of the residual CO219, see also Fig. 7.
For Helianthus, at a cm/ca ratio of 0.81, we observed an increase in Δ47 from 0.24 ‰ to 0.68 ‰ (an increase by 0. 44 ‰) for a CO2 drawdown of 100 µmol mol−1. Eiler and Schauble19 reported an increase in Δ47 from 0.65 to 0.75 (an increase by 0.1 ‰, i.e. ΔA Δ47 ~ 0.54 ‰) due to photosynthetic gas exchange for a CO2 drawdown of 72 µmol mol−1 (from 390 to 318) using Antirrhinum majus with a cm/ca ratio of 0.85, which is consistent with our results.
Using the leaf cuvette model, we quantitatively estimated the expected Δ47 discrimination based on the equations discussed in Sect. “Photosynthetic Δ47 discrimination" and the derived concentration gradient between the atmosphere and the site of CO2–H2O exchange (cm/ca). For the C3 species, we obtained an excellent agreement between the observed and the predicted change in Δ47, except for one outlier for the experiments with Hedera (Fig. 6 and Figure S5). For Zea, the observed change between outgoing and ingoing CO2 of − 0.01 ± 0.01 ‰ (the error is standard deviation for the three measurements) was slightly lower than the expected change in Δ47 of about + 0.01 ‰. However, the difference was still of the order of our measurement precession.
The correlation between ΔAΔ47 and the cm/ca ratio and the overall good agreement between observed and predicted ΔAΔ47 confirm the hypothesis of Eiler and Schauble19 that CO2–H2O exchange and kinetic fractionation control the discrimination. For high CO2 back-diffusion fluxes, i.e., high cm/ca ratios, the magnitude of the in- and outgoing CO2 flux is almost the same so that the kinetic term due to diffusion cancels out and the Δ47 value of the residual CO2 is close to the thermodynamic equilibrium value for the respective leaf temperature, i.e. Δ47 = 0.95 ‰ at 20 °C (Fig. 2). At room temperature, the sensitivity of Δ47 to temperature is about 0.005 ‰/°C so that small variations in temperature do not have a large effect. For cm/ca ratios close to zero, the back-diffusion flux is small and the kinetic fractionation term due to diffusion into the intercellular airspace induces a negative discrimination in Δ47 of up to − 0.08 ‰ if the ingoing CO2 has a Δ47 close to 0 ‰.
As mentioned above and illustrated in Fig. 3, we used CO2 with an artificially diminished Δ47 value in the gas exchange experiments in order to increase the signal, i.e., the Δ47 difference between incoming and outgoing CO2. After having verified the Eiler and Schauble19 mechanism, we use our leaf cuvette model to quantify the effect of photosynthesis on ΔAΔ47 for CO2 with typical ambient Δ47 values. In ambient air, Δ47 of CO2 is usually close to or lower than the Δ47 value of CO2 at the CO2–H2O exchange site (Δ47 = 0.95 ‰).
Figure 7 illustrates the calculated dependence of ΔAΔ47, as well as Δ47a on cm/ca during gas exchange for Δ47e values between 0.6 ‰ and 1.1 ‰ to get Δ47a values between 0.6 ‰ and 1.05 ‰ close to the Δ47 values of atmospheric CO2 reported in literature19,20,21,22. A negative ΔAΔ47 means that \(_{{47{\text{a}}}} < _{{47{\text{e}}}}\) (Eq. 9), i.e., photosynthetic gas exchange would decrease ambient Δ47 values, whereas a positive ΔAΔ47 would increase ambient Δ47 values. Figure 7a shows that ΔAΔ47 is mostly negative, thus photosynthetic gas exchange generally acts to decrease Δ47 except for situations in which ambient Δ47 is at the low end of reported values and cm/ca ratios are very high (indicating very slow assimilation rates).
At very low cm/ca ratio, i.e., the diffusion limited case, ΔAΔ47 is controlled by the diffusional fractionation. Figure 7a shows that ΔAΔ47 converges to − 0.5 ‰ as cm/ca approaches 0, independent of the incoming CO2. As indicated in Fig. 2, the theoretically calculated Δ47 fractionation associated with diffusion is Δ47a–Δ47e = − 0.3 ‰ when all CO2 that enters the stomata is assimilated. In our leaf cuvette model where we assume a CO2 drawdown from 500 to 400 ppm (ζ = 500 / (500–400) = 5), this translates to ΔAΔ47 ≈ ζ × (Δ47a—Δ47e) =− 1.5 ‰). The fact that the model assumes that 2/3 of the CO2 leave the stomata again without exchanging isotopes explains quantitatively why the diffusion limited endmember is ζ × (Δ47a—Δ47e)/3 = − 0.5 ‰, independent of the Δ47 of CO2 entering the cuvette. Figure 7a shows that in this limit the value of Δ47a depends very strongly on Δ47e.
For the other extreme scenario cm/ca ≈ 1, Δ47a converges to the Δ47 value of CO2 at the CO2–H2O exchange site (Δ47 = 0.95 ‰), independent of the Δ47 value of incoming CO2. This reflects the exchange dominated case in Fig. 2. In this case, ΔAΔ47 strongly depends on Δ47e value of the CO2, for instance ΔAΔ47 will be ≈ 0.00 ‰ and ≈ − 0.75 ‰ for Δ47e of 0.95 ‰ and 1.1 ‰, respectively. This is similar to what Adnew et al.13 showed for the 17O-excess of CO2 in gas exchange experiments.
The leaf cuvette model calculations show that in principle photosynthetic gas exchange can deplete or enrich the Δ47 depending on the initial Δ47 value of the CO2 in the air surrounding the leaf, leaf temperature (via the Δ47 value at the exchange site) and the fraction of CO2 exchanged and diffused back to the atmosphere. However, photosynthesis will enrich Δ47 only if the Δ47 value of the CO2 entering the leaf is far lower than the Δ47 value of the CO2 at the CO2–H2O exchange site. In addition, Fig. 7b shows that when \(\Delta _{{47{\text{e}}}}\) is lower than the equilibrium value of 0.95 ‰, photosynthetic gas exchange cannot lead to \(\Delta _{{47{\text{a}}}}\) values above the equilibrium value. Thus, according to our leaf cuvette model, photosynthesis cannot lead to Δ47 values that are higher than the thermodynamic equilibrium (unless the incoming, i.e. ambient Δ47 values are already higher, in which case they would be reduced). This agrees well with most reported Δ47 values of atmospheric CO2 which are lower than expected from the CO2–H2O equilibrium at the surface temperature19,20,21.
As a result, an enrichment of up to 0.08 ‰ in Δ47 relative to the thermodynamic equilibrium value reported by Laskar and Liang22 for Δ47 measurements of CO2 sampled in a greenhouse cannot be explained by photosynthesis based on our leaf cuvette model results, even at the extreme scenario of cm/ca ~ 1. In addition, our results show that photosynthetic driven CO2–H2O isotope exchange affects Δ47 and δ18O in a similar way (Fig. 6, Figure S3 and S4) as observed for a simple CO2–H2O equilibration experiment32,33. This does not support the greenhouse experiment results of Laskar and Liang22 where they concluded that photosynthesis decouples δ18O and Δ47. We suggest that other processes than photosynthetic gas exchange affected the greenhouse gas experiments reported earlier22. Further experiments in similar environments should be carried out to investigate this in more detail.
Our results provide the experimental verification of the isotope exchange model suggested by Eiler and Schauble19. In particular, we determine how ΔAΔ47 varies as a function of cm/ca. At high cm/ca ratio (high back-diffusion flux), the effect of fractionation due to diffusion is negligible and the Δ47 of atmospheric CO2 will be driven towards the Δ47 of CO2 at the CO2–H2O exchange site. At low cm/ca ratio, the diffusion fractionation dominates and photosynthetic gas exchange will generally lower Δ47. For the real atmosphere with Δ47 values slightly lower than the thermodynamic equilibrium set by CO2–H2O exchange, photosynthetic gas exchange cannot increase Δ47 above this equilibrium value.
Our results also show that Δ47 and 18O discrimination are affected in similar ways during photosynthesis, but in contrast to 18O, the clumped isotope composition is independent of the δ18O of bulk leaf water. This means that it is not necessary to know the precise isotopic composition of water at the CO2–H2O exchange site for calculating ΔAΔ47. Furthermore, a disequilibrium in Δ47 is often identified more readily than in δ18O, since Δ47 depends mainly on CO2–H2O exchange temperature. As a result, measurements of Δ47 during air-leaf gas exchange experiments may be an alternative method to determine the mesophyll conductance to the site of CO2–H2O exchange and/or the degree of equilibration between CO2–H2O inside the leaf. The limitation to this approach is that the Δ47 signals are very small and it requires high precision measurements to constrain the relevant parameters significantly under ambient conditions.
For Helianthus, we found gm13 values of 0.27 ± 0.1 mol m−2 s−1 bar−1 at a PPFD of 200 μmol m−2 s−1, 0.54 ± 0.1 mol m−2 s−1 bar−1 at a PPFD of 700 μmol m−2 s−1 and 0.43 ± 0.05 mol m−2 s−1 bar−1 at a PPFD of 1800 μmol m−2 s−1, in good agreement with values reported in previous studies41,43,67,68. These observations confirm earlier findings that the mesophyll conductance is generally lower at low light intensities (Flexas et al., 2007), although we did not observe any significant difference between mid and high light conditions. For Hedera, we found a gm13 value of 0.20 ± 0.02 mol m−2 s−1 bar−1 at a PPFD of 700 μmol m−2 s−1, which is in good agreement with the maximum mesophyll conductance of 0.14 ± 0.01 mol m−2 s−1 bar−1 for evergreen angiosperms, including observations from the Hedera species41,43,51,69. The higher mesophyll conductance for Helianthus compared to Hedera might be due to the high mesophyll porosity and thin cell walls of mesophyll cells which facilitate easier movement of CO2 within intercellular airspaces and across cell walls as reported for evergreen woody plants70,71.
Mesophyll conductance (gm18) of Helianthus did not show a clear dependency on PPFD (Table 1 and Figure S2) with on average a value of 0.68 mol m−2 s−1 bar−1, which is in good agreement with the few values reported in the literature41,72. For maize, gm18 = 0.57 mol m−2 s−1 bar−1, which is within the wide range of 0.169 to 0.9 mol m−2 s−1 bar−1 reported in literature41,73,74,75. For Helianthus and Hedera, gm18 is on average 1.7 times gm13 confirming that CO2–H2O exchange occurs in the mesophyll cell, i.e., before the carboxylation site, in agreement with previous findings41,47,49,54.
Data availability
All the data used in this manuscript are presented in the form of Figures and Tables.
References
Affek, H. P. & Yakir, D. The stable isotopic composition of atmospheric CO2. 2 edn, 179–212 (Elsevier, 2014).
Ciais, P., Tans, P. P., Trolier, M., White, J. W. C., Francey, R. J. A large northern hemisphere terrestrial CO2 sink indicated by the 13C/12C ratio of atmospheric CO2 Science 269, 4 (1995).
Ciais, P. et al. A three-dimensional synthesis study of δ18O in atmospheric CO2: 1 Surface fluxes. J. Geophys. Res. Atmos. 102, 5857–5872. https://doi.org/10.1029/96jd02360 (1997).
Cuntz, M., Ciais, P., Hoffmann, G. & Knorr, W. A comprehensive global three-dimensional model of δ18O in atmospheric CO2: 1. Validation of surface processes. J. Geophys. Res. Atmos. 108, 24 (2003).
Cuntz, M. A dent in carbon’s gold standard. Nature 477, 547–548 (2011).
Welp, L. R. et al. Interannual variability in the oxygen isotopes of atmospheric CO2 driven by El Niño. Nature 477, 579–582. https://doi.org/10.1038/nature10421 (2011).
Adnew, G. A. et al. Determination of the triple oxygen and carbon isotopic composition of CO2 from atomic ion fragments formed in the ion source of the 253 Ultra High-Resolution Isotope Ratio Mass Spectrometer. Rapid Commun. Mass Spectrom. 33, 17 (2019).
Hoag, K. J., Still, C. J., Fung, I. Y. & Boering, K. A. Triple oxygen isotope composition of tropospheric carbon dioxide as a tracer of terrestrial gross carbon fluxes. Geophys. Res. Lett. https://doi.org/10.1029/2004gl021011 (2005).
Thiemens, M. K., Chakraborty, S. & Jackson, T. L. Decadal Δ17O record of tropospheric CO2: verification of a stratospheric component in the troposphere. J. Geophys. Res. Atmos. 119, 8 (2014).
Hofmann, M. E. G. et al. Atmospheric measurements of Δ17O in CO2 in Göttingen, Germany reveal a seasonal cycle driven by biospheric uptake. Geochim. Cosmochim. Ac. 199, 143–163. https://doi.org/10.1016/j.gca.2016.11.019 (2017).
Liang, M.-C., Mahata, S., Laskar, A. H., Thiemens, M. H. & Newman, S. Oxygen isotope anomaly in tropospheric CO2 and implications for CO2 residence time in the atmosphere and gross primary productivity. Sci. Rep. 7, 13180. https://doi.org/10.1038/s41598-017-12774-w (2017).
Koren, G. et al. Global 3-D Simulations of the Triple Oxygen Isotope Signature Δ17O in Atmospheric CO2. J. Geophys. Res. Atmos. 124, 28 (2019).
Adnew, G. A., Pons, T. L., Koren, G., Peters, W. & Röckmann, T. Leaf-scale quantification of the effect of photosynthetic gas exchange on Δ17O of atmospheric CO2. Biogeosciences 17, 3903–3922 (2020).
Liang, M.-C. & Mahata, S. Oxygen anomaly in near surface carbon dioxide reveals deep stratospheric intrusion. Sci. Rep. 5, 11352. https://doi.org/10.1038/srep11352 (2015).
Mahata, S., Chung-Ho, W., Bhattacharya, S. K. & Mao-Chang, L. Near Surface CO2 triple oxygen isotope composition. Terrest. Atmos. Ocean. Sci. 27, 7 (2016).
Laskar, A. M., Mahata, S., Bhattacharya, S. K. & Liang, M.-C. Triple oxygen and clumped isotope compositions of CO2 in the middle troposphere. Earth Space Sci. 6, 1205–1219 (2019).
Levin, I. a. K., Bernd and Schmidt, Martina and Sartorius, Hartmut. A novel approach for independent budgeting of fossil fuel CO2 over Europe by 14CO2 observations. Geophys. Res. Lett., 2194 (2003).
Turnbull, J. C. et al. Assessment of fossil fuel carbon dioxide and other anthropogenic trace gas emissions from airborne measurements over Sacramento, California in spring 2009. Atmos. Chem. Phys. 11, 705–721 (2011).
Eiler, J. M. & Schauble, E. 18O13C16O in Earth’s atmosphere. Geochim. Cosmochim. Acta 68, 10 (2004).
Affek, H. P. & Eiler, J. M. Abundance of mass 47 CO2 in urban air, car exhaust, and human breath. Geochim. Cosmochim. Acta. 70, 12. https://doi.org/10.1016/j.gca.2005.08.021 (2006).
Affek, H. P., Xu, X. & Eiler, J. M. Seasonal and diurnal variations of 13C18O16O in air: Initial observations from Pasadena CA. Geochim. Cosmochim. Acta 71, 5033–5043. https://doi.org/10.1016/j.gca.2007.08.014 (2007).
Laskar, A. H. & Liang, M.-C. Clumped isotopes in near-surface atmospheric CO2 over land, coast and ocean in Taiwan and its vicinity. Biogeosciences 13, 7 (2016).
Röckmann, T., Popa, M. E., Krol, M. C. & Hofmann, M. E. G. Statistical clumped isotope signatures. Sci. Rep. 6, 31947 (2016).
Wang, Z., Schauble, E. A. & Eiler, J. M. Equilibrium thermodynamics of multiply substituted isotopologues of molecular gases. Geochim. Cosmochim. Acta. 68, 4779–4797 (2004).
Dennis, K. J., Affek, H. P., Passey, B. H., Schrag, D. P. & Eiler, J. M. Defining an absolute reference frame for ‘clumped’isotope studies of CO2. Geochim. Cosmochim. Acta 75, 7117–7131 (2011).
Affek, H. P., Xu, X. & Eiler, J. M. Seasonal and diurnal variations of 13C18O16O in air: Initial observations from Pasadena CA. Geochim. Cosmochim. Acta 71, 10 (2007).
Francey, R. J. & Tans, P. P. Latitudinal variation in oxygen-18 of atmospheric CO2. Nature 327, 2 (1987).
Farquhar, G. D. et al. Vegetation effects on the isotope composition of oxygen in atmospheric CO2. Nature 363, 439–443 (1993).
Gillon, J. S. & Yakir, D. Influence of carbonic anhydrase activity in terrestrial vegetation on the 18O content of atmospheric CO2 Science 291, 3 (2001).
Wingate, L. et al. The impact of soil microorganisms on the global budget of δ18O in atmospheric CO2. PNAS 106, 4 (2009).
Ogée, J., Wingate, L. & Genty, B. Estimating mesophyll conductance from measurements of C18OO photosynthetic discrimination and carbonic anhydrase activity. Plant Physiol. 178, 728–752 (2018).
Affek, H. P. Clumped isotopic equilibrium and the rate of isotope exchange between CO2 and water. Am J. Sci. 313, 16. https://doi.org/10.2475/04.2013.02 (2013).
Clog, M., Stolper, D. & Eiler, J. M. Kinetics of CO2 (g)–H2O (l) isotopic exchange, including mass 47 isotopologues. Chem. Geol. 395, 10 (2015).
Dai, A. Recent climatology, variability, and trends in global surface humidity. J. Clim. 19, 17 (2006).
Daëron, M., Blamart, D., Peral, M. & Affek, H. P. Absolute isotopic abundance ratios and the accuracy of Δ47 measurements. Chem. Geol. 442, 13. https://doi.org/10.1016/j.chemgeo.2016.08.014 (2016).
Craig, H. Isotopic standards for carbon and oxygen and correction factors for mass-spectrometric analysis of carbon dioxide. Geochim Cosmochim Ac. 12, 16. https://doi.org/10.1016/0016-7037(57)90024-8 (1957).
Brand, W. A., Assonov, S. S. & Coplen, T. B. Correction for the 17O interference in δ13C measurements when analyzing CO2 with stable isotope mass spectrometry (IUPAC Technical Report). Pure Appl. Chem. 82, 1719–1733. https://doi.org/10.1351/pac-rep-09-01-05 (2010).
Schauer, A. J., Kelson, J., Saenger, C. & Huntington, K. W. Choice of 17O correction affects clumped isotope (Δ47) values of CO2 measured with mass spectrometry. Rapid Commun. Mass Spectrom. 30 (2016).
Ubierna, N. et al. Critical review: incorporating the arrangement of mitochondria and chloroplasts into models of photosynthesis and carbon isotope discrimination. Photosynth. Res. 141, 26 (2019).
Cousins, A. B., Mullendore, D. L., Sonawane, B. V. Recent developments in mesophyll conductance in C3, C4 and CAM plants Plant J. 101, 816–830 (2020).
Adnew, G. A. The effect of photosynthetic gas exchange on Δ17O of atmospheric CO2. PhD thesis. doi:https://doi.org/10.33540/412 (2020).
Farquhar, G. D., Ehleringer, J. R. & Hubick, K. T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 40, 503–537 (1989).
Flexas, J., Ribas-Carbo, M., Diaz-Espejo, A., Galmes, J. & Medrano, H. Mesophyll conductance to CO2: current knowledge and future prospects. Plant Cell Environ. 31, 19 (2008).
Busch, F. A., Holloway-Phillips, M., Stuart-Williams, H. & Farquhar, G. D. Revisiting carbon isotope discrimination in C3 plants shows respiration rules when photosynthesis is low. Nat. Plants 6, 245–258 (2020).
Farquhar, G. D. & Richards, R. A. Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Funct. Plant Biol. 11, 11 (1984).
Farquhar, D. G. & Lloyd, J. Carbon and oxygen isotope effects in the exchange of carbon dioxide between terrestrial plants and the atmosphere. 33 (Acadamic Press Inc., 1993).
Barbour, M. M., Evans, J. R., Simonin, K. A. & Caemmerer, S. v. Online CO2 and H2O oxygen isotope fractionation allows estimation of mesophyll conductance in C4 plants, and reveals that mesophyll conductance decreases as leaves age in both C4 and C3 plants. New Phytol., 875–889 (2016).
Evans, J. R., Sharkey, T. D., Berry, J. A. & Farquhar, G. D. Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 diffusion in leaves of higher plants. Funct Plant. Biol. 13, 11 (1986).
Gillon, J. S. & Yakir, D. Internal conductance to CO2 diffusion and C18OO discrimination in C3 leaves. Plant Physiol. 123, 13 (2000).
Caemmerer, S. v., Ghannoum, O., Pengelly, J. J. L. & Cousins, A. B. Carbon isotope discrimination as a tool to explore C4 photosynthesis. J. Exp. Bot. 65, 3459 (2014).
Pons, T. L. et al. Estimating mesophyll conductance to CO2: methodology, potential errors, and recommendations. J. Exp. Bot. 60, 7 (2009).
Holloway-Phillips, M., Cernusak, L. A., Stuart-Williams, H., Ubierna, N. & Farquhar, G. D. Two-source δ18O method to validate the CO18O-photosynthetic discrimination model: implications for mesophyll conductance. Plant Physiol. 181, 15 (2019).
Cernusak, L. A., Farquhar, G. D., Wong, S. C. & Stuart-Williams, H. Measurement and interpretation of the oxygen isotope composition of carbon dioxide respired by leaves in the dark. Plant Physiol. 136, 13 (2004).
Gillon, J. S. & Yakir, D. Naturally low carbonic anhydrase activity in C4 and C3 plants limits discrimination against C18OO during photosynthesis. Plant Cell Environ. 23, 12 (2000).
Song, X., Simonin, K. A., Loucos, K. E. & Barbour, M. M. Modelling non-steady-state isotope enrichment of leaf water in a gas-exchange cuvette environment. Plant Cell Environ. 38, 10 (2015).
Parkinson, K. J. A simple method for determining the boundary layer resistance in leaf cuvettes. Plant Cell Environ 8, 223–226 (1985).
Laskar, A. H., Mahata, S. & Liang, M.-C. Identification of anthropogenic CO2 using triple oxygen and clumped isotopes. Environ. Sci. Technol. 50, 18. https://doi.org/10.1021/acs.est.6b02989 (2016).
Defliese, W. F. & Lohmann, K. C. Non-linear mixing effects on mass-47 CO2 clumped isotope thermometry: patterns and implications. Rapid Commun. Mass Spectrom. 29, 901–909 (2017).
Koren, G., Adnew, G. A., Röckmann, T. & Peters, W. Leaf conductance model for 17O in CO2, https://git.wur.nl/leaf_model. (2020).
Pons, T. L. & Welschen, R. A. M. Technical report; Overestimation of respiration rates in commercially available clamp-on leaf chambers. Complications with measurement of net photosynthesis. Plant, Cell Environ. 25, 1367–1372. https://doi.org/10.1093/jxb/erp081 (2002).
von Caemmerer, S. & Farquhar, G. D. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153, 376–387 (1981).
Werner, R. A., Rothe, M. & Brand, W. A. Extraction of CO2 from air samples for isotopic analysis and limits to ultra high precision δ18O determination in CO2 gas. Rapid Commun. Mass Spectrom. 15, 2152–2167 (2001).
Werner, R. A. & Brand, W. A. Referencing strategies and techniques in stable isotope ratio analysis. Rapid Commun Mass Spectrom 15, 501–519. https://doi.org/10.1002/rcm.258 (2001).
Huntington, K. W. et al. Methods and limitations of ‘clumped’CO2 isotope (Δ47) analysis by gas-source isotope ratio mass spectrometry. J. Mass Spectrom. 44, 11 (2009).
Cernusak, L. A. et al. Stable isotopes in leaf water of terrestrial plants. Plant Cell Environ. 39, 1087–1102. https://doi.org/10.1111/pce.12703 (2016).
Farquhar, G. D., Cernusak, L. A. & Barnes, B. Heavy water fractionation during transpiration. Plant Physiol. 143, 7 (2007).
Hanba, Y. T., Kogami, H. & Terashima, I. The effect of internal CO2 conductance on leaf carbon isotope ratio. Isotopes Environ. Health Stud 39, 5–13. https://doi.org/10.1080/1025601031000102233 (2003).
Laisk, A. & Loreto, F. Determining photosynthetic parameters from leaf CO2 exchange and chlorophyll fluorescence (ribulose-1, 5-bisphosphate carboxylase/oxygenase specificity factor, dark respiration in the light, excitation distribution between photosystems, alternative electron transport rate, and mesophyll diffusion resistance. Plant Physiol. 110, 9 (1996).
Loreto, F., Harley, P. C., Marco, G. D. & Sharkey, T. D. Estimation of mesophyll conductance to CO2 flux by three different methods. Plant Physiol. 98, 6 (1992).
Veromann-Jürgenson, L.-L., Tosens, T., Laanisto, L. & Niinemets, ü. Extremely thick cell walls and low mesophyll conductance: welcome to the world of ancient living!. Journal of Experimental Botany 68 (2017).
Niinemets, Ü. Does the touch of cold make evergreen leaves tougher?. Tree Physiol. 36, 5 (2016).
Shrestha, A., Song, X. & Barbour, M. M. The temperature response of mesophyll conductance, and its component conductances, varies between species and genotypes. Photosynth. Res., 18 (2019).
Ubierna, N., Gandin, A., Boyd, R. A. & Cousins, A. B. Temperature response of mesophyll conductance in three C4 species calculated with two methods: 18O discrimination and in vitro Vpmax. New Phytol. 214, 66–80 (2017).
Kolbe, A. R. & Cousins, A. B. Mesophyll conductance in Zea mays responds transiently to CO2 availability: implications for transpiration efficiency in C4 crops. New Phytol. 217, 11 (2018).
Ubierna, N., Gandin, A. & Cousins, A. B. The response of mesophyll conductance to short-term variation in CO2 in the C4 plants Setaria viridis and Zea mays Journal of experimental botany 69, 11 (2018).
Acknowledgements
GAA is supported by EU Horizon 2020 ERC-ASICA project with a research grant number 64908 and project ALWPP.2016.013 of the Dutch Science Foundation NWO. GK is supported by EU Horizon 2020 ERC-ASICA project with a research grant number 649087. MEGH supported by Dutch Science Foundation NWO with Grant Number 822.01.014 for clumped isotopes in atmospheric CO2 project.
Author information
Authors and Affiliations
Contributions
M.E.G.H. and T.R. designed the experiment, T.P. and M.E.G.H. performed the leaf cuvette experiment and air sampling. G.A.A., T.P., M.E.G.H. and T.R. evaluated the data. G.K. provided the leaf cuvette model calculations. G.A.A., M.E.G.H. and T.R. wrote the manuscript with contributions from T.P., G.K., M.Z., and L.J.L.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Adnew, G.A., Hofmann, M.E.G., Pons, T.L. et al. Leaf scale quantification of the effect of photosynthetic gas exchange on Δ47 of CO2. Sci Rep 11, 14023 (2021). https://doi.org/10.1038/s41598-021-93092-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-93092-0
- Springer Nature Limited