Abstract
Surface texture of a dental material may cause nonaesthetic appearance, secondary caries and periodontal problem. Factors influencing surface roughness include the type of the material, its filler, and exposure to bacterial accumulation. The aim of this study was to investigate surface roughness of glass ionomer cement (GIC) and resin composite (CR). A total of 112 specimens consisting of Fuji II LC (microfilled GIC), Ketac N100 (nanofilled GIC), Z250 (microfilled composite) and Z350 (nanofilled composite) were used. All specimens were packed in acrylic mould, light cured for 40 s and polished with Sof-Lex discs. The samples were divided in<to two groups of 7 and 24 hrs (n=7). Ra values were assessed at baseline and after bacterial colonization using Atomic Force Microscope (AFM, Ambios, USA). The data was analyzed using SPSS version 20 and the level of significance had been set at p < 0.05. After 7 hrs of Streptococcus Mutans culture, only Fuji II LC showed a significant change in the Ra with p value of 0.031. Furthermore, Ra value of Fuji II LC was significantly different from the other test materials with P=0.000. There was also a significant difference in Z250 when compared to Ketac Nano.(Fuji II>Ketac Nano>Filtek Z250). After 24-hrs culture with Streptococcus Mutans, all materials were significantly different P=0.000 from each other, except between Z350 and Z250. (Fuji II>Ketac Nano>Filtek Z250=Filtek Z350). There was no significant difference between 7 and 24 hrs in all tested materials. In conclusion, in the early stages of bacterial growth; after 7 and 24 hrs culture with Streptococcus Mutans. the microfilled GIC shows the highest surface roughness, followed by nanofilled GIC and the resin composites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
I. Introduction
S URFACE texture of a dental material has a major influence on plaque accumulation, discoloration, and the aesthetical appearance of the restorations [1]. One of the determining factors for surface texture of a restorative material is the size of filler particles. Nowadays, there are many types of glass ionomers and resin composites available in the market which are based on the filler sizes. There are macrofilled, micronfilled, microhybrid and the recent technology is nanofilled. Glass ionomers are usually used in high caries risk patients due to their fluoride releasing property. However, one of the drawbacks of conventional glass ionomers is their large particle size, which contributes to the high surface roughness. Currently, nanotechnology is used to provide some value added features to glass ionomers. Nanofilled dental restorative material can offer high translucency, high polish and retention similar to those of microfills while maintaining physical properties and wear resistance equivalent to several commercial hybrid materials [2].
Another factor influencing the surface texture of a restorative material is the accumulation of plaque on its surface. Streptococcus mutans are among the bacteria consisting the dental biofilm. Their virulence is mainly due to their high adhesion capability, acidogenicity and aciduric properties, and these characteristics could be responsible for surface damage to restorations [3]. The amount of biofilm accumulation varies according to the particle size of fillers and monomer components of the resin matrix [4]. It was proven that the nanofilled composite cause reduction in the biofilm formation [5]. In our previous study[6], it was also found that biofilm thickness was higher in the microfilled GIC compared to the microfilled resin composite after 24 hrs in vivo. Although the GIC has fluoride releasing properties and antibacterial effects, the authors suggested that its surface characteristic plays a major important factor in determining its plaque retentiveness in the early periods. Montanaro et al (2004) also found GIC and ormocer group was highly susceptible to bacterial adherence compared to resin composite group [7]. They concluded that the release of fluoride from some of the test surfaces did not appear capable to reduce early bacterial adhesion.
In addition, Quirynen and Bollen, (1995) concluded both surface free energy and surface roughness of intra-oral hard surfaces have a major impact on the initial adhesion and the retention of oral microorganisms. However at supragingival plaque the demand for smooth surfaces with a low surface-free energy is required in order to minimise plaque formation. Wettability of the material surfaces also plays a role in microbial adhesion. Surfaces with high wettability or hydrophilic surface tend to adhere more microorganism compared to low wettability or hydrophobic surface [8].
Other factors such as incubation of bacteria and its duration of incubation will also determine the surface roughness of the material. The formation of biofilm is a complex process [7]. The mechanism of plaque accumulation is initiated from adsorption of proteins and bacteria to form a film on the tooth surface. There is the effect of van der Waals and electrostatic forces between microbial surfaces and the film to create reversible adhesion to the teeth. Next step of the process of biofilm formation involves the adhesion of the microbial cells, when bacteria begin to anchor to the surface [9]. Colonisation of the cell-free layer by bacteria, particularly by S. sanguis and S. mutans strains occurs within 24 hours, and plaque becomes physically visible between 12-24 hours. Further processes include the progressive build-up of plaque substance by bacterial polysaccharides, and the proliferation of filamentous and other bacteria as the plaque matures.
Studies on the surface roughness after bacterial accumulation, in relation to the nanofilled glass ionomers are still scarce. In our previous study [10], it was found that after 30 days of S. Mutans culture, Nanofilled glass-ionomer has less surface roughness than microfilled glass-ionomer, and its surface finish is similar to nanofilled composite. However, the effect of the early stages of bacterial accumulation on the surface roughness of the nanofilled glass ionomer is limited. Therefore, this study was conducted to assess the surface roughness of the nanofilled glass-ionomer cultured with S.mutans biofilm for 7 and 24 hours.
II. Materials and methods
A. Specimen Preparation
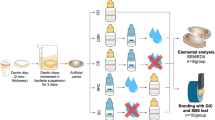
The materials used in this study are shown in Table 1. Acrylic resin moulds with the internal diameter of 5 mm and a thickness of 2 mm were used to prepare the specimens. The mould was placed on a clean glass slab and the test materials mixed according to manufacturer’s instruction and packed into the mould, covered with a Mylar strip and pressed flat with a microscopic glass slide. The disks were photo-polymerized according to manufacturer’s instruction using Elipar™ Free Light 2 (3M ESPE, USA) LED light curing unit, with a light intensity of 1000 mW/cm2. Following light curing, the specimens were placed into 37°C humidor for 24 hrs [11].
Fifty six similar disks, with 14 disks of each test material were prepared for testing. Convenient random sampling was used to divide the 56 disks of each test material into 2 groups (n=7), according to the Streptococcus mutans incubation period of either 7 hrs or 24 hrs. Sof-Lex™ disk (3M ESPE, USA) coarse (55 μm), medium (40 μm), fine (24 μm), and ultra fine (8 μm) aluminium oxide were used to polish all specimens using the same slow-speed handpiece at 10,000 rpm [11]. The polishing procedure consisted of repetitive strokes of ten seconds per step, to prevent heat buildup and formation of grooves. A conscious effort was made to standardize the strokes, downward force, and the number of strokes for each polishing procedure.
Baseline surface roughness of the specimens was evaluated using AFM (AMBIOS Technology Inc., USA). The measurements were made in three different locations, and the means of these measurements were considered as a surface roughness of each sample. The specimens were sterilized with 70% Ethanol solution and distilled water, and then run through with UV light for 30 min.
Stock of Streptococcus mutans was prepared in brain heart infusion broth solution. Then, the broth were incubated with temperature at 37°C for 48 hrs to increase population of Streptococcus mutans. Then, 40 ml of broth solution was placed in sterile containers using pipette. Specimens (type of tooth coloured materials) were placed in the containers containing broth solution, and incubated at 37°C for 7 hrs and 24 hrs depending on group.
After incubation the specimens were taken out from the containers and dried. Images of the specimens were taken using SEM. Then, the specimens were washed with 70% Ethanol solution and distilled water, and dried. The reading of surface roughness (Ra) for each specimen was taken again, using AFM.
III. Results
Comparison of means and standard deviations the of Ra values (nm) of the test materials at baseline, and after 7 hrs of Streptococcus Mutans culture is shown in Table 2. Fuji II LC showed a significant change in the Ra value after being cultured with Streptococcus Mutans for 7 hrs with p value of 0.031.
Table 3 shows the means and standard deviations of the Ra Values (nm) between the test materials after 7 hrs of S. Mutans culture. The post-hoc Tukey test shows that there were significant difference between Fuji II LC when it was compared to the other three materials with P=0.000. There was also a significant difference in Z250 when compared to Ketac Nano with P=0.018. Fig. 1 illustrates the morphology of the test materials.
However, after 24 hrs of culture with Streptococcus Mutans, there were no significant changes in the Ra values of all tested materials, as shown in Table 4.
When the comparison was made after 24 hrs culture with Streptococcus Mutans, all materials were significantly different P=0.000 from each other, except between Z350 and Z250 with P=0.979, as shown in Table 5 and Fig. 2.
There was no significant difference in the Ra values of all tested materials when the comparison was made between 7 hrs and 24 hrs of culture with S. Mutans. (Table 6).
IV. Discussion
There was a significant increase in the surface roughnesof Fuji II LC after 7 hrs of culture with S. Mutans. When the comparison was made between test materials at 7 hrs, again, Fuji II LC showed the roughest surface compared to the other materials. Our result is in agreement with Montanaro et al. (2004) they concluded that the release of fluoride from some of the test surfaces did not appear capable to reduce early bacterial adhesion in GIC and ormocer [7]. After 7 hrs of culture, the bacteria start to produce acid, which may soften the surface of the GIC, and cause the plucking out effect of Fuji II LC, which has larger filler, leaving its surface rougher.
Ketac N100 showed a higher surface roughness than Filtek Z250 after 7 hrs of culture with S. Mutans. Even though Ketac N100 contains nano particles, however, the resin composition between the two materials are different, in which Ketac N100 only contains HEMA, thus the softening effect occurs more in Ketac N100.
There was no change in the surface roughness in all test materials when the comparison was made between baseline and after 24 hrs of culture with S. Mutans. However, when the comparison was made between the test materials at 24 hrs, Fuji II LC showed the highest surface roughness, followed by Ketac N100. Filtek Z250 and Filtek Z350 both showed the lowest surface roughness. This significance difference may due to the fact that resin composite naturally has smooth surface compared to GIC. Therefore, less bacterial accumulation occurs in resin composite compared to GIC, which contribute to the less surface roughness of CR. Einwag et al. (1990) examined the influence of the surface roughness of dental filling materials on plaque accumulation and found that Streptococcus mutans adhered more frequently to rough cements than to filling materials [12].
It is observed that the nanofilled GIC showed significantly less surface roughness than microfilled type. Again, this may be explained by the plucking out effect of Fuji II LC, which has larger filler, leaving its surface rougher.
There was no significant difference in the surface roughness of all tested materials between 7 hrs and 24 hrs of bacterial incubation. This result may be explained by the bacterial growth curve theory. The curve consists of several phases which are lag phase, log (exponential) phase, stationary phase and death phase. The period between 7 hrs and 24 hrs may fall in the stationary phase, in which the S. Mutans population did not increase.
Our limitations of this study whereby all the procedures were done in vitro which needs further observation for oral simulating environment, such as using saliva for future study.
V. Conclusion
In conclusion, in the early stages of bacterial growth, after 7 and 24 hrs culture with Streptococcus Mutans. the microfilled GIC shows the highest surface roughness, followed by nanofilled GIC and the resin composites.
Significance of the study in assisting clinicians, it is recommended that for patient with high risk of plaque accumulation to use resin composite followed by nanofilled GIC, instead of using microfilled GIC as their first choice.
Acknowledgment
We would like to express our special thanks to all individuals that help us during our research conducted.
References
M. Morgan, “Finishing and polishing of direct posterior resin restorations,” Pract Proced Aesthet Dent, vol. 16, no. 3, pp. 211–217, 2004.
S. B. Mitra, D. Wu and B. N. Holmes, “An application of nanotechnology in advanced dental materials,” J Am Dent Assoc, vol. 134, no. 10, pp. 1382–1390, 2003.
M. Hannig, L. Kriener, W. Hoth-Hannig, C. Becker-Willinger and H. Schmidt, “Influence of nanocomposite surface coating on biofilm formation in situ,” J Nanoscie and Nanotech, vol. 7, no. 12, pp. 4642-4648, 2007.
M. Ono, T. Nikaido, M. Ikeda, S. Imai, N. Hanada, J. Tagami and K. Matin, “Surface properties of resin composite materials relative to biofilm formation,” Dent Mater J, vol. 26, no. 5, pp. 613–622, 2007.
M. Quirynen, H. C. Van der Mei, C. M. Bollen, L. H. Van den Bossche, G. I. Doornbusch, D. van Steenberghe and H. J. Busscher, “The influence of surface-free energy on supra- and subgingival plaque microbiology. An in vivo study on implants,” J Perio, vol. 65, no. 2, pp. 162–167, 1994.
D Mohamad, M Omar, S Ahmad, “Confocal Laser Scanning Microscopic (CLSM) study of early plaque formed on tooth-coloured restorative materials,” J Micros Malays, vol.3, pp. 79–87, 2007.
L. Montanaro, D. Campoccia, S. Rizzi, M. E. Donati, L. Breschi, C. Prati, C. R. Arciola, “Evaluation of Streptoccocus mutans on dental restorative materials,” Biomater, vol. 25, pp. 4457–4463, 2004.
M. Quirynen* and C. M. L. Bollen. “The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man,” Journal of Clinical Periodontology. vol. 22, no. 1, pp. 1–14, 1995
M. Hannig, “Transmission electron microscopic study of in vivo pellicle formation on dental restorative materials,” Eur J Oral Sci. vol.105 pp. 422-33, 1997.
Z. H. Nema, Z.Ab.Ghani, D. Mohamad, S. M. Masudi, “Surface Roughness of Nano-Glassionomer Polished With Different Polishing Methods After 30 Days S. Mutans Biofilm Culture.” Archives of BioCeramic Research, vol 10, pp. 78–82, 2010.
Z. Ab-Ghani, H. Ngo and J. Mclntyre, Effect of remineralization/demineralization cycles on mineral profiles of fuji ix fast in vitro using electron probe microanalysis, Austr Dent J. vol 52, no. 4. 2007.
J. Einwag, A.Ulrich and F. Gehring, “In-vitro plaque accumulation on different filling materials,” Oral pro-phylaxe, vol. 12, No. 1, pp. 22–25, 1990.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
D Mohamad was born in Pasir Puteh, Kelantan, Malaysia on 30th May 1975. She joined Shool of Dental Sciences, USM in 2005 as a lecturer and was appointed as a senior lecturer in 2008. Her studies at undergraduate and postgraduate levels were both in the United Kingdom. She graduated with BEng Chem Eng (Hon) from The University of Birmingham in 1998. Later in 2002 she obtained an MSc in Polymer Science and Technology from UMIST, Manchester. She was awarded her PhD in 2005 from The University of Manchester in the field of Biomaterials.
She has published in several indexed and non-indexed journals including a few journals of high impact factors.
Dr. Mohamad is a Vice President of Malaysian Biomaterials Society since 2010 and a member of Young Scientist Network under the umbrella of Akademi Sains Malaysia. She has won several medals during innovation exhibitions at international level.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohamad, D., Ab-Ghani, Z., Sidek, M. et al. Surface Roughness of Tooth Coloured Materials after Streptococcus Mutans Culture. GSTF J Adv Med Res 1, 13 (2014). https://doi.org/10.7603/s40782-014-0013-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.7603/s40782-014-0013-x