Abstract
This study investigated the influence of silver diamine fluoride (SDF) on the shear bond strength (SBS) to artificial carious dentin and GIC restorations with various SDF application protocols. Artificial caries were prepared on human dentin discs using bacteria model. These samples were randomly allocated to five groups (n = 10/group) according to the following treatment: (1) control group (CD): no treatment (2) CSR: dentin conditioner, SDF, and rinsing (3) CS: dentin conditioner and SDF (4) SRC: SDF, rinsing and dentin conditioner, and (5) SC: SDF and dentin conditioner. The treated-dentin surface was bonded with GIC and subjected to SBS test. Mean SBS was analyzed using one-way ANOVA. Surface morphology and elemental contents after surface treatment were examined (n = 3/group) by scanning electron microscopy with energy-dispersive X-ray spectroscopy (SEM/EDX). There was no significant difference in the mean SBS among CD (2.45 ± 0.99 MPa), CSR (1.76 ± 0.65 MPa), and SRC (2.64 ± 0.95 MPa). Meanwhile, the mean SBS of CS (0.35 ± 0.21 MPa) was significantly lower than the control and SRC group. SEM/EDX demonstrated deeper silver penetration in CSR and CS groups when compared to SRC and SC groups. SDF-modified GIC restorations resulted in significantly lower bond strength in CS and SC groups. The findings suggested treating the carious dentin surface with CSR and SRC protocol. SDF-treated carious dentin should be rinsed off prior to restore with GIC.
Similar content being viewed by others
Introduction
Deep carious lesions can be challenging to restore in clinical practice. Traditional guidelines advocate nonselective caries removal to control cariogenic activity and provide well-mineralized dentin to achieve successful and long-lasting restorations1. This method can easily lead to accidental pulpal exposure, which is considered a highly unfavorable prognostic factor1,2. Recently, so-called “selective carious tissue removal” was proposed. With this technique, peripheral caries are completely removed, whereas the firm and leathery carious dentin over the pulpal surface is left. Therefore, the chance of pulp exposure is minimized2. However, leaving carious dentin may compromise restoration longevity, mainly because of mechanical and adhesive reasons3,4,5,6. Ricucci et al.7 demonstrated that the residual bacteria underneath the restoration provoked an inflammatory response with an absence of clinical symptoms during the entire observation period. Therefore, there is a risk of clinical failure due to reinfection or pulpitis when viable bacteria are present in dentin7,8.
Silver diamine fluoride (SDF) is a topical fluoride used to clinically prevent and arrest dental caries in young children9,10,11,12 and older adults13,14,15. The combination of silver and fluoride has a synergistic effect that arrests active dentin caries16. Regarding the antibacterial effect, silver ions react with the thiol groups of amino and nucleic acids, disrupting bacterial metabolic and reproductive pathways and inducing cell death17,18. Furthermore, the reactivation of SDF (zombie effect) is also observed when dead silver-containing bacterial cells make contact with and kill living bacterial cells. This reservoir effect helps explain why silver deposited on bacteria and dentin proteins within a cavity has sustained antimicrobial effects19. Meanwhile, fluoride ions promote the remineralization of dental tissues by forming fluorapatite and fluorohydroxyapatite on the tooth surface16. Due to these benefits, it has been suggested to apply SDF before restoration to prevent secondary caries lesions and improve the biomechanical and biochemical properties of carious dentin20,21.
SDF has been recommended for application to residual carious dentin prior to permanent restoration with glass-ionomer cement (GIC) in a silver modified atraumatic restorative treatment (SMART) procedure22,23,24. This technique is often used in transitional or definitive restorations for high-risk caries or less cooperative patients, including providing care outside the dental office25. However, the results from previous studies are inconsistent26,27,28,29,30,31,32. This could be due to the lack of consensus regarding the protocol for SDF application, (39) which might be related to the success of the bonding performance. To date, no studies have directly compared the effectiveness of surface conditioners and rinsing steps immediately after SDF treatment to the bonding ability of GICs on carious dentin. Therefore, the aim of this study was to investigate the influence of SDF on the shear bond strength (SBS) of artificial carious dentin and GIC restorations with various SDF application protocols. The null hypothesis in the current study was that the application protocol does not affect the SBS of GIC and the amount of silver remnant.
Materials and methods
Specimen preparation
The protocol of this study was reviewed by the Ethics Committee for Human Research of the local university (HE622155). Sixty-five extracted human third molars were used in this study. All teeth selected for the experiment exhibited mature features of an intact crown and a root without a crack line on the surface. If either cavitated lesions or existing restoration was detected, the tooth was excluded from the study. All teeth were curetted to remove gingival tissues and calcium remnants and subsequently rinsed with distilled water prior to storage in 0.1% thymol solution at room temperature and used within 6 months.
The occlusal dentin disc, approximately 2 mm thick, was prepared perpendicularly to the long axis of teeth with a model trimmer to expose the flat dentin surface at a mid-coronal level. The first cut on the crown was created 2 mm from the cementoenamel junction, and the second cut was performed 2 mm from the first cut and parallel to it. Then, all dentin discs were disinfected under UV light for 1 h on each side before the experiment.
Cariogenic biofilm challenge
The microorganisms used for the cariogenic biofilm challenge were Streptococcus mutans (ATCC 25,175) and Lactobacillus acidophilus (ATCC 4356). The bacteria were cultivated on brain heart infusion (BHI) agar plates and de Man Rogosa and Sharpe (MRS) agar plates until isolated colonies were visible (5% CO2 concentration, 37 °C for 48 h). Next, the grown colonies were collected and separately resuspended in tubes containing MRS broth and incubated for 24 h. At the end of this culture period, the concentration of each bacteria was adjusted to 107 CFU/mL using a spectrophotometer wavelength of 600 nm (the optical density was 0.5). Subsequently, each bacterial suspension was equally mixed (1:1 ratio of mixture) in 5% sucrose solution and incubated for at least 1 h or until the pH was 5–6. Finally, 1 mL of bacterial suspension was incubated with each dentin disc in a 12-well plate. The plates were incubated for 3 days, and the medium was refreshed daily to induce the formation of carious lesions.
Experimental treatment
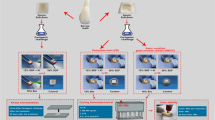
A schematic illustration of the study design is shown in Fig. 1. Sixty-five carious dentin discs were divided into five subgroups, and the dentin surfaces were treated with the following protocols, as shown in Table 1.
A schematic illustration representing the procedures used in the current study. SDF 38% silver diamine fluoride; GIC conventional glass ionomer cement; conditioner conditioner, 10% polyalkenoic acid; CD Carious dentin; CSR dentin conditioner + SDF + rinsing with water; CS dentin conditioner + SDF + no rinsing; SRC SDF + rinsing with water + dentin conditioner; SC SDF + dentin conditioner.
Assessment of shear bond strength (SBS) and fracture mode
Immediately after dentin discs were treated with the protocol, the biofilm on the carious dentin surface was removed by ultrasonication before GIC restoration. After that, a plastic tube 4 mm in diameter and 2 mm in height was placed on the treated surface. GICs were mixed using a rotational capsule mixing unit (Fuji IX GP Extra Capsule, GC America Inc.) for 10 s, and then the mixture was injected into a plastic tube to form a cylindrical button. The GIC was compressed with a mylar strip to ensure good adaptation. After 5 min of setting, the tube was removed, and the GIC-dentin specimens were stored at 100% relative humidity and incubated at 37 °C for 24 h to ensure GIC maturation before the bond strength test.
The SBS test (n = 10/group) was performed with a universal testing machine with a knife-edge loading head (Universal testing machine; LR30K, Lloyd instruments, UK). A shear force was applied perpendicularly to the GIC–dentin interface with loading at a fixed rate of 1 mm/minute. The maximum load used to debond GICs was recorded in newtons. The bond strength was expressed in megapascals (MPa) by dividing the load at failure by the bonded surface area in square millimeters.
The fracture surface was examined under an optical microscope at 20 × magnification. The failure modes were categorized into three types:
-
Type 1: adhesive failure between dentin and GICs and dentin surfaces.
-
Type 2: cohesive failure in GICs or dentin.
-
Type 3: mixed failure; partially adhesive failure and partially cohesive failure.
Assessment of surface morphology and elemental analysis
Fifteen carious dentin discs were prepared as previously described. After treatment according to the 5 experimental groups (n = 3), all specimens were stored at 100% relative humidity and incubated at 37 °C for 24 h. Then, the specimens were carefully fractured (long axis) by a scalpel blade and a hammer and dried at room temperature for 24 h.
One half of the fractured specimen was examined through elemental mapping analysis to detect the presence and distribution of silver, fluoride, phosphorous, and calcium ions of the precipitate on the surface of the specimens (n = 3/group) via energy–dispersive X–ray spectrometry (EDX) under scanning electron microscopy (SEM) at a magnification of 2500x (SEM, SU3800, Hitachi Ltd., Tokyo, Japan).
The exposed cross-section surface of the other half of the fractured specimen was examined using elemental line analysis to determine the deposition of silver ions and other ions at the targeted line via EDX under SEM. The targeted line, which was 250 μm in length from the surface of the specimen toward the pulp, was randomly selected.
Statistical analysis
Data were analyzed using SPSS Premium V.28 for Mac OS (IBM, Armonk, NY, USA) for statistical analysis. The SBS data were reported in the study as means and SDs. The normality of data was assessed using the Shapiro–Wilk test. For normally distributed results, one-way ANOVA followed by the Dunnett T3 test post hoc multiple-comparison test was employed to analyze the SBS data. In addition, the failure mode was descriptively reported as a percentage. For all the analyses, the significance level was set at P–value < 0.05.
The post hoc power analysis was performed using G*Power version 3.1.9.6 (University of Dusseldorf, Dusseldorf, Germany). The effect size of the SBS experiment was calculated from the results obtained in the current study, which demonstrated that the sample size used in each test exhibited a power > 0.95 at alpha = 0.05.
Finally, the SEM/EDX images of silver ions and other deposition data were used for descriptive analysis.
Ethics approval
All procedures performed in this study were in accordance with the principles of the Declaration of Helsinki. The ethics committee of Khon Kaen University approved this study with the code HE622115.
Informed consent
All specimens could not identify the participants, and their information was anonymous. For the above reasons, the need for informed consent has been waived by Khon Kaen University Ethics Committee for Human Research.
Results
Assessment of shear bond strength (SBS) and fracture mode
No pretest failure was observed in this study. The means and standard deviations of SBS are presented in Fig. 2. The highest SBS (2.64 ± 0.95 MPa) was observed in the SRC group, whereas the lowest SBS (0.35 ± 0.21 MPa) was observed in the CS group. Only the CSR and SRC groups demonstrated no significant difference compared to the CD group (CSR; P = 0.521, SRC; P > 0.05, respectively).
Mean of SBS (MPa) and percentage of fracture mode of GIC to carious dentin after SDF application with various surface treatment methods. Different letters indicate statistically significant differences at P < 0.05 with Dunnett T3 post hoc test. SBS shear bond strength; CD Carious dentin; CSR dentin conditioner + SDF + rinsing with water; CS dentin conditioner + SDF + no rinsing; SRC SDF + rinsing with water + dentin conditioner; SC SDF + dentin conditioner; Ad adhesive failure, and Mix mix failure. All data analyzed during this study are included in the Supplementary Information.
Adhesive failure was the most prevalent type of failure in all groups regardless of the surface treatment (Fig. 2 and Supplementary Information). Notably, an increase in mixed failure was observed in the groups treated with conditioner after SDF treatment (SRC and SC).
Assessment of surface morphology and elemental analysis
Figure 3 shows representative images of the SEM/EDX analysis. In the CD group, the smear layer and smear plug on the surface were absent (Fig. 3a). In addition, all dentinal tubules were opened with demineralized peritubular dentin. The CD group showed a decrease in calcium and phosphate compared to healthy dentin (Supplementary Information). Notably, silver deposition was not detected in the CD group (Fig. 3f and k).
SEM at 2500X magnification (a–e), EDX elemental mapping (f–j) and EDX spectra of carious dentin (k–o), after SDF application with various surface treatment methods. CD Carious dentin; CSR dentin conditioner + SDF + rinsing with water; CS dentin conditioner + SDF + no rinsing; SRC SDF + rinsing with water + dentin conditioner; SC SDF + dentin conditioner; Ag silver (pink); F fluoride (red); Ca calcium (blue); P phosphorus (green). White arrows indicate the exposed calcium ion.
For the experimental groups, the entire surfaces were covered with a silver–enriched layer (Fig. 3g–j), especially the CS (Fig. 3h) and SC (Fig. 3j) groups, in which other elements underneath the silver layer were barely detected. Moreover, the CSR and SRC groups exhibited a loose silver layer, leading to the detection of the colocalization of silver and calcium in some areas in the CSR group (Fig. 3g) and the robust detection of the same in the SRC group (Fig. 3i). Notably, all dentinal tubules were opened in the CSR and SRC groups (Fig. 3b and d, respectively).
The SEM/EDX elemental line analysis is shown in Fig. 4a–e. All groups revealed a decrease in calcium and phosphate until approximately 80 μm depth compared to sound dentin, which presented a high intensity of calcium and phosphate along the cross-sectional surface (Supplementary Information). In addition, a silver-enriched layer of approximately 20 μm was found on the carious dentin surface in the CSR and CS groups (Fig. 4b and c, respectively), which was thicker than that in the SRC and SC groups at approximately 10 μm thickness (Fig. 4d and e, respectively).
SEM at 500X magnification (a–e) and EDX line–scan representing element profile (Ag, F, Ca, and P element) along the path (line in a–e). CD Carious dentin; CSR dentin conditioner + SDF + rinsing with water; CS dentin conditioner + SDF + no rinsing; SRC SDF + rinsing with water + dentin conditioner; SC SDF + dentin conditioner; Ag silver.
Discussion
This study investigated the influence of various SDF treatment protocols on artificial carious dentin induced by biofilms before restoration with GIC. According to the results of this study, normal dentin presented higher SBS than carious dentin (Supplementary Information). The SBS of GIC of the carious dentin (CD) group was not significantly different from those of the SRC and CSR groups (P > 0.05). However, significantly lower SBS was observed in the CS and SC groups (P < 0.05). Therefore, the null hypothesis was rejected.
It was reported that a silver phosphate was formed on the SDF-treated dentin surface after SDF application33, and free silver particles extended into the dentinal tubules, totally or partially obstructing them34. These mechanisms might affect both the mechanical interlocking and chemical bonding of the GIC to SDF-modified dentin. Therefore, many publications have recommended eliminating excess silver precipitation to minimize the negative effect of SDF on dentin29,30,32,35,36,37. However, previous studies have reported contradictory outcomes regarding the nonstandard protocol for SDF application. Some studies demonstrated that SDF had no effect on the bond strength of GIC to dentin26,27,29,30, while others reported that SDF reduced the bond strength32,38. These inconsistent results might be due to the different surface treatment protocols used in the previous studies.
This study reveals that the bond strength of GIC to artificial carious dentin was affected by SDF and the different surface treatment procedures. The SDF application without removing excess silver precipitate through a rinsing step, as in the CS and SC groups, led to a significantly lower bond strength compared to that in the untreated carious dentin surface (CD group). This finding is in agreement with previous studies32,38. This could be due to residual silver that hinders the adhesion between GIC and underlying dentin. This is supported by our EDX analysis demonstrating the dense silver-enriched layer that covered the dentin in the CS and SC groups (Fig. 3c and e, respectively). Such a layer can alter the surface energy and the degree of wetness on the dentin surface39, impairing the binding of GIC to the underlying dentin. Moreover, this layer probably also prevents intimate contact between GIC and dentin. Therefore, there is less opportunity for bonding between the carboxylate groups in the GIC and the calcium ions from the hydroxyapatite40. Finally, the SDF-treated surfaces without rinsing treatment may present excessive alkalinity due to the high pH of SDF36. Such pH can impede the acid–base reaction of GIC and adversely affect the tooth-GIC interface40.
Regarding the CSR and SRC groups, no statistical significance compared to the untreated carious dentin surface was detected. This is supported by previous studies demonstrating no effect of SDF on the bond strength when a rinsing step was applied before GIC restoration26,27,29,30. The adverse effect on the bond strength of GIC seems to be eliminated by rinsing SDF off before GIC restoration. However, the sequence of dentin conditioner application (before or after SDF application) did not affect the bond strength of GIC to SDF-treated carious dentin.
When the rinsing step was applied after SDF application, the excess free silver ions might be removed by the rinsing water from the superficial surface. Therefore, a looser silver-enriched layer and obvious open dentinal tubules were observed in the CSR and SRC groups (Fig. 3g and i, respectively). This technique may be practical for modifying SDF-treated surfaces, allowing freshly placed GIC to penetrate the underlying dentin, leading to a proper micromechanical interlocking and chemical interaction39. Additionally, EDX elemental mapping revealed the presence of calcium ions on the SDF-treated surfaces in the CSR (Fig. 4b) and SRC groups (Fig. 4d). This result might facilitate the chance of developing the chemical bond between calcium ions from the hydroxyapatite and carboxylate functional groups from the GIC41, which likely improves the SBS in the SRC and CSR groups. On the other hand, no calcium ions were detected in the SC and CS groups, which is consistent with previous studies36,42,43. Therefore, a rinsing step after SDF application is strongly recommended to minimize free silver precipitation and reduce the alkalinity of the SDF-treated surface. This protocol may enhance the bond strength for long-lasting restoration, including maintaining the SDF therapeutic effect due to the residual silver precipitates on the SDF-treated surface.
When SDF is applied to the tooth structure, silver ions are released from the diamine silver ion complex, leading to the penetration and deposition of silver particles into the tooth structure44. This study showed that the silver-enriched layer thickness on artificial carious dentin was up to 20 µm using EDX line scanning analysis. However, a difference in silver-enriched layer thickness among groups was observed, which might depend on the order of PAA application. As demonstrated in Fig. 3a (CD group), PAA is a mild acid45 that can remove the smear layer and facilitate partial demineralization. Therefore, if SDF is applied on the PAA-treated carious dentin surface, the zone of exposed collagen is subsequently infiltrated with silver particles46,47 to form a thicker silver-enriched layer, as found in the CS and CSR groups. This finding indicated that dentin surface conditioning with PAA before SDF application could exert a positive effect. This probably promotes silver ion deposition deep into artificial carious dentin, resulting in a thorough silver-based antimicrobial effect48,49. Furthermore, the remaining silver ions probably continue to react with hydroxyapatite, forming silver phosphate (Ag3PO4). This compound might transform into other compounds and release phosphate ions to initiate apatite formation, leading to the remineralization of demineralized dentin50. Moreover, the presence of stable silver precipitates can introduce antimicrobial and anticariogenic properties and, thus, may be very useful for improving the resistance of restorations to secondary caries. Additionally, various forms of particle silver aggregates may improve the mechanical properties of this altered carious dentin substrate. However, the mechanism of particle formation might need further investigation.
The clinical implication of this finding is that if SDF is used to treat residual caries prior to GIC restoration, the bond strength of GIC on the carious dentin might be impaired. However, the beneficial effect of rinsing SDF off was demonstrated, which seems to eliminate this adverse effect. Therefore, for SDF-modified GIC restorations, it is suggested to apply SDF and rinse it off before PAA application, as in the SCR group. Applying PAA before SDF, as in the CSR group, is also favorable since it facilitates more effective silver ion absorption into the collagen network. However, the effect of SDF precipitated on dentin in long-term studies or randomized clinical trials might be necessary.
Conclusion
SDF-modified GIC restoration resulted in significantly lower bond strength in the CS and SC groups. The findings supported the treatment of carious dentin surface with CSR and SRC protocols.
References
Li, T., Zhai, X., Song, F. & Zhu, H. Selective versus non-selective removal for dental caries: A systematic review and meta-analysis. Acta Odontol. Scand. 76, 135–140. https://doi.org/10.1080/00016357.2017.1392602 (2018).
Bjørndal, L., Simon, S., Tomson, P. L. & Duncan, H. F. Management of deep caries and the exposed pulp. Int. Endod. J. 52, 949–973. https://doi.org/10.1111/iej.13128 (2019).
Nicoloso, G. F. et al. The bonding performance of a universal adhesive to artificially-created caries-affected dentin. J. Adhes. Dent. 19, 317–321. https://doi.org/10.3290/j.jad.a38890 (2017).
Kucukyilmaz, E. et al. Fluoride release/recharging ability and bond strength of glass ionomer cements to sound and caries-affected dentin. Niger. J. Clin. Pract. 20, 226–234. https://doi.org/10.4103/1119-3077.178917 (2017).
Burrow, M. F., Bokas, J., Tanumiharja, M. & Tyas, M. J. Microtensile bond strengths to caries-affected dentine treated with Carisolv®. Aust. Dent. J. 48, 110–114. https://doi.org/10.1111/j.1834-7819.2003.tb00018.x (2003).
Hevinga, M. A. et al. Does incomplete caries removal reduce strength of restored teeth?. J. Dent. Res. 89, 1270–1275. https://doi.org/10.1177/0022034510377790 (2010).
Ricucci, D. et al. Pulp and dentine responses to selective caries excavation: A histological and histobacteriological human study. J. Dent. 100, 103430. https://doi.org/10.1016/j.jdent.2020.103430 (2020).
Bin-Shuwaish, M. S. Effects and effectiveness of cavity disinfectants in operative dentistry: A literature review. J. Contemp. Dent. Pract. 17, 867–879. https://doi.org/10.5005/jp-journals-10024-1946 (2016).
Yee, R. et al. Efficacy of silver diamine fluoride for arresting caries treatment. J. Dent. Res. 88, 644–647. https://doi.org/10.1177/0022034509338671 (2009).
Zhi, Q. H., Lo, E. C. M. & Lin, H. C. Randomized clinical trial on effectiveness of silver diamine fluoride and glass ionomer in arresting dentine caries in preschool children. J. Dent. 40, 962–967. https://doi.org/10.1016/j.jdent.2012.08.002 (2012).
Duangthip, D., Wong, M. C. M., Chu, C. H. & Lo, E. C. M. Caries arrest by topical fluorides in preschool children: 30-month results. J. Dent. 70, 74–79. https://doi.org/10.1016/j.jdent.2017.12.013 (2018).
Fung, M. H. T. et al. Randomized clinical trial of 12% and 38% silver diamine fluoride treatment. J. Dent. Res. 97, 171–178. https://doi.org/10.1177/0022034517728496 (2018).
Tan, H. P. et al. A randomized trial on root caries prevention in elders. J. Dent. Res. 89, 1086–1090. https://doi.org/10.1177/0022034510375825 (2010).
Zhang, W., McGrath, C., Lo, E. C. M. & Li, J. Y. Silver diamine fluoride and education to prevent and arrest root caries among community-dwelling elders. Caries Res. 47, 284–290. https://doi.org/10.1159/000346620 (2013).
Li, R. et al. Randomized clinical trial on arresting dental root caries through silver diammine fluoride applications in community-dwelling elders. J. Dent. 51, 15–20. https://doi.org/10.1016/j.jdent.2016.05.005 (2016).
Mei, M. L., Lo, E. C. M. & Chu, C. H. Arresting dentine caries with silver diamine fluoride: What’s behind it?. J. Dent. Res. 97, 751–758. https://doi.org/10.1177/0022034518774783 (2018).
Thibodeau, E. A., Handelman, S. L. & Marquis, R. E. Inhibition and killing of oral bacteria by silver ions generated with low intensity direct current. J. Dent. Res. 57, 922–926. https://doi.org/10.1177/00220345780570091901 (1978).
Oppermann, R. V., Rølla, G., Johansen, J. R. & Assev, S. Thiol groups and reduced acidogenicity of dental plaque in the presence of metal ions in vivo. Eur. J. Oral Sci. 88, 389–396. https://doi.org/10.1111/j.1600-0722.1980.tb01244.x (1980).
Wakshlak, R. B. K., Pedahzur, R. & Avnir, D. Antibacterial activity of silver-killed bacteria: The “zombies” effect. Sci. Rep. 5, 9555. https://doi.org/10.1038/srep09555 (2015).
Wu, D. I. et al. Effect of silver diamine fluoride (SDF) application on microtensile bonding strength of dentin in primary teeth. Pediatr. Dent. 38, 148–153 (2016).
Kucukyilmaz, E., Savas, S., Akcay, M. & Bolukbasi, B. Effect of silver diamine fluoride and ammonium hexafluorosilicate applications with and without Er:YAG laser irradiation on the microtensile bond strength in sound and caries-affected dentin. Lasers Surg. Med. 48, 62–69. https://doi.org/10.1002/lsm.22439 (2016).
Fa, B. A., Jew, J. A., Wong, A. & Young, D. Silver modified atraumatic restorative technique (SMART): An alternative caries prevention tool. Stomatol. Edu J. 3, 243–249. https://doi.org/10.25241/STOMAEDUJ.2016.3(3-4).ART.15 (2016).
Bridge, G., Martel, A.-S. & Lomazzi, M. Silver diamine fluoride: Transforming community dental caries program. Int. Dent. J. 71, 458–461. https://doi.org/10.1016/j.identj.2020.12.017 (2021).
Modasia, R. & Modasia, D. Application of silver diamine fluoride as part of the Atraumatic restorative technique. BDJ Stud. 28, 42–43. https://doi.org/10.1038/s41406-021-0199-1 (2021).
Krämer, N. et al. Glass ionomer cement inhibits secondary caries in an in vitro biofilm model. Clin. Oral Investig. 22, 1019–1031. https://doi.org/10.1007/s00784-017-2184-1 (2018).
Braz, P. V. F. et al. The effect of silver diamine fluoride and cleaning methods on bond strength of glass-ionomer cements to caries-affected dentin. Am. J. Dent. 33, 196–200 (2020).
Wang, A. S., Botelho, M. G., Tsoi, J. K. H. & Matinlinna, J. P. Effects of silver diammine fluoride on microtensile bond strength of GIC to dentine. Int. J. Adhes. Adhes. 70, 196–203. https://doi.org/10.1016/j.ijadhadh.2016.06.011 (2016).
Koizumi, H., Hamama, H. H. & Burrow, M. F. Effect of a silver diamine fluoride and potassium iodide-based desensitizing and cavity cleaning agent on bond strength to dentine. Int. J. Adhes. Adhes. 68, 54–61. https://doi.org/10.1016/j.ijadhadh.2016.02.008 (2016).
Zhao, I. S. et al. Effect of silver diamine fluoride and potassium iodide on shear bond strength of glass ionomer cements to caries-affected dentine. Int. Dent. J. 69, 341–347. https://doi.org/10.1111/idj.12478 (2019).
Puwanawiroj, A., Trairatvorakul, C., Dasanayake, A. P. & Auychai, P. Microtensile bond strength between glass ionomer cement and silver diamine fluoride-treated carious primary dentin. Pediatr. Dent. 40, 291–295 (2018).
Gupta, J. et al. Effect of silver diamine fluoride-potassium iodide and 2% chlorhexidine gluconate cavity cleansers on the bond strength and microleakage of resin-modified glass ionomer cement. J. Conserv. Dent. 22, 201–206. https://doi.org/10.4103/JCD.JCD_485_18 (2019).
Knight, G. M. & McIntyreMulyani, J. M. The effect of silver fluoride and potassium iodide on the bond strength of auto cure glass ionomer cement to dentine. Aust. Dent. J. 51, 42–45. https://doi.org/10.1111/j.1834-7819.2006.tb00399.x (2006).
Patel, J., Anthonappa, R. P. & King, N. M. Evaluation of the staining potential of silver diamine fluoride: In vitro. Int. J. Paediatr. Dent. https://doi.org/10.1111/ipd.12401 (2018).
Knight, G. M. et al. Differences between normal and demineralized dentine pretreated with silver fluoride and potassium iodide after an in vitro challenge by Streptococcus mutans. Aust. Dent. J. 52, 16–21. https://doi.org/10.1111/j.1834-7819.2007.tb00460.x (2007).
van Duker, M. et al. Effect of silver diamine fluoride and potassium iodide on bonding to demineralized dentin. Am. J. Dent. 32, 143–146 (2019).
Lutgen, P., Chan, D. & Sadr, A. Effects of silver diammine fluoride on bond strength of adhesives to sound dentin. Dent. Mater. J. 37, 1003–1009. https://doi.org/10.4012/dmj.2017-401 (2018).
Oliveira, B. H., Cunha-Cruz, J., Rajendra, A. & Niederman, R. Controlling caries in exposed root surfaces with silver diamine fluoride: A systematic review with meta-analysis. J. Am. Dent. Assoc. 149, 671-679.e1. https://doi.org/10.1016/j.adaj.2018.03.028 (2018).
Ng, E. et al. Shear bond strength of glass ionomer cement to silver diamine fluoride-treated artificial dentinal caries. Pediatr. Dent. 42, 221–225 (2020).
Nicholson, J. W. Adhesion of glass-ionomer cements to teeth: A review. Int. J. Adhes. Adhes. 69, 33–38. https://doi.org/10.1016/j.ijadhadh.2016.03.012 (2016).
Khor, M. M. et al. SMART: Silver diamine fluoride reduces microtensile bond strength of glass ionomer cement to sound and artificial caries-affected dentin. Dent. Mater. J. 41, 698–704. https://doi.org/10.4012/DMJ.2021-319 (2022).
Fukuda, R. et al. Bonding efficacy of polyalkenoic acids to hydroxyapatite, enamel and dentin. Biomaterials 24, 1861–1867. https://doi.org/10.1016/S0142-9612(02)00575-6 (2003).
TambaraFröhlich, T., Botton, G., De, R. & Rocha, O. Bonding of glass-ionomer cement and adhesives to silver diamine fluoride-treated dentin: An updated systematic review and meta-analysis. J. Adhes. Dent. 24, 29–38. https://doi.org/10.3290/j.jad.b2701679 (2022).
Fröhlich, T. T., de Oliveira Rocha, R. & Botton, G. Does previous application of silver diammine fluoride influence the bond strength of glass ionomer cement and adhesive systems to dentin? Systematic review and meta-analysis. Int. J. Paediatr. Dent. 30, 85–95. https://doi.org/10.1111/IPD.12571 (2020).
Mei, M. L. et al. The inhibitory effects of silver diamine fluoride at different concentrations on matrix metalloproteinases. Dent. Mater. 28, 903–908. https://doi.org/10.1016/j.dental.2012.04.011 (2012).
Yoshioka, M. et al. Adhesion/decalcification mechanisms of acid interactions with human hard tissues. J. Biomed. Mater. Res. 59, 56–62. https://doi.org/10.1002/jbm.1216 (2002).
Inoue, S. et al. Effect of remaining dentin thickness and the use of conditioner on micro-tensile bond strength of a glass-ionomer adhesive. Dent. Mater. 17, 445–455. https://doi.org/10.1016/S0109-5641(01)00003-3 (2001).
Abdalla, A. I. Morphological interface between hybrid ionomers and dentin with and without smear-layer removal. J. Oral Rehabil. 27, 808–814. https://doi.org/10.1046/j.1365-2842.2000.00601.x (2000).
Mei, M. L. et al. Antibacterial effects of silver diamine fluoride on multi-species cariogenic biofilm on caries. Ann. Clin. Microbiol. Antimicrob. https://doi.org/10.1186/1476-0711-12-4 (2013).
Karched, M., Ali, D. & Ngo, H. In vivo antimicrobial activity of silver diammine fluoride on carious lesions in dentin. J. Oral Sci. 61, 19–24. https://doi.org/10.2334/josnusd.17-0366 (2019).
Zhao, I. S. et al. Mechanisms of silver diamine fluoride on arresting caries: A literature review. Int. Dent. J. 68, 67–76. https://doi.org/10.1111/idj.12320 (2018).
Funding
Supportive Funding from the Research and Innovation unit, Faculty of Dentistry, Khon Kaen University, Thailand (DTR 6404).
Author information
Authors and Affiliations
Contributions
The authors contributions to the present study were the following: J.R.Y. and S.T.N. conceived and planned the experiments, performed the experiment, and collected the data; K.T. and P.J. carried out the experiment; P.P. and S.B. contributed to sample preparation; P.S. contributed to data interpretation, writing-original draft; writing-review and editing; C.H.P. contributed to conceptualization and study design, contributed to data interpretation, performed the statistical analysis and contributed to writing-original draft; writing-review, and editing. All authors critically revised the manuscript and agreed to the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rinsathon, J., Wiriyasuebpong, S., Thariya, K. et al. Bonding performance of glass ionomer cement to carious dentin treated with different surface treatment protocols using silver diamine fluoride. Sci Rep 13, 14233 (2023). https://doi.org/10.1038/s41598-023-41511-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-41511-9
- Springer Nature Limited