Abstract
Introduction:
Calotropis procera is a member of the plant family Asclepiadaceae, a shrub about 6m high and is widely distributed in the kingdom of Saudi Arabia. This study aimed to show some medicinal potentials and biological activity of Calotropis procera and to discover new natural, safe and effective materials available in the Saudi Arabia environment.
Marerial and Methods:
The leaves extracts and latex of Calotropis procera (C. procera) were investigated for its anti- hyperglycemic effect in Male Wister Albino rats. Diabetes was induced by administration of single dose of streptozotocin (STZ, 50 mg/kg, I.P). Fourty two male albino rats, weighting 150-200 gm divided into seven groups, each consisted of 6 rats as follows: Group I : Normal control, Group II: Diabetic control, Group III: Diabetic rats given Glibenclamide 600 μg/kg, Group IV: Diabetic rats given aqueous leaves extracts C. procera 200mg/kg b. wt, Group V: Diabetic rats given chloroform leaves extracts C. procera 200mg/kg b. wt, Group VI: Diabetic rats given ethanol leaves extracts C. procera 200mg/kg b. wt, Group VII: Diabetic rats given latex of C. procera 200mg/kg b. wt.The leaves extracts and latex of Calotropisprocera were administered as single dose per day to diabetes-induced rats for a period of 15 days.The effect of C. proceraon blood glucose level was measured in the diabetic rats. Serum lipid profile (Total cholesterol, triglycerides, low density, and high density lipoprotein) also were measured. The activities were also compared to that effect produced by a standard anti-diabetic agent, glibenclamide 500μg/kg.
Results and Discussion:
The results showed a significant decrease in the mean level of blood glucose and serum cholesterol, Triglycrides, HDL, LDL. Calotropis procera appears to be a rich source of phytoconstituents that activate and inhence a pharmacological response of different parts of the body and this study need further studies to shows the complete properties of the plant
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is a syndrome caused by inherited and/or acquired deficiency in of insulin, or by ineffectiveness of insulin, such a deficiency results in increased concentration of glucose in the blood. Diabetes mellitus is a syndrome characterized by chronic hyperglycemia.
Besides hyperglycemia, several other factors including dislipidemia or hyperlipidemia are involved in the development of micro and macro vascular complications of diabetes which are the major causes of morbidity and death (Larner and Haynes Jr, 1985). Despite considerable progress in the treatment of diabetes by oral hypoglycemic agents, search for newer drugs continues because the existing synthetic drugs have several limitations. In recent times, there has been a renewed interest in the plant remedies (plants, 2012). Calotropis procera (Sodom apple) is a member of the plant family asclepiadaceae, a shrub about 6m high and is widely distributed in West Africa and other parts of the tropics (Alam and Ali, 2009; Irvine, 1961). The plant is erect, tall, large, much branched and perennial with milky latex throughout. In India, the secretions from the root bark is traditionally used for the treatment of skin diseases, enlargements of abdominal viscera and intestinal worms (Parrotta, 2001). The milky latex used in the treatment of cutaneous diseases such as ringworm, syphilitic sores and leprosy (Burkill, 1985). In Nigeria traditional medicine, C. procera is either used alone or with other herbs to treat common diseases such as fevers, rheumatism, indigestion, cold, eczema and diarrohea. In addition preparations from latex with honey are used as antirabies and also in the treatment of toothache and cough (Burkill, 1985). Leaf extracts, chopped leaves and latex of C. procera have shown great promise as a nematicide in vitro and in vivo (Khirstova and Tissot, 1995). The potentials of C.procera leaves in water treatment and its ability to reduce total viable count have also been reported (Shittu, 2004).
Traditional doctors in West Africa have claimed to have successfully used the plant to cure many diseases. Anti malaria and skin infection activities of C. procera have been documented(Ayoola et al., 2008). The morphological studies revealed the leaves to be subsessile, 6-15 cm by 4.5-8 cm, broadly ovate, ovateoblong, elliptical, or obovate, acute, pubescent when young and glabrous on both sides on maturity C. procera are reported to have anti inflammation, antimicrobial, anticancer anti-diabetic, antioxidant properties (Kumar et al., 2010).
C. procera grows widely in warm and urbanizing regions it is can found easily in desert areas that cover most of Saudi Arabia. In Saudi Arabia the plant is commonly used in traditional medicine for the treatment of variety of diseases including fever, constipation, muscular spasm and joint pain (Al-Yahya, 1990).
Materials and methods
Collection of Plant material
The fresh leaves and latex of C. procera were collected from the Dawadmi Kingdom of Saudi Arabia. Calatropisprocera leaves were air dreid under shed and blend into powder form by using electric blender (Moulinex). This powder was stored in glass air tight jar and kept under room temperature.
The fresh latex of C. procera was collected in sterile 250 ml conical flask by making incision at stem near the leave attachment and stored in ice bag to maintain homogeneity and activity during transport.
Preparation of different Plant Extract by cold maceration method
The leaves and latex were collected from the aerial parts of the plant and dried under shad (Kumar vl, 2004). The leaves were grinded to a fine powder. The extracts of water, chloroform and ethanol were prepared by soaking 100g each the dry powdered leaves in 1 litter of water, chloroform and ethanol at room temperature for 4 days with continuous stirring. The extracts were filtered, through a Whitman filter paper and then placed in water bath (Oluwatoyin Eunice, October-December 2013).
Animals
Male albino rats (150-200g) of 3-4 months old were used in this study. They were kept in the departmental animal house at 27°C and relative humidity 44 – 56 %, light and dark cycles of 10 and 12 h respectively for one week before and during the experiments. Animals were provided with standard rodent pellet diet and the food was withdrawn 18-24 h before the experiment though water was allowed ad libitum. Animal experiments were carried out following the guidelines of the animal ethical committee of the institute.
Preliminary phytochemical screening
The extracts were subjected to preliminary screening for various active phytochemical constituents as per the methods mentioned in Practical pharmacognosy (Kokate, 1994).
Acute toxicity test
All extracts of leaves and latex of Calotropisprocera-were screened for acute toxicity, following the standard method (OECD/OCDE No: 425). Albino rats of either sex weighing 180-200gm were used in this study. Animals were maintained on normal diet and water prior to and during the course of experiment. The dose of aqueous, chloroform, ethanol leaves extracts and latex of C. procera were prepared with 1% gum acacia and was administered orally. The acute toxicity was tested at the doses of 300 and 2000mg/kg.
Experimental design: Long-term experiment
Induction of diabetes in rats
Diabetes was induced in rats by intra peritoneal (i.p) injection of streptozotocin at a dose of 50 mg/kg, dissolved in 0.9% ice-cold saline (Pulok, 2002). Fourtyeight hours after streptozotocin administration, blood samples were drawn from tail and glucose levels determined to confirm diabetes. The diabetic rats exhibiting blood glucose levels higher than 200 mg/dl were selected for the studies. After that, a total of 42 rats were used (36 diabetic surviving rats, 6 control rats) for the execution of the experiment. The rats were divided as follows into seven groups each contain 6 animals and all the doses for oral administration were prepared in 1% gum acacia.
Group I: Normal control (Vehicle treated)
Group II: Diabetic control (Received 0.5 ml of 1% gum acacia)
Group III: Diabetic rats given Glibenclamide 600 μg/kg
Group IV: Diabetic rats given aqueous leaves extracts C. procera200mg/kg b. wt
Group V: Diabetic rats given chloroform leaves extracts C. procera200mg/kg b. wt
Group VI: Diabetic rats given ethanol leaves extracts C. procera200mg/kg b. wt
Group VII: Diabetic rats given latex of C. procera200mg/ kg b. wt
Blood samples were collected from the tail for glucose estimation just before drug administration on the first day and 1 h after drug administration on days 4, 7, 10 and 15. Blood samples were collected and centrifuged at 3000 r.p.m for 10 minutes to separate serum. Biochemical parameters were assessed in method of calorimetric enzymatic using commercial kits (Boehringer Mannheim Diagnostica GmbH, Mannheim, Germany).
Statistical evaluation
All the data are presented as mean ±SEM, n=6. The differences between groups were evaluated by oneway analysis of variance (ANOVA) followed by the Dunnette multiple comparisons test. P<0.01 was considered to be significant.
Results
Preliminary phytochemical test
Phytochemical studies indicated that extracts of leaves of C. proceracontains alkaloids, flavanoids, glycosides, saponins and terpenes.
Acute toxicity studies
In performing preliminary test for pharmacological activity in rats, aqueous extract did not produce any significant changes in the behavioral or neurological responses up-to 2500 g/kg b. wt. acute toxicity studies revealed the non-toxic nature of the aqueous, chloroform, ethanol leaves extracts and latex of Calotropisprocera.
Antihyperglycemic activity
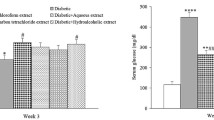
The Effect of leaves extracts and latex of Calotropisprocera on blood glucose level compared to streptozotocin- induced diabetic rats are presented in Table 1 . Blood glucose levels of the STZ treated rats were significantly higher than those in normal rats. In STZ (50 mg/kg) induced rats, the blood glucose level significantly increased from 92.12±1.45 to 241.3±2.36 mg/dl. Aqueous, chloroform, ethanol leaves extracts and latex of Calotropis procera (200 mg/kg) given up-to 15 days. After aqueous, chloroform, ethanol leaves extracts and latex treatment, the blood glucose levels were decreased from 239.2±1.98 to 168.3±1.65 and 241.5±1.72 to 155.9±1.28, 240.2±2.85 to 156.2±1.85 and 238.9±2.16 to 149.1±1.65 mg/kg respectively. Whereas in glibenclamide treated rats, blood glucose levels were decreased from 240.6±1.52 to 144.2±2.23 mg/kg.
Antihyperlipidaemic activity
The lipid profiles in control and experimental rats are depicted in Table 2 in STZ induced diabetic rats, there was a significant (p<0.001) increase of total cholesterol, triglycerides, phospholipids, and low density lipoproteins (LDL) and significant (p<0.001) decreases in high density lipoprotein (HDL) cholesterol in serum compared with normal control. The extracts-treated rats indicated significantly (p<0.001) decreased total cholesterol, triglycerides, phospholipids and LDL and significantly (p<0.001) increased HDL.
Discussion
Calotropis procera is well known plant in many different region worldwide including India, Vietnam, Afghanistan, Kenya, Pakistan, Algeria, Niger, Nigeria, Iran, Iraq, Kuwait, Oman, United Arab Emirates, Yemen and Saudi Arabia (Chatterjee and Pakrashi, 1994).
The previous studies showed that the plant leaves contain calotrpin and calotropegnin while the latex contains uzarigenin, and terpenol ester. In addition, Calotropis procera contains many other compounds that have different pharmacological activities. A lot of pharmacological activities have been reported in scientific literatures like analgesic activity of dry latex (Saber et al., 1969), antifertility activity of the roots (Saxena and Saxena, 1979) anti -tumor potential of the roots (Rajani and Gupta, 2009), anthelmintic activity of the flowers (Larhsini et al. 1997) and dry latex have anti-hyperglycemic effect (Silvania, 2005).
In these study the aims was tostudy some medicinal potentials and biological activity of crude aquouse, chloroform and ethanolic extract of Calotropis procera leaves in addition to its latex.
The results showed that there was a significant decrease in the mean serum glucose level after the treatment of the animals by the aqueous, chloroform or ethanolic leave extract and the latex of Calotropis procera 200mg/kg dissolved in 1% gum acacia.
The results also showed that there was a significant increase in the mean levels of serum cholesterol, triglycrides, HDL and LDL in diabetic control group but after the treatment with the aqueous, Chloroform or ethanolic leave extract and the latex of Calotropis procera 200mg/kg there was significant decrease in whole lipid profile except HDL which showed that there was significant increase as (Garg, 1979).
Conclusion
In this study we try to make an overview on one of plants that have some traditional uses and evaluate its properties and we found that the probable mode of action of aqueous, chloroform, ethanol leaves extract in addition to latex of Calotropis procera appears to be through it is a rich source of phytoconstituents which can be used as an alternative treatment for the management of diabetes and its complications.
Conflict of interests
All authors have declared that there is no conflict persists in this article.
Acknowledgements
The authors are thankful to Shaqra University, Ministry of Higher Education, Kingdom of Saudi Arabia for funding this research and providing platform to encourage research and developments amongst the students, staff and society. We are also very much thankful to the Department of Clinical Laboratory Science, College of Applied Medical Science Dawadmi, Shaqra University Kingdom of Saudi Arabia for their help with lab facility. We may not proceed to this present work if they do not allow us. We again thank their ethical committee for approving our in vivo protocol.
Competing interests
The authors declare that they have no competing interests.
References
Ahmed, U.A.M., Zuhua, S., Bashier, N.H.H., Muafi, K., Zhongping, H., and Yuling, G. (2006). Evaluation of insecticidal potentialities of aqueous extracts from Calotropis procera Ait. against Henosepilachna elaterii Rossi. Journal of Applied Sciences 6, 2466–2470.
Al-Yahya, M.A., Al-Meshal, I.A., Mossa, J.S., Al-Badr, A.A. and Tariq, M. Saudi Plants (1990). A phytochemical and biological approach. General directorate of research grants programs. KACST, Riyadh, pp. 75–80.
Alam, P., and Ali, M. (2009). Phytochemical investigation of Calotropis procera Ait roots. Indian journal of chemistry Section B, Organic including medicinal 48, 443.
Ayoola, G.A., Coker, H.A., Adesegun, S.A., Adepoju-Bello, A.A., Obaweya, K., Ezennia, E.C., and Atangbayila, T.O. (2008). Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Tropical Journal of Pharmaceutical Research 7, 1019–1024.
Burkill, H.M. (1985). The useful plants of west tropical Africa. Edition 2. Vol. 1: families AD. Kew, Royal Botanic Gardens.
Chatterjee, A., and Pakrashi, S.C. (1994). The treatise on Indian medicinal plants: vol. 3. New Delhi: Council of Scientific and Industrial Research 274pillus, col illus ISBN 8172360800 En Icones Plant records Geog 6.
Garg, A. (1979). Effect of AK Calotropis procera (Ait.) R. Br. flower extract on testicular function of the Indian desert male gerbil Meriones hurrianae Jerdon: a biochemical & histological study. Indian journal of experimental biology 17, 859–862.
Irvine, F.R. (1961). Woody plants of Ghana. Woody plants of Ghana.
Khirstova, P. and Tissot, M. (1995). Soda Anthroqinone pulping of Hibiscus sabdariffa (Karkadeh) and C.procera from Sudan. Bioresource Technology 53: 672–677.
Kokate, C.K. (1994). Practical pharmacognosy. Vallabh Prakashan, New Delhi 4, 110–111.
Kumar, G., Karthik, L., and Rao, K.V.B. (2010). Antibacterial activity of aqueous extract of Calotropis gigantea leaves–an in vitro study. Int J Pharm Sci Rev Res 4, 141–144.
Kumar vl, k.v. (2004). Extraction of latex of calotropis procera and a process for the preparation thereof. indian patent application.
Larhsini, M., Bousaid, M., Lazrek, H.B., Jana, M., and Amarouch, H. (1997). Evaluation of antifungal and molluscicidal properties of extracts of Calotropis procera. Fitoterapia 68, 371–373.
Larner, J., and Haynes Jr, R.C. (1985). Insulin and oral hypoglycemic drugs; glucagon. The pharmacological basis of therapeutics 7, 1490–1516.
Mathur, R., Gupta, S.K., Mathur, S.R., and Velpandian, T. (2009). Antitumor studies with extracts of Calotropis procera (Ait.) R. Br. root employing Hep2 cells and their possible mechanism of action. Indian journal of experimental biology 47, 343.
Mohd. Mazid, Taqi Ahmed Khan, Firoz Mohammad. 2012. Medicinal Plants of Rural India: A Review of Use by Indian Folks. Indo Global Journal of Pharmaceutical Sciences, 2(3): 286–304.
Oluwatoyin Eunice, B., Bello Oluwasesan, M. and Dada Adewumi, O (October-December 2013). nvestigation of aqueous extracts of leaves of calotropis procera as a natural nematicide against root knot nematode infection on abelmoschus esculentus Moench’s yield. International Journal of Plant, Animal and Environmental Sciences 4.
Parrotta, J.A. (2001). Healing plants of peninsular India (CABI publishing).
Saber, A.H., Maharan, G.H., Rizkallah, M.M., and Ste Saber, A.H. (1969). Sterols and pentacyclic triterpenes of Calotropis procera. Bull Fac Pharm Cairo Univ 7, 91–104.
Saxena, V.K., and Saxena, Y.P. (1979). Isolation and study of triterpenoids from Calotropis procera. J Res Indian Med Yoga Homeopathy 14, 152–154.
Shittu, B.O. (2004). POTENTIAL OF Calotropis procera LEAVES FOR WASTEWATER TREATMENT. College of Natural Sciences Proceedings, 97–108.
Soares, P.M., Lima, S.R., Matos, S.G., Andrade, M.M., Patrocínio, M.C.A., de Freitas, C.D.T., Ramos, M.V., Criddle, D.N., Cardi, B.A., and Carvalho, K.M. (2005). Antinociceptive activity of Calotropis procera latex in mice. Journal of ethnopharmacology 99, 125–129.
Yoganarasimhan, S.N. (2011). Medicinal plants of India. Regional research institute (Ay.) Bangalore, Tamil Ayurvedic uses and Pharmacological activities of Calotropis procera Linn. Asian Journal of Traditional Medicines 6.
Cite this article as:
Abd Alrheam, A., & Shehri, Z. (2015). Ethanopharmacological study of the aqueous, chloroform, ethanol leaves extracts and latex of Calotropisprocera in diabetic rats. Biomedical Research And Therapy, 2(11): 396–401.
Author information
Authors and Affiliations
Corresponding author
Additional information
Clinical Laboratory department, Al-Dawadmi College of Applied Medical Sciences, Shaqra Universty –KSA, Saudi Arabia
* Corresponding author: a.ismail@su.edu.sa
Open Access
This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alrheam, A., Shehri, Z. Ethanopharmacological study of the aqueous, chloroform, ethanol leaves extracts and latex of Calotropis procera in diabetic rats. Biomed Res Ther 2, 27 (2015). https://doi.org/10.7603/s40730-015-0027-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.7603/s40730-015-0027-8