Abstract
Neutrophil and platelet are essential arms of the innate immune response. In sepsis, platelet abnormal activation as well as neutrophil paralysis are well recognized. For platelet, it is characterized by the contribution to disseminated intravascular coagulation (DIC) and the enhanced inflammation response. In terms of neutrophil, its dysfunction is manifested by the impaired recruitment and migration to the infectious foci, abnormal sequestration in the remote organs, and the delayed clearance. More recently, it has been apparent that together platelet-neutrophil interaction can induce a faster and harder response during sepsis. This article focuses on the activation of platelet, dysfunction of neutrophil, and the interaction between them during sepsis and profiles some of the molecular mechanisms and outcomes in these cellular dialogues, providing a novel strategy for treatment of sepsis.
Similar content being viewed by others
Introduction
Sepsis is a combination of clinical manifestations of systemic inflammation specifically related to an infectious insult[1] and the inflammatory dynamic of it, in term of the current hypothesis, includes an initial systemic inflammatory response syndrome (SIRS) followed temporally by a compensatory anti-inflammatory response syndrome (CARS)[2–4] then with a continuously, highly mixed anti-inflammtory response syndrome (MARS).[5] Given a profoundly impairment and life threatening of sepsis, there is an imperative to understand the concrete pathophysiology during sepsis and over the time the understanding is evolving. Mortality from sepsis continues to be high. Totally, the mechanism of sepsis is complex and the late therapies targeting a single molecular fail to cure the disease, for example, the monoclonal antibodies against tumor necrosis factor (TNF)-α,[6] the receptor antagonists of interleukin (IL)-1β[7] and the antibodies to endotoxin.[8] Hence, understanding the intricate and heterogeneous of sepsis addresses a better approach the problem of sepsis. Besides shock and multi-organ dysfunction occurring following the intense inflammatory reaction to sepsis, complications arising from sepsis-related platelet activation and platelet-neutrophil interaction contribute to the morbidity and mortality from sepsis. This review explores the basis for sepsis-related platelet activation, neutrophil dysfunction, and platelet-neutrophil interaction and discusses their clinical implications for the treating intensivist.
Sepsis-induced platelet activation
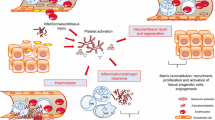
Platelets, small (approximately 3–5 µm) anucleate cells derived from bone marrow megakaryocytes, were primitively recognized to sense damaged vessel endothelium and accumulate at the site of the vessel injury to initiate blood clotting. Recently, there is increasing evidence suggesting their indispensable role in regulating inflammatory response.[9–11] During sepsis, platelets are immoderately activated by various pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), which amplify inflammatory response through complicated mechanisms. And the response triggered by the interaction between platelets and various PAMPs and DAMPs is through the platelet receptors, mainly glycoprotein (GP)IIb-IIIa (mediating the crosslinking of platelet by fibrinogen to promote aggregation),[12] GPIbα (inducing platelet activation mainly by the von Willebrand factor [vWF]), FcγRIIa (enhancing the function of GPIIb-IIIa and GPIbα in an lgG-independent manner),[13,14] complement receptors (increasing upon activation,[15] and inducing platelet aggregation in a complement-dependent process[16]) and toll-like receptors (TLRs). Of note, GPIIb-IIIa, GPIbα, and FcγRIIa play a crucial role in platelet activation, adhesion and aggregation. For TLRs, especially TLR4 and TLR2, they can activate platelet to release immunomodulatory agents (like TNF-α[17]) and promote other cells activation, such as neutrophils, endothelial cells. Inappropriate activated platelets are major contributors to the initiation of disseminated intravascular coagulation (DIC) that is initiated by tissue factor (TF), leading to the platelet adhesion induced by the receptors (like p-selectin) and ligands, like P-selectin glycoprotein ligand (PSGL)-1, interaction,[18] the formation of thrombin, fibrin, and intravascular thrombi,[19] which reduce oxygen supplement and enhance inflammatory cytokine networks.[20,21] Additionally, various pro-inflammatory factors in platelets granules are released into the surrounding environment or transferred to plasma membrane, such as interleukins, monocyte chemoattactant protein (MCP)-1, platelet factor (PF)-4, to activate more remote platelets and immune cells.[22–24] Activated platelets can also release some microparticles.[25] Circulation microparticles are membrane-derived nano-fragments (0.05-1 µm) which contain a storage pool of TF and express P-selectin and platelet glycoprotein IIb-IIIa. As described above, these bioactive molecules may play deleterious role in the dissemination of coagulopathy and inflammatory responses in sepsis.[26]
Neutrophil dysfunction in sepsis
Neutrophil originates from the bone marrow with a consequent egress to the blood, recruits and migrates to the inflammatory site, then culminates in clearance. The life of neutrophil has been described, all of which are uncontrolled altered during sepsis.
The mature neutrophil within the bone marrow can rapidly egress in the early phrase of sepsis, increasing the circulation numbers by tenfold within a matter of hours compared with the normal condition.[27] The release of neutrophils from bone marrow to the infection site has been historically attributed to the chemotactic factors including leukotriene B4, C5a, chemokine interleukin IL-8,[27–30] and the bacterial products. And the chemokine (C-X-C motif) ligand (CXCL)12, a recently new and pivotal chemoattractant serve to retain neutrophil within the marrow, are also involved in this process.[31–34] The granulocyte colony-stimulating factor (G-CSF) which is indirectly mobilized neutrophil through shifting the balance between stromal cell-derived factor (SDF)-1 and CXCR2 ligands in bone marrow[35] are also associated with this phenomenon, triggering a release of neutrophil into the circulation.
Infection is an alarming condition that renders host to defend. Neutrophil, as the fist line cell against the bacterial and fungal pathogens, recruits to the site of infection. The classical leukocyte recruitment cascade involves the following recognized steps: Tethering, rolling, adhesion, crawling, and, finally, transmigration. This process is a sequential, multistep adhesion cascade in which various cytomembrane molecules are sophisticated interactived (reviewed in Table 1[36]). However, during sepsis, this response is dysregulated with the abnormal accumulation of neutrophil, impaired recruitment of neutrophils to the infectious foci, and damaged neutrophil migration. Of note, the neutrophil cell membrane altered, becoming more rigid and less deformed, and this change in rigidity increases proportionally with sepsis severity.[37] As a result, neutrophils sequester in the capillary beds, especially those in lung and liver sinusoids and the process will lead to microvascular occlusion, resulting in the tissue ischemia and subsequently multiple organ failure.[37,38] Nitric oxide (NO) and its producer inducible nitric oxide synthase (iNOS), the sine qua non in neutrophil migration impairment, downregulate neutrophil migration mainly from the following three aspects:
-
1.
The iNOS inhibits leukocyte β-integrins and selectins as well as downregulates vascular cell adhesion molecule (VCAM)-1[39,40] and;
-
2.
NO interacts with other molecules like reaction oxygen species (ROS), forming peroxynitrite that can decrease neutrophil chemotactic activity[41] and leukocyteendothelium interaction which relays on P-selectin[42,43];
-
3.
NO can induce heme oxygenase (HO)-1 expression, one that can impair neutrophil rolling and adhesion.[44,45]
Besides, the enhanced level of carbon monoxide (CO) and bilirubin in serum and exhaled breath of septic patients[46,47] also indicate that HO-1 pathway plays a role in this pathology. The proteins on the cell-surface and the nuclear as the receptor like C-X-C chemokine receptor (CXCR) type 2, C-C chemokine receptor (CCR) type 2, and peroxisome proliferator-activated receptor (PPAR)γ, mediate the impairment in neutrophil migration. Their participation can be justified by:
-
1.
Decreased expression of CXCR2 on neutrophil isolated from septic patients[48] and the upregulation of CCR2 on circulating murine netrophil during sepsis[49] have been found, which are due, at least in part, to the TLR signaling[50–52];
-
2.
The expression of PPARγ increased in the isolated neutrophil from not only septic mice but septic patients.[53]
Another novel finding is that the direction of neutrophil migration was error during sepsis[54] and the consequence is complex in vivo. The precise mechanism about how neutrophils direct to the target destination is incompletely understood.
To maintain the homeostasis of neutrophils, the key thing is a fine management of the balance between the income and outcome neutrophils. Homeostatic removal of neutrophils mainly gives the credit to the macrophages[55] and to a small extent by the dendritic cells and lymph nodes. In it neutrophil undergoing apoptosis allows removal by scavenger macrophage[56] and constitutive apoptosis of neutrophil is an essential factor for keeping neutrophil homeostasis. However, in patients with sepsis the apoptosis of neutrophil is delayed[57–59], which may contribute to tissue injury associated with the multiple organ dysfunction syndrome (MODS) of sepsis. The mechanisms that govern this process are not completely understood and the recent investigation found that the inflammation mediators, i.e., granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-18[60,61], which regulate the pro- and anti-apoptosis genes leading to the change of apoptosis relevant factors expression: B-cell lymphoma (BCL)-2 members,[62] the sFas, Dad1,[63] etc., can manage it. Remarkably, additional upstream regulatory factors of these apoptosis factors are involved in the delayed apoptosis of neutrophil in sepsis. In addition the destructed mitochondrial transmembrane potential and the reduced activity of caspase 3,9[59] also dampen the apoptosis. Along with the death combined with a formation of neutrophil extracellular traps (NETs) which contain nuclear components (like deoxyribonucleic acid, DNA and histones) are decorated by various proteins.[64] During sepsis, NETs present like a double-edged swords: They can trap microorganisms [64] through NET-localized molecules; moreover, they exert detrimental effects that contribute to tissue damage.[65]
Platelet-neutrophil interaction during sepsis
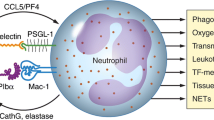
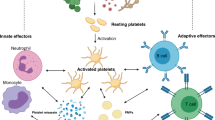
Platelets and neutrophils have the potential to promote inflammatory response during sepsis independently of each other, but together platelet-neutrophil interactions can induce a faster and harder response.[11,65] In the early phase of sepsis, possibility of collisions between platelets and leukocytes is promoted by the rheological margination of neutrophil exiting the central core of the blood vessel. With further activation by septic inflammatory stimuli (PAMPs and DAMPs), platelet-neutrophil interactions are extensively formed.[66] It is well accepted that activated platelets adhere to neutrophils through a rapid surface expression of a granular protein P-selectin that binding to the high affinity counter ligand PSGL-1 expressed on neutrophils.[67–69] Engagement of PSGL-1 leads to further neutrophil activation of the β2-integrins, CD11a/CD18, LFA-1 (αLβ2), CD11b/CD18 and Mac-1 (αMβ2) that do not require additional stimuli,[70–72] which result in massive neutrophil migration and accumulation in distal organs such as lung and liver to cause tissue injury. Related to this, Clark et al. found that isolated human neutrophils require 2–4 hours stimulation to release NET, however it took a few minutes when interact with lipopolysaccharide (LPS)-stimulated platelets under flow.[65] Further studies discover that platelet-induced NET release is dependent on lymphocyte function-associated antigen (LFA)-1 interaction both in murine and human sepsis.[73] Although NET formation is critical for ensnare bacteria, it can also provide a stimulus and scaffold for thrombus formation, by promoting platelet and RBC adhesion and by concentrating effector proteins and coagulation factors involved in clotting to aggravate DIC and tissue damage during sepsis.[73,74]
The interaction between CD40 and its ligand CD40L activates various pathways in immune and non-immune cells related to inflammation and was shown to be critical for the development of sepsis.[75,76] Activated by septic stimulation, expression of CD40L is severely increased on platelet surface and shed into circulation to interact with immune cells.[75,77] Platelet-derived CD40L can be sensed by CD40 on endothelial cell to induce upregulation of intercellular Adhesion Molecule (ICAM)1 and VCAM1 and release of CCL2, thereby indirectly promoting leukocyte recruitment to inflammatory sites.[78] In addition, platelet-derived CD40L can directly interact with neutrophil CD40 and enhance the neutrophil activation and ROS generation.[79] Another way in which platelets interact with neutrophils during sepsis is through triggering receptor expressed on myeloid cells (TREM)1.[80] In the presence of LPS neutrophils and platelets interact through TREM1 activation, which increases neutrophil-mediated production of ROS and secretion of IL-8.[81] TREM-like transcript (TLT)1, an orphan receptor only expressed in the α-granules of platelets and megakaryocytes, is newly demonstrated to be significantly upregulated in the plasma of patients with sepsis and correlated with the outcome in these patients.[82] These observations suggest that TREM1 ligand TREM-like family may have synergetic effects on interaction of neutrophils and platelets during sepsis[9] [Figure 1].
Platelet-neutrophil interaction during sepsis. During sepsis, activated platelets attach to neutrophils via a selectin dependent process, namely the release and expression P-selectin of platelet from a-granules which binds to the counter ligand P-selectin glycoprotein ligand (PSGL) expressed on neutophils. Besides that, activated platelet can expression CD40L and then shed it into circulation. Triggering receptor expressed on myeloid cells (TREM)1, triggering receptor expressed on myeloid cells together with CD40L interact with neutrophils which can further promote the activation of neutophils and its generation of reaction oxygen species (ROS). For platelet-expressed CD40L, it can also interact with CD40 on endothelial cells to stimulate the endothelial cell to a pro-inflammatory phenotype: upregulation of intercellular adhesion molecule (ICAM)1 and vascular cell adhesion molecule (VCAM)1, thereby driving neutrophil recruitment. The platelet can also mediate the formation of neutrophil extracellular trap (NET) via the interaction of lymphocyte function-associated antigen (LFA)-1, which can trap free bacteria and enhance the platelet and red blood cell (RBC) adhesion to promote thrombus formation.
Increasing evidences have proved that the interactions between platelet and neutrophil play a major role in the development of organ failure both in septic patients and experimental animals. In patients with sepsis, enhanced platelet-neutrophil interaction was determined by increased platelet-leukocyte conjugates in blood using a double-labeling flow cytometry technique and this interaction correlated with the severity of septic organ dysfunction.[83] Platelets mediate excessive neutrophil recruitment in lung and acute lung injury via CD40L/Mac-1 pathway in murine abdominal sepsis.[79] Neutrophil-dependent recruitment of platelets in the liver microcirculation impairs sinusoidal perfusion and may contribute to the liver dysfunction in murine abdominal sepsis.[84]
Therapeutic potential for platelet-neutrophil interaction in sepsis
Clinical therapeutic strategy for platelet or neutrophil alone has been applied for several decades and achieved a great success. However, therapeutic strategy targeting platelet-neutrophil interaction in sepsis is barely seen. Come a long way in understanding of molecular and cellular basis of platelet-neutrophil interaction in sepsis, a growing body of studies focuses on the interference with platelet-neutrophil interaction in sepsis. Ogura H et al. reported P-selectin-dependent platelet-neutrophil interaction is involved in the outcome of severely septic patients and P-selectin blockade markedly inhibited this interaction.[85] Exposed to cecal ligation and puncture (CLP), CD40L gene-deficient mice show a significantly inhibited platelet-neutrophil interaction and alleviated pulmonary damage.[79] Experimental inhibition of PSGL-1 significantly abolished CLP-induced platelet-neutrophil aggregation which has no effect on neutrophil expression of Mac-1.[10] Owing to crucial role on platelet-neutrophil interaction, TREM1-silenced mice are highly resistant to a lethal endotoxin challenge and partial silencing of TREM1 in the bacterial peritonitis model produces a significant survival benefit.[86] Exciting times lies ahead, with the improving awareness of intracellular machinery, we are on the cusp of converting new lessons from intravital studies into novel effective treatment options.
Conclusion
Sepsis, frequently occurs after hemorrhage, trauma, burn, or abdominal surgery, remains a major challenge both for clinicians and researchers. Despite many years of intensive research and numerous clinical studies, its pathophysiology is still incompletely understood, and some specific treatments have not been successful in clinical trials. This is mainly due to the fact that sepsis can be characterized as a complex and dynamic disease process. Targeting platelet-neutrophil interaction is a promising field for sepsis management and infection control. Developing sepsis-specific platelet-neutrophil interaction for patients is a path strewn with obstacles, but an exciting and promising area of research. Understanding of sepsis-induced platelet-neutrophil interaction offers vast opportunities for improving the mortality and morbidity from sepsis. We expect that this novel strategy will continue to be clinically assessed and potentially exploited for the more effective future treatment of sepsis.
Reference
Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet 2005;365:63–78.
Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 2003;348:138–50.
Oberholzer A, Oberholzer C, Moldawer LL. Sepsis syndromes: Understanding the role of innate and acquired immunity. Shock 2001;16:83–96.
Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol 2008;8:776–87.
Osuchowski MF, Craciun F, Weixelbaumer KM, Duffy ER, Remick DG. Sepsis chronically in MARS: Systemic cytokine responses are always mixed regardless of the outcome, magnitude, or phase of sepsis. J Immunol 2012;189:4648–56.
Fisher CJ Jr, Agosti JM, Opal SM, Lowry SF, Balk RA, Sadoff JC, et al. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein. The Soluble TNF Receptor Sepsis Study Group. N Engl J Med 1996;334:1697–702.
Opal SM, Fisher CJ Jr, Dhainaut JF, Vincent JL, Brase R, Lowry SF, et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: A phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit Care Med 1997;25:1115–24.
Angus DC, Birmingham MC, Balk RA, Scannon PJ, Collins D, Kruse JA, et al. E5 murine monoclonal antiendotoxin antibody in gram-negative sepsis: a randomized controlled trial. E5 Study Investigators. JAMA 2000;283:1723–30.
Semple JW, Italiano JE Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol 2011;11:264–74.
Asaduzzaman M, Lavasani S, Rahman M, Zhang S, Braun OO, Jeppsson B, et al. Platelets support pulmonary recruitment of neutrophils in abdominal sepsis. Crit Care Med 2009;37:1389–96.
Li Z, Yang F, Dunn S, Gross AK, Smyth SS. Platelets as immune mediators: Their role in host defense responses and sepsis. Thromb Res 2011;127:184–8.
Bennett JS. Structure and function of the platelet integrin alphaIIbbeta3. J Clin Invest 2005;115:3363–9.
Boylan B, Gao C, Rathore V, Gill JC, Newman DK, Newman PJ. Identification of FcgammaRIIa as the ITAM-bearing receptor mediating alphaIIbbeta3 outside-in integrin signaling in human platelets. Blood 2008;112:2780–6.
Sullam PM, Hyun WC, Szöllösi J, Dong Jf, Foss WM, Lopez JA. Physical proximity and functional interplay of the glycoprotein Ib-IX-V complex and the Fc receptor FcgammaRIIA on the platelet plasma membrane. J Biol Chem 1998;273:5331–6.
Peerschke EI, Murphy TK, Ghebrehiwet B. Activation-dependent surface expression of gC1qR/p33 on human blood platelets. Thromb Haemost 2003;89:331–9.
Miajlovic H, Loughman A, Brennan M, Cox D, Foster TJ. Both complement- and fibrinogen-dependent mechanisms contribute to platelet aggregation mediated by Staphylococcus aureus clumping factor B. Infect Immun 2007;75:3335–43.
Aslam R, Speck ER, Kim M, Crow AR, Bang KW, Nestel FP, et al. Platelet Toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-alpha production in vivo. Blood 2006;107:637–41.
Braun OO, Slotta JE, Menger MD, Erlinge D, Thorlacius H. Primary and secondary capture of platelets onto inflamed femoral artery endothelium is dependent on P-selectin and PSGL-1. Eur J Pharmacol 2008;592:128–32.
Pawlinski R, Wang JG, Owens AP 3rd, Williams J, Antoniak S, Tencati M, et al. Hematopoietic and nonhematopoietic cell tissue factor activates the coagulation cascade in endotoxemic mice. Blood 2010;116:806–14.
Tyml K. Critical role for oxidative stress, platelets, and coagulation in capillary blood flow impairment in sepsis. Microcirculation 2011;18:152–62.
Secor D, Li F, Ellis CG, Sharpe MD, Gross PL, Wilson JX, et al. Impaired microvascular perfusion in sepsis requires activated coagulation and P-selectin-mediated platelet adhesion in capillaries. Intensive Care Med 2010;36:1928–34.
Kowalska MA, Rauova L, Poncz M. Role of the platelet chemokine platelet factor 4 (PF4) in hemostasis and thrombosis. Thromb Res 2010;125:292–6.
Breland UM, Michelsen AE, Skjelland M, Folkersen L, Krohg-Sørensen K, Russell D, et al. Raised MCP-4 levels in symptomatic carotid atherosclerosis: An inflammatory link between platelet and monocyte activation. Cardiovasc Res 2010;86:265–73.
Park HB, Yang JH, Chung KH. Characterization of the cytokine profile of platelet rich plasma (PRP) and PRP-induced cell proliferation and migration: Upregulation of matrix metalloproteinase-1 and -9 in HaCaT cells. Korean J Hematol 2011;46:265–73.
Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol 1967;13:269–88.
Woth G, Tőkés-Füzesi M, Magyarlaki T, Kovács GL, Vermes I, Mühl D. Activated platelet-derived microparticle numbers are elevated in patients with severe fungal (Candida albicans) sepsis. Ann Clin Biochem 2012;49:554–60.
Furze RC, Rankin SM. Neutrophil mobilization and clearance in the bone marrow. Immunology 2008;125:281–8.
Jagel MA, Hugli TE. Neutrophil chemotactic factors promote leukocytosis. A common mechanism for cellular recruitment from bone marrow. J Immunol,1992;148:1119–28.
Terashima T, English D, Hogg JC, van Eeden SF. Release of polymorphonuclear leukocytes from the bone marrow by interleukin-8. Blood 1998;92:1062–9.
Jagels MA, Chambers JD, Arfors KE, Hugli TE. C5a- and tumor necrosis factor-alpha-induced leukocytosis occurs independently of beta 2 integrins and L-selectin: Differential effects on neutrophil adhesion molecule expression in vivo. Blood 1995;85:2900–9.
Delano MJ, Kelly-Scumpia KM, Thayer TC, Winfield RD, Scumpia PO, Cuenca AG, et al. Neutrophil mobilization from the bone marrow during polymicrobial sepsis is dependent on CXCL12 signaling. J Immunol 2011;187:911–8.
Kovach MA, Standiford TJ. The function of neutrophils in sepsis. Curr Opin Infect Dis 2012;25:321–7.
Devi S, Wang Y, Chew WK, Lima R, A-González N, Mattar CN, et al. Neutrophil mobilization via plerixafor-mediated CXCR4 inhibition arises from lung demargination and blockade of neutrophil homing to the bone marrow. J Exp Med 2013;210:2321–36.
Christopher MJ, Link DC. Regulation of neutrophil homeostasis. Curr Opin Hematol 2007;14:3–8.
Sadik CD, Kim ND, Luster AD. Neutrophils cascading their way to inflammation. Trends Immunol 2011;32:452–60.
Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013;13:159–75.
Skoutelis AT, Kaleridis V, Athanassiou GM, Kokkinis KI, Missirlis YF, Bassaris HP. Neutrophil deformability in patients with sepsis, septic shock, and adult respiratory distress syndrome. Crit Care Med 2000;28:2355–9.
Drost EM, Kassabian G, Meiselman HJ, Gelmont D, Fisher TC. Increased rigidity and priming of polymorphonuclear leukocytes in sepsis. Am J Respir Crit Care Med 1999;159:1696–702.
Benjamim CF, Silva JS, Fortes ZB, Oliveira MA, Ferreira SH, Cunha FQ. Inhibition of leukocyte rolling by nitric oxide during sepsis leads to reduced migration of active microbicidal neutrophils. Infect Immun 2002;70:3602–10.
Benjamim CF, Ferreira SH, Cunha FQ. Role of nitric oxide in the failure of neutrophil migration in sepsis. J Infect Dis 2000;182:214–23.
Clements MK, Siemsen DW, Swain SD, Hanson AJ, Nelson-Overton LK, Rohn TT, et al. Inhibition of actin polymerization by peroxynitrite modulates neutrophil functional responses. J Leukoc Biol 2003;73:344–55.
Torres-Dueñas D, Celes MR, Freitas A, Alves-Filho JC, Spiller F, Dal-Secco D, et al. Peroxynitrite mediates the failure of neutrophil migration in severe polymicrobial sepsis in mice. Br J Pharmacol 2007;152:341–52.
Lefer DJ, Scalia R, Campbell B, Nossuli T, Hayward R, Salamon M, et al. Peroxynitrite inhibits leukocyte-endothelial cell interactions and protects against ischemia-reperfusion injury in rats. J Clin Invest 1997;99:684–91.
Naughton P, Hoque M, Green CJ, Foresti R, Motterlini R. Interaction of heme with nitroxyl or nitric oxide amplifies heme oxygenase-1 induction: involvement of the transcription factor Nrf2. Cell Mol Biol (Noisy-le-grand) 2002;48:885–94.
Freitas A, Alves-Filho JC, Secco DD, Neto AF, Ferreira SH, Barja-Fidalgo C, et al. Heme oxygenase/carbon monoxide-biliverdin pathway down regulates neutrophil rolling, adhesion and migration in acute inflammation. Br J Pharmacol 2006;149:345–54.
Banks JG, Foulis AK, Ledingham IM, Macsween RN. Liver function in septic shock. J Clin Pathol 1982;35:1249–52.
Zegdi R, Perrin D, Burdin M, Boiteau R, Tenaillon A. Increased endogenous carbon monoxide production in severe sepsis. Intensive Care Med 2002;28:793–6.
Cummings CJ, Martin TR, Frevert CW, Quan JM, Wong VA, Mongovin SM, et al. Expression and function of the chemokine receptors CXCR1 and CXCR2 in sepsis. J Immunol 1999;162:2341–6.
Speyer CL, Gao H, Rancilio NJ, Neff TA, Huffnagle GB, Sarma JV, et al. Novel chemokine responsiveness and mobilization of neutrophils during sepsis. Am J Pathol 2004;165:2187–96.
Alves-Filho JC, Freitas A, Souto FO, Spiller F, Paula-Neto H, Silva JS, et al. Regulation of chemokine receptor by Toll-like receptor 2 is critical to neutrophil migration and resistance to polymicrobial sepsis. Proc Natl Acad Sci U S A 2009;106:4018–23.
Zou L, Feng Y, Zhang M, Li Y, Chao W. Nonhematopoietic toll-like receptor 2 contributes to neutrophil and cardiac function impairment during polymicrobial sepsis. Shock 2011;36:370–80.
Souto FO, Alves-Filho JC, Turato WM, Auxiliadora-Martins M, Basile-Filho A, Cunha FQ. Essential role of CCR2 in neutrophil tissue infiltration and multiple organ dysfunction in sepsis. Am J Respir Crit Care Med 2011;183:234–42.
Reddy RC, Narala VR, Keshamouni VG, Milam JE, Newstead MW, Standiford TJ. Sepsis-induced inhibition of neutrophil chemotaxis is mediated by activation of peroxisome proliferator-activated receptor-{gamma}. Blood 2008;112:4250–8.
Kurihara T, Jones CN, Yu YM, Fischman AJ, Watada S, Tompkins RG, et al. Resolvin D2 restores neutrophil directionality and improves survival after burns. FASEB J 2013;27:2270–81.
Gordy C, Pua H, Sempowski GD, He YW. Regulation of steady-state neutrophil homeostasis by macrophages. Blood 2011;117:618–29.
Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: Clearance of apoptotic cells regulates immune responses. Nat Rev Immunol 2002;2:965–75.
Jimenez MF, Watson RW, Parodo J, Evans D, Foster D, Steinberg M, et al. Dysregulated expression of neutrophil apoptosis in the systemic inflammatory response syndrome. Arch Surg 1997;132:1263–70.
Keel M, Ungethüm U, Steckholzer U, Niederer E, Hartung T, Trentz O, et al. Interleukin-10 counterregulates proinflammatory cytokine-induced inhibition of neutrophil apoptosis during severe sepsis. Blood 1997;90:3356–63.
Taneja R, Parodo J, Jia SH, Kapus A, Rotstein OD, Marshall JC. Delayed neutrophil apoptosis in sepsis is associated with maintenance of mitochondrial transmembrane potential and reduced caspase-9 activity. Crit Care Med 2004;32:1460–9.
Paunel-Görgülü A, Zörnig M, Lögters T, Altrichter J, Rabenhorst U, Cinatl J, et al. Mcl-1-mediated impairment of the intrinsic apoptosis pathway in circulating neutrophils from critically ill patients can be overcome by Fas stimulation. J Immunol 2009;183:6198–206.
Akhtar S, Li X, Kovacs EJ, Gamelli RL, Choudhry MA. Interleukin-18 delays neutrophil apoptosis following alcohol intoxication and burn injury. Mol Med 2011;17:88–94.
Paunel-Görgülü A, Kirichevska T, Lögters T, Windolf J, Flohé S. Molecular mechanisms underlying delayed apoptosis in neutrophils from multiple trauma patients with and without sepsis. Mol Med 2012;18:325–35.
Makishima T, Yoshimi M, Komiyama S, Hara N, Nishimoto T. A subunit of the mammalian oligosaccharyltransferase, DAD1, interacts with Mcl-1, one of the bcl-2 protein family. J Biochem 2000;128:399–405.
Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science 2004;303:1532–5.
Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med 2007;13:463–9.
Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol 2010;31:318–24.
Asaduzzaman M, Rahman M, Jeppsson B, Thorlacius H. P-selectin glycoprotein-ligand-1 regulates pulmonary recruitment of neutrophils in a platelet-independent manner in abdominal sepsis. Br J Pharmacol 2009;156:307–15.
Lam FW, Burns AR, Smith CW, Rumbaut RE. Platelets enhance neutrophil transendothelial migration via P-selectin glycoprotein ligand-1. Am J Physiol Heart Circ Physiol 2011;300:H468–75.
Kornerup KN, Salmon GP, Pitchford SC, Liu WL, Page CP. Circulating platelet-neutrophil complexes are important for subsequent neutrophil activation and migration. J Appl Physiol (1985) 2010;109:758–67.
Konstantopoulos K, Neelamegham S, Burns AR, Hentzen E, Kansas GS, Snapp KR, et al. Venous levels of shear support neutrophil-platelet adhesion and neutrophil aggregation in blood via P-selectin and beta2-integrin. Circulation 1998;98:873–82.
Blanks JE, Moll T, Eytner R, Vestweber D. Stimulation of P-selectin glycoprotein ligand-1 on mouse neutrophils activates beta 2-integrin mediated cell attachment to ICAM-1. Eur J Immunol 1998;28:433–43.
Yeo EL, Sheppard JA, Feuerstein IA. Role of P-selectin and leukocyte activation in polymorphonuclear cell adhesion to surface adherent activated platelets under physiologic shear conditions (an injury vessel wall model). Blood 1994;83:2498–507.
McDonald B, Urrutia R, Yipp BG, Jenne CN, Kubes P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe 2012;12:324–33.
von Brühl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med 2012;209:819–35.
Lorente L, Martín MM, Varo N, Borreguero-León JM, Solé-Violán J, Blanquer J, et al. Association between serum soluble CD40 ligand levels and mortality in patients with severe sepsis. Crit Care 2011;15:R97.
Tuinman PR, Juffermans NP. Soluble CD40 ligand, a mediator of sepsis or of transfusion-related adverse effects? Crit Care 2011;15:429.
Rahman M, Zhang S, Chew M, Syk I, Jeppsson B, Thorlacius H. Platelet shedding of CD40L is regulated by matrix metalloproteinase-9 in abdominal sepsis. J Thromb Haemost 2013;11:1385–98.
Li G, Sanders JM, Bevard MH, Sun Z, Chumley JW, Galkina EV, et al. CD40 ligand promotes Mac-1 expression, leukocyte recruitment, and neointima formation after vascular injury. Am J Pathol 2008;172:1141–52.
Rahman M, Zhang S, Chew M, Ersson A, Jeppsson B, Thorlacius H. Platelet-derived CD40L (CD154) mediates neutrophil upregulation of Mac-1 and recruitment in septic lung injury. Ann Surg 2009;250:783–90.
Derive M, Bouazza Y, Sennoun N, Marchionni S, Quigley L, Washington V, et al. Soluble TREM-like transcript-1 regulates leukocyte activation and controls microbial sepsis. J Immunol 2012;188:5585–92.
Haselmayer P, Grosse-Hovest L, von Landenberg P, Schild H, Radsak MP. TREM-1 ligand expression on platelets enhances neutrophil activation. Blood 2007;110:1029–35.
Washington AV, Gibot S, Acevedo I, Gattis J, Quigley L, Feltz R, et al. TREM-like transcript-1 protects against inflammation-associated hemorrhage by facilitating platelet aggregation in mice and humans. J Clin Invest 2009;119:1489–501.
Russwurm S, Vickers J, Meier-Hellmann A, Spangenberg P, Bredle D, Reinhart K, et al. Platelet and leukocyte activation correlate with the severity of septic organ dysfunction. Shock 2002;17:263–8.
Singer G, Urakami H, Specian RD, Stokes KY, Granger DN. Platelet recruitment in the murine hepatic microvasculature during experimental sepsis: Role of neutrophils. Microcirculation 2006;13:89–97.
Ogura H, Kawasaki T, Tanaka H, Koh T, Tanaka R, Ozeki Y, et al. Activated platelets enhance microparticle formation and platelet-leukocyte interaction in severe trauma and sepsis. J Trauma 2001;50:801–9.
Gibot S, Massin F, Marcou M, Taylor V, Stidwill R, Wilson P, et al. TREM-1 promotes survival during septic shock in mice. Eur J Immunol 2007;37:456–66.
Acknowledgements
This work was supported by National Natural Science Foundation of China, No. 30772256, 81071546 and No. 81272148, and Jiangsu Provincial Natural Science Foundation, No. BK2012703.
Author information
Authors and Affiliations
Corresponding author
Additional information
How to cite this article: Wang X, Qin W, Sun B. New strategy for sepsis: Targeting a key role of platelet-neutrophil interaction. Burn Trauma 2014;2:114–20.
Source of Support: This work was supported by National Natural Science Foundation of China, No. 30772256, 81071546 and No. 81272148, and Jiangsu Provincial Natural Science Foundation, No. BK2012703. Conflict of Interest: None declared.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made
About this article
Cite this article
Wang, X., Qin, W. & Sun, B. New strategy for sepsis: Targeting a key role of platelet-neutrophil interaction. Burn Trauma 2, 114–120 (2014). https://doi.org/10.4103/2321-3868.135487
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.4103/2321-3868.135487