Abstract
Introduction
CD40 Ligand (CD40L) and its soluble counterpart (sCD40L) are proteins that exhibit prothrombotic and proinflammatory properties on binding to their cell surface receptor CD40. The results of small clinical studies suggest that sCD40L levels could play a role in sepsis; however, there are no data on the association between sCD40L levels and mortality of septic patients. Thus, the aim of this study was to determine whether circulating sCD40L levels could be a marker of adverse outcome in a large cohort of patients with severe sepsis.
Methods
This was a multicenter, observational and prospective study carried out in six Spanish intensive care units. Serum levels of sCD40L, tumour necrosis factor-alpha and interleukin-10, and plasma levels of tissue factor were measured in 186 patients with severe sepsis at the time of diagnosis. Serum sCD40L was also measured in 50 age- and sex-matched controls. Survival at 30 days was used as the endpoint.
Results
Circulating sCD40L levels were significantly higher in septic patients than in controls (P = 0.01), and in non-survivors (n = 62) compared to survivors (n = 124) (P = 0.04). However, the levels of CD40L were not different regarding sepsis severity. Logistic regression analysis showed that sCD40L levels >3.5 ng/mL were associated with higher mortality at 30 days (odds ratio = 2.89; 95% confidence interval = 1.37 to 6.07; P = 0.005). The area under the curve of sCD40L levels >3.5 ng/mL as predictor of mortality at 30 days was 0.58 (95% CI = 0.51 to 0.65; P = 0.03).
Conclusions
In conclusion, circulating sCD40L levels are increased in septic patients and are independently associated with mortality in these patients; thus, its modulation could represent an attractive therapeutic target.
Similar content being viewed by others
Introduction
Severe sepsis is a common, expensive, and frequently fatal condition, leading to as many deaths annually as acute myocardial infarction [1]. Thus, a continuous search for new biomarkers in sepsis is necessary to aid early diagnosis and stratification of its severity.
CD40 Ligand (CD40L) and its soluble counterpart (sCD40L) are proteins that exhibit prothrombotic and proinflammatory properties on binding to their cell surface receptor CD40 [2, 3]. CD40L is a member of the tumour necrosis factor (TNF) family and is expressed as a transmembrane protein in activated platelets [4, 5]. CD40L exerts several pro-inflammatory [6, 7] and procoagulant [8–13] effects.
Higher levels of sCD40L have been found in patients with acute coronary syndrome [14, 15], stroke [16], systemic lupus erythematosus [17], and chronic lymphocytic leukemia [18]. The role of sCD40L in sepsis has hardly been studied. In some animal models, an increase in sCD40L was reported after the development of sepsis [19, 20]. In humans, higher sCD40L levels were found in 49 patients with meningococcal sepsis and 15 patients with African tick bite fever compared with controls [21, 22]. In other small series with pulmonary tuberculosis, higher sCD40L levels were found in patients with more severe disease [23, 24]. A study including 35 septic patients found higher circulating sCD40L levels in non-surviving than in surviving patients [25]; however, there are no data on the association between circulating sCD40L levels and mortality of septic patients.
We hypothesized that circulating sCD40L levels could be associated with an adverse outcome in patients with severe sepsis. The primary objective of this study was to determine the association between the circulating sCD40L levels and mortality, and the secondary objective to determine the association between circulating sCD40L, inflammatory and prothrombotic markers in these patients.
Materials and methods
Design and subjects
A multicenter, observational, prospective study was carried out in six Spanish ICUs. The study was approved by the Institutional Review Boards of the six hospitals and written informed consent from the patients or from the family members was obtained. A total of 186 patients with severe sepsis and 50 age- and sex-matched healthy controls were included.
The diagnosis of sepsis and severe sepsis was established according to the International Sepsis Definitions Conference [26]. Severe sepsis was defined as sepsis complicated by organ dysfunction. Sepsis was defined as a documented or suspected infection (defined as a pathologic process induced by a microorganism) and some of the following parameters: I) General parameters: fever (core temperature higher than 38.3°C), hypothermia (core temperature lower than 36.0°C), tachycardia (heart rate higher than 90 beats/minute), tachypnea (respiratory rate higher than 30 breaths/minute), altered mental status, significant edema or positive fluid balance (higher than 20 ml/kg over 24 hours), hyperglycemia (plasma glucose higher than 110 mg/dl) in the absence of diabetes; II) Inflammatory parameters: leukocytosis (white blood cell count higher than 12,000/mm3), leukopenia (white blood cell count lower than 4,000 mm3), normal white blood cell count with a percentage of immature forms higher than 10%, plasma C-reactive protein >2 standard deviations above the normal value, plasma procalcitonin >2 standard deviations above the normal value; III) Hemodynamic parameters: arterial hypotension (systolic blood pressure lower than 90 mmHg, mean arterial blood pressure lower than 70 mmHg, or decrease of systolic blood pressure from the baseline higher than 40 mmHg), mixed venous oxygen saturation higher than 70%, cardiac index higher than 3.5 l/min/m2; IV) Organ dysfunction: arterial hypoxemia (pressure of arterial oxygen/fraction inspired oxygen (PaO2/FIO2) ratio <300), acute oliguria (urine output <0.5 ml/kg/h for at least two hours), creatinine increase ≥0.5 mg/dl, thrombocytopenia (platelet count <100,000/mm3), hyperbilirubinemia (total bilirubin >4 mg/dl); V) Tissue perfusion parameters: hyperlactatemia (>3 mmol/l), decreased capillary refill or mottling.
Exclusion criteria were: age <18 years, pregnancy, lactation, human immunodeficiency virus (HIV), solid or haematological tumour, or immunosuppressive, steroid or radiation therapy.
Variables recorded
The following variables were recorded for each patient: sex, age, diabetes mellitus, chronic obstructive pulmonary disease (COPD), site of infection, creatinine, leukocytes, lactatemia, platelets, Acute Physiology and Chronic Health Evaluation II (APACHE II) score [27], Sepsis-related Organ Failure Assessment (SOFA) score [28]. We assessed survival at 30 days as the endpoint.
Blood samples were collected from 186 patients with severe sepsis at the time of the diagnosis and from 50 age- and sex-matched controls.
Serum levels of sCD40L
Venous blood samples were collected in serum separator tubes (SST) and centrifuged within 30 minutes at 1,000*g for 15 minutes. The serum was removed and frozen at -80°C until measurement. Serum sCD40L levels were assayed by specific ELISA (Bender MedSystems, Vienna, Austria) according to the manufacturer's instructions in the Atherosclerosis Research Laboratory of CIMA-University of Navarra (Pamplona, Spain).
Plasma levels of TF
Venous blood samples were collected in citrate collected plasma tubes and centrifuged within 30 minutes at 1,000*g for 15 minutes. The plasma was removed and frozen at -80°C until measurement. Assays for TF antigen were performed by specific ELISA (Imubind Tissue Factor ELISA™, American Diagnostica, Inc, Stanford, CT, USA) in the Laboratory Department of the Hospital Universitario de Canarias (La Laguna, Santa Cruz de Tenerife, Spain).
Serum levels of TNF-α and IL-10
Serum separator tubes (SST) were used to determine TNF-α and IL-10 serum levels. Venous blood samples were taken and centrifuged within 30 minutes at 1,000 g for 15 minutes, and the serum was removed and frozen at -80°C until measurement. TNF-α and IL-10 serum levels were measured by a solid-phase, chemiluminiscent immunometric assay kits (Immulite®, Siemens Healthcare Diagnostics Products, Llanberis, UK) in the Laboratory Deparment of the Hospital Universitario de Canarias (La Laguna, Santa Cruz de Tenerife, Spain).
Statistical analysis
In a pilot study with 30 patients with severe sepsis, we found that surviving patients show lower circulating levels of sCD40L (3.83 ± 1.44 ng/mL) than non-survivors (4.37 ± 1.52 ng/mL). We calculated to include 186 patients in a cohort study to demonstrate significant differences in the circulating levels of sCD40L between groups, for a power of 80% and a 5% type I error rate.
Continuous variables are reported as medians and interquartile ranges. Categorical variables are reported as frequencies and percentages. Comparisons of continuous variables between groups were carried out using Wilcoxon-Mann-Whitney test. Comparisons between groups on categorical variables were carried out with chi-square test. The association between continuous variables was carried out using Spearman's rank correlation coefficient or Spearman's rho coefficient. The cut-off 3.5 ng/mL was selected using a likelihood method as previously described [29]. Receiver operation characteristic (ROC) curves using lactate, APACHE score, sCD40L ≥3.5 ng/mL as independent variables, and exitus at 30 days as a dependent variable were obtained. To calculate the standard error of the area under the curves we used the method of Delong et al. [30]. Survival curves at 30 days, using sCD40L levels ≥ or lower than 3.5 ng/mL, were represented using the Kaplan-Meier method and were compared by log-rank test. Multivariate logistic regression analysis was applied to determine the independent contribution of sCD40L levels, lactate levels, APACHE-II score and thrombocytopenia to the prediction of the mortality during the 30-day period. Odds ratio and its 95% confidence intervals were calculated as measurement of the clinical impact of the predictor variables. A P-value of less than 0.05 was considered statistically significant. Statistical analyses were performed with SPSS 17.0 (SPSS Inc., Chicago, IL, USA), NCSS 2000 (Kaysville, UT, USA), and Statistic 8.0 (Tulsa, OK, USA).
Results
Baseline characteristics of 186 patients with severe sepsis and 50 age- and sex-matched controls are shown in Table 1. Higher sCD40L levels were observed in the group of patients with severe sepsis compared with controls (P = 0.01) (Table 1).
Non-surviving septic patients (n = 62) showed higher sCD40L levels (P = 0.04) than survivors (n = 124) after the 30-day follow-up. Non-surviving patients also showed a higher incidence of diabetes mellitus (P = 0.02), higher lactatemia (P < 0.001), higher SOFA (P < 0.001) and APACHE-II (P < 0.001) scores, and lower platelet count (P = 0.002) and IL-10 (P < 0.001) than surviving patients (Table 2).
We did not find differences in 30-day survival between those patients that received statins before sepsis compared with those that did not receive statins (Table 2). After the diagnosis of sepsis, none of the patients continued receiving statins.
We did not find significant differences in sCD40L serum levels according to sex, diabetes mellitus status, COPD, use of statins before sepsis diagnosis, personal history of ischemic heart disease, need for mechanical ventilation and presence of septic shock (Table 3). Neither did we find significant differences in sCD40L serum levels according to the site and source of infection (Table 4).
Logistic regression analysis showed that serum sCD40L levels ≥3.5 ng/mL, lactatemia, APACHE-II score and platelet count <60,000/mm3 were associated with death at Day 30 (Table 5).
Survival analysis showed that patients with sCD40L levels ≥3.5 ng/mL presented higher mortality during the 30-day period than patients with lower levels (Chi-square: 4.50; P = 0.03) (Figure 1).
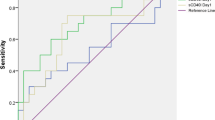
The areas under the curves (AUC) for each predictor of mortality were the following: sCD40L ≥3.5 ng/mL (AUC = 0.58; 95% CI = 0.51 to 0.65; P = 0.03), lactatemia (AUC = 0.66; 95% CI = 0.59 to 0.73; P < 0.001) and APACHE-II (AUC = 0.70; 95% CI = 0.62 to 0.76; P < 0.001) (Figure 2).
In the group of septic patients there were found an association between serum sCD40L levels and tissue factor levels (rho = 0.26; P = 0.005) (Figure 3) and platelet count (rho = 0.26; P < 0.001); but no association with lactatemia, TNF-α and IL-10 levels, SOFA or APACHE-II scores were observed (Table 6).
Discussion
The main finding of the present study is that serum sCD40L levels were independently associated with mortality at 30 days in a large series of septic patients.
We found higher serum sCD40L levels in patients with severe sepsis than in healthy controls, in agreement with previous studies [21, 22, 25]. We also found higher circulating sCD40L levels in non-surviving than in surviving patients, as previously reported by Nolan et al. in a small series [25]. In addition, we found that serum sCD40L levels ≥3.50 ng/mL were associated with higher death during the 30-day period in the multiple logistic regression analysis. Impaired prognosis was previously reported in patients with acute coronary artery syndrome and higher sCD40L levels [31]; however, our study is the first reporting an impaired prognosis in patients with severe sepsis and higher sCD40L levels.
The role of sCD40L in sepsis remains unclear; but it is possible that there are similarities with findings observed in coronary artery disease [2]. CD40L is stored in α-granules in unstimulated platelets but rapidly translocates to the platelet surface when platelets are activated, where it is cleaved and released into circulation as sCD40L. The sCD40L binds to circulating monocytes through its receptor CD40, promoting their adhesion to vascular endothelium. The sCD40L also binds to CD40 on endothelial cell surfaces. Activated endothelial cells produce the overexpression of transcriptional factors such as nuclear factor-kappa B (NF-kß) [32], with subsequent up-regulation of proinflammatory and prothrombotic factors. Thus, sCD40L could have prothrombotic effects via induction of TF [8–11], diminishing thrombomodulin expression [10, 11], and binding to the glycoprotein IIb/IIIa platelet receptor [12, 13]. All these effects could facilitate the development of vascular thrombosis, organ dysfunction and death.
We report for first time an association between sCD40L and TF levels in patients with severe sepsis, which has been previously described in culture of vascular endothelial cells [8–11]. However, the observed association between sCD40L levels and mortality could not been explained by the TF levels, since there were no significant differences between non-surviving and surviving patients. It is possible that other reported prothrombotic effects of sCD40L, such as reduced thrombomodulin expression [10, 11] and binding to the glycoprotein IIb/IIIa platelet receptor [12, 13] could lead to vascular thrombosis and, finally, death in patients with severe sepsis.
We found a positive association between serum sCD40L levels and platelet count, possibly because platelets are the major source of sCD40L in circulation [4, 5]. However, we did not find this association in thrombocytopenic patients, which may be explained by different underlying mechanisms in septic patients [33, 34], such as immune destruction by platelet antibodies, hematophagocytosis in the bone marrow, reduced production due to bone marrow depression and non-immune destruction by the interaction of activated platelets with endothelium.
We failed to find an association between sCD40L levels and sepsis severity criteria such as lactatemia, APACHE II score, SOFA score, TNF-α or IL-10 levels. This is in agreement with the results by Nolan et al. [25] reporting no correlation between sCD40L and the concentrations of IL-6, IL-12 or APACHE II. The lack of correlation may be due to a true absence of relationship or that sCD40L levels are underestimated in more severe disease (for example, dilution, leakage to the intersticial space and urin, increased uptake at sites of inflammation, and so on). In addition, sCD40L would most likely response earlier to changes in inflammatory activity that the APACHE, which is a composite of multiple parameters.
Whereas the strength of our study was the relatively large sample size compared with previous reports assessing sCD40L levels in septic patients, some limitations should be recognized. We determined a single testing point for sCD40L levels and we were, therefore, unable to establish the time course of serum sCD40L levels. Data on other coagulation factors were not analyzed. We determined sCD40 levels only in serum and not in plasma samples to evaluate possible differences due to there has been reported higher sCD40L levels in serum than in plasma levels [35], and in platelet rich plasma than in platelet poor plasma [36, 37]. There has been reporting the association between sCD40L levels and other cytokines as IL-12 or interferon-gamma; however, we have not explored this possible association [6, 38]. Neither, we have explored procalcitonin to determine its association with sCD40L levels. There are been reported an association between sCD40L levels and severity of acute coronary artery syndrome [31] and between troponin and severity of sepsis [39–41]; however, we have not investigated markers of cardiac damage to explore its association with sCD40L levels. The time until the diagnosis of sepsis can influence the levels of sCD40L observed; however, we have not report it. The serum blood samples for the determination of sCD40L were obtained at moment of sepsis diagnosis and APACHE II was calculated at 24 hours of admission to ICU; thus, we did know if this time-gap can affect in the association between both variables. We have not found significant difference in the survival at 30 days with the use of statins previously to the diagnosis of severe sepsis; and it was not possible to explore the effect of this agent group during the sepsis because in all patients was it suspended.
From a therapeutic perspective, the use of sCD40L modulators could be used as a new class of drugs for the treatment of severe sepsis [42–46]. In one study including patients with coronary artery disease, the use of statins decreased circulating sCD40L levels [42]. Besides, the results of some studies suggest that the use of statins could improve the prognosis in patients with infectious episodes [43–46]. However, in some human and animal studies the use of antibody against CD40L was associated with platelet activation and thromboembolic complications [47–49].
Conclusions
In conclusion, circulating sCD40L levels are increased in septic patients and are independently associated with mortality in these patients; thus, its modulation could represent an attractive therapeutic target.
Key messages
-
Patients with severe sepsis showed higher circulating sCD40L levels than healthy controls.
-
Non-survivor septic patients showed higher circulating sCD40L levels than survivor ones.
-
Modulation of circulating sCD40L levels could represent an attractive therapeutic target in sepsis.
Abbreviations
- APACHE:
-
Acute Phisiology and Chronic Health Evaluation
- ICU:
-
Intensive Care Unit
- sCD40L:
-
soluble CD40 Ligand
- SOFA:
-
Sepsis-related Organ Failure Assessment score.
References
Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR: Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001, 29: 1303-1310. 10.1097/00003246-200107000-00002
Antoniades C, Bakogiannis C, Tousoulis D, Antonopoulos AS, Stefanadis C: The CD40/CD40 ligand system: linking inflammation with atherothrombosis. J Am Coll Cardiol 2009, 54: 669-677. 10.1016/j.jacc.2009.03.076
Ferroni P, Santilli F, Guadagni F, Basili S, Davì G: Contribution of platelet-derived CD40 ligand to inflammation, thrombosis and neoangiogenesis. Curr Med Chem 2007, 14: 2170-2180. 10.2174/092986707781389664
Aukrust P, Damås JK, Solum NO: Soluble CD40 ligand and platelets: self-perpetuating pathogenic loop in thrombosis and inflammation? J Am Coll Cardiol 2004, 43: 2326-2328. 10.1016/j.jacc.2004.03.023
Anand SX, Viles-Gonzalez JF, Badimon JJ, Cavusoglu E, Marmur JD: Membrane-associated CD40L and sCD40L in atherothrombotic disease. Thromb Haemost 2003, 90: 377-384.
Mach F, Schönbeck U, Sukhova GK, Bourcier T, Bonnefoy JY, Pober JS, Libby P: Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for CD40-CD40 ligand signaling in atherosclerosis. Proc Natl Acad Sci USA 1997, 94: 1931-1936. 10.1073/pnas.94.5.1931
Noelle RJ, Roy M, Shepherd DM, Stamenkovic I, Ledbetter JA, Aruffo A: A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc Natl Acad Sci U S A 1992, 89: 6550-6554. 10.1073/pnas.89.14.6550
Zhou L, Stordeur P, de Lavareille A, Thielemans K, Capel P, Goldman M, Pradier O: CD40 engagement on endothelial cells promotes tissue factor-dependent procoagulant activity. Thromb Haemost 1998, 79: 1025-1028.
Hezi-Yamit A, Wong PW, Bien-Ly N, Komuves LG, Prasad KS, Phillips DR, Sinha U: Synergistic induction of tissue factor by coagulation factor Xa and TNF: evidence for involvement of negative regulatory signaling cascades. Proc Natl Acad Sci USA 2005, 102: 12077-12082. 10.1073/pnas.0504526102
Miller DL, Yaron R, Yellin MJ: CD40L-CD40 interactions regulate endothelial cell surface tissue factor and thrombomodulin expression. J Leukoc Biol 1998, 63: 373-379.
Slupsky JR, Kalbas M, Willuweit A, Henn V, Kroczek RA, Müller-Berghaus G: Activated platelets induce tissue factor expression on human umbilical vein endothelial cells by ligation of CD40. Thromb Haemost 1998, 80: 1008-1014.
Prasad KS, Andre P, He M, Bao M, Manganello J, Phillips DR: Soluble CD40 ligand induces beta3 integrin tyrosine phosphorylation and triggers platelet activation by outside-in signaling. Proc Natl Acad Sci USA 2003, 100: 12367-12371. 10.1073/pnas.2032886100
André P, Prasad KS, Denis CV, He M, Papalia JM, Hynes RO, Phillips DR, Wagner DD: CD40L stabilizes arterial thrombi by a beta3 integrin--dependent mechanism. Nat Med 2002, 8: 247-252. 10.1038/nm0302-247
Aukrust P, Muller F, Ueland T, Berget T, Aaser E, Brunsvig A, Solum NO, Forfang K, Frøland SS, Gullestad L: Enhanced levels of soluble and membrane-bound CD40 ligand in patients with unstable angina: possible reflection of T lymphocyte and platelet involvement in the pathogenesis of acute coronary syndromes. Circulation 1999, 100: 614-620.
Varo N, de Lemos JA, Libby P, Morrow DA, Murphy SA, Nuzzo R, Gibson CM, Cannon CP, Braunwald E, Schonbeck U: Soluble CD40L: risk prediction after acute coronary syndromes. Circulation 2003, 108: 1049-1052. 10.1161/01.CIR.0000088521.04017.13
Garlichs CD, Kozina S, Fateh-Moghadam S, Tomandl B, Stumpf C, Eskafi S, Raaz D, Schmeier A, Yilmaz A, Ludwig J, Neundörfer B, Daniel WG: Upregulation of CD40-CD40 Ligand (CD154) in patients with acute cerebral ischemia. Stroke 2003, 34: 1412-1418. 10.1161/01.STR.0000074032.64049.47
Vakkalanka RK, Woo C, Kirou KA, Koshy M, Berger D, Crow MK: Elevated levels and functional capacity of soluble CD40 ligand in systemic lupus erythematosus sera. Arthritis Rheum 1999, 42: 871-881. 10.1002/1529-0131(199905)42:5<871::AID-ANR5>3.0.CO;2-J
Clodi K, Asgary Z, Zhao S, Kliche KO, Cabanillas F, Andreeff M, Younes A: Coexpression of CD40 and CD40 ligand in B-cell lymphoma cells. Br J Haematol 1998, 103: 270-275. 10.1046/j.1365-2141.1998.01031.x
Ding Y, Chung CS, Newton S, Chen Y, Carlton S, Albina JE, Ayala A: Polymicrobial sepsis induces divergent effects on splenic and peritoneal dendritic cell function in mice. Shock 2004, 22: 137-144. 10.1097/01.shk.0000131194.80038.3f
Rahman M, Zhang S, Chew M, Ersson A, Jeppsson B, Thorlacius H: Platelet-derived CD40L (CD154) mediates neutrophil upregulation of Mac-1 and recruitment in septic lung injury. Ann Surg 2009, 250: 783-790. 10.1097/SLA.0b013e3181bd95b7
Inwald DP, Faust SN, Lister P, Peters MJ, Levin M, Heyderman R, Klein NJ: Platelet and soluble CD40L in meningococcal sepsis. Intensive Care Med 2006, 32: 1432-1437. 10.1007/s00134-006-0250-2
Damås JK, Jensenius M, Ueland T, Otterdal K, Yndestad A, Frøland SS, Rolain JM, Myrvang B, Raoult D, Aukrust P: Increased levels of soluble CD40L in African tick bite fever: possible involvement of TLRs in the pathogenic interaction between Rickettsia africae, endothelial cells, and platelets. J Immunol 2006, 177: 2699-2706.
Mizusawa M, Kawamura M, Takamori M, Kashiyama T, Fujita A, Usuzawa M, Saitoh H, Ashino Y, Yano I, Hattori T: Increased synthesis of anti-tuberculous glycolipid immunoglobulin G (IgG) and IgA with cavity formation in patients with pulmonary tuberculosis. Clin Vaccine Immunol 2008, 15: 544-548. 10.1128/CVI.00355-07
Chegou NN, Black GF, Kidd M, van Helden PD, Walzl G: Host markers in QuantiFERON supernatants differentiate active TB from latent TB infection: preliminary report. BMC Pulm Med 2009, 9: 21. 10.1186/1471-2466-9-21
Nolan A, Weiden M, Kelly A, Hoshino Y, Hoshino S, Mehta N, Gold JA: CD40 and CD80/86 act synergistically to regulate inflammation and mortality in polymicrobial sepsis. Am J Respir Crit Care Med 2008, 177: 301-308. 10.1164/rccm.200703-515OC
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G, International Sepsis Definitions Conference: 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med 2003, 29: 530-538.
Knaus WA, Draper EA, Wagner DP, Zimmerman JE: APACHE II. a severity of disease classification system. Crit Care Med 1985, 13: 818-829. 10.1097/00003246-198510000-00009
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG, for the Working Group on Sepsis-related Problems of the European Society of Intensive Care Medicine: The Sepsis-related Organ Failure Assessment (SOFA) score to describe organ dysfunction/failure. Intensive Care Med 1996, 22: 707-710. 10.1007/BF01709751
Hintze JL: ROC curves. In NCSS 6.0 User's Guide-II. Kaysville, Utah: Number Cruncher Statistical Systems; 1995:1099-1110.
DeLong ER, DeLong DM, Clarke-Pearson DL: Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988, 44: 837-845. 10.2307/2531595
Dominguez-Rodriguez A, Abreu-Gonzalez P, Garcia-Gonzalez MJ, Kaski JC: Soluble CD40 ligand:interleukin-10 ratio predicts in-hospital adverse events in patients with ST-segment elevation myocardial infarction. Thromb Res 2007, 121: 293-299. 10.1016/j.thromres.2007.04.007
Chen Y, Chen J, Xiong Y, Da Q, Xu Y, Jiang X, Tang H: Internalization of CD40 regulates its signal transduction in vascular endothelial cells. Biochem Biophys Res Commun 2006, 345: 106-117. 10.1016/j.bbrc.2006.04.034
Warkentin TE, Aird WC, Rand JH: Platelet-endothelial interactions: sepsis, HIT, and antiphospholipid syndrome. Hematology Am Soc Hematol Educ Program 2003, 497-519.
Levi M: Platelets at a crossroad of pathogenic pathways in sepsis. J Thromb Haemost 2004, 2: 2094-2095. 10.1111/j.1538-7836.2004.01004.x
Weber M, Rabenau B, Stanisch M, Elsaesser A, Mitrovic V, Heeschen C, Hamm C: Influence of sample type and storage conditions on soluble CD40 ligand assessment. Clin Chem 2006, 52: 888-891. 10.1373/clinchem.2005.062083
Cognasse F, Boussoulade F, Chavarin P, Acquart S, Fabrigli P, Lamy B, Garraud O: Release of potential immunomodulatory factors during platelet storage. Transfusion 2006, 46: 1184-1189. 10.1111/j.1537-2995.2006.00869.x
Khan SY, Kelher MR, Heal JM, Blumberg N, Boshkov LK, Phipps R, Gettings KF, McLaughlin NJ, Silliman CC: Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury. Blood 2006, 108: 2455-2462. 10.1182/blood-2006-04-017251
Kalady MF, Onaitis MW, Emani S, Abdel-Wahab Z, Tyler DS, Pruitt SK: Sequential delivery of maturation stimuli increases human dendritic cell IL-12 production and enhances tumor antigen-specific immunogenicity. J Surg Res 2004, 116: 24-31. 10.1016/j.jss.2003.09.003
John J, Woodward DB, Wang Y, Yan SB, Fisher D, Kinasewitz GT, Heiselman D: Troponin-I as a prognosticator of mortality in severe sepsis patients. J Crit Care 2010, 25: 270-275. 10.1016/j.jcrc.2009.12.001
Røsjø H, Varpula M, Hagve TA, Karlsson S, Ruokonen E, Pettilä V, Omland T, FINNSEPSIS Study Group: Circulating high sensitivity troponin T in severe sepsis and septic shock: distribution, associated factors, and relation to outcome. Intensive Care Med 2011, 37: 77-85. 10.1007/s00134-010-2051-x
Mehta NJ, Khan IA, Gupta V, Jani K, Gowda RM, Smith PR: Cardiac troponin I predicts myocardial dysfunction and adverse outcome in septic shock. Int J Cardiol 2004, 95: 13-17. 10.1016/j.ijcard.2003.02.005
Li J, Zhao SP, Peng DQ, Xu ZM, Zhou HN: Early effect of pravastatin on serum soluble CD40L, matrix metalloproteinase-9, and C-reactive protein in patients with acute myocardial infarction. Clin Chem 2004, 50: 1696-1699. 10.1373/clinchem.2003.030940
Tleyjeh IM, Kashour T, Hakim FA, Zimmerman VA, Erwin PJ, Sutton AJ, Ibrahim T: Statins for the prevention and treatment of infections: a systematic review and meta-analysis. Arch Intern Med 2009, 169: 1658-1667. 10.1001/archinternmed.2009.286
Thomsen RW, Riis A, Kornum JB, Christensen S, Johnsen SP, Sørensen HT: Preadmission use of statins and outcomes after hospitalization with pneumonia: population-based cohort study of 29,900 patients. Arch Intern Med 2008, 168: 2081-2087. 10.1001/archinte.168.19.2081
Hsu J, Andes DR, Knasinski V, Pirsch J, Safdar N: Statins are associated with improved outcomes of bloodstream infection in solid-organ transplant recipients. Eur J Clin Microbiol Infect Dis 2009, 28: 1343-1351. 10.1007/s10096-009-0787-4
Dobesh PP, Klepser DG, McGuire TR, Morgan CW, Olsen KM: Reduction in mortality associated with statin therapy in patients with severe sepsis. Pharmacotherapy 2009, 29: 621-630. 10.1592/phco.29.6.621
Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB: Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med 2000, 6: 114. 10.1038/72162
Robles-Carrillo L, Meyer T, Hatfield M, Desai H, Dávila M, Langer F, Amaya M, Garber E, Francis JL, Hsu YM, Amirkhosravi A: Anti-CD40L immune complexes potently activate platelets in vitro and cause thrombosis in FCGR2A transgenic mice. J Immunol 2010, 185: 1577-1583. 10.4049/jimmunol.0903888
Sidiropoulos PI, Boumpas DT: Lessons learned from anti-CD40L treatment in systemic lupus erythematosus patients. Lupus 2004, 13: 391-397. 10.1191/0961203304lu1032oa
Acknowledgements
This research was supported, in part, by funding from the Rafael Clavijo Foundation for Biomedical Research (Tenerife, Spain) and the "UTE project CIMA" (University of Navarra, Spain).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
LL was responsible of conceiving, designing and coordinating the study, making substantial contributions to acquisition of data, analysis and interpretation of data, and drafting the manuscript. MMM, JSV, JB, LL, CD, EP, FB, JMFA, JF and CL have made substantial contributions to acquisition of data and provided useful suggestions. NV, JO and JAR carried out the determination of sCD40L, and made substantial contributions to analysis and interpretation of data. JMB and EGM carried out the determination of tissue factor, TNF-alpha and IL-10, and made substantial contributions to analysis and interpretation of data. AJ made substantial contributions to analysis and interpretation of data. JAP contributed to study design, and made substantial contributions to analysis and interpretation of data. All authors read and approved the manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Lorente, L., Martín, M.M., Varo, N. et al. Association between serum soluble CD40 ligand levels and mortality in patients with severe sepsis. Crit Care 15, R97 (2011). https://doi.org/10.1186/cc10104
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/cc10104