Abstract
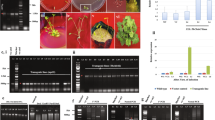
Many lines of evidence indicate that the increased activity of enzymes involved in phenolic compound biosynthesis and the subsequent accumulation of these compounds correlate with plant resistance to abiotic and biotic stresses. The production of anthocyanin pigment gene (PAP1) was previously identified using an activation tagging approach in Arabidopsis thaliana. A. thaliana PAP1-Dominant (pap1-D) mutant was generated by activation tagging. Throughout development, the pap1-D plant exhibited massive and widespread activation of genes encoding enzymes involved in phenylpropanoid natural product biosynthesis. Due to increased anthocyanin levels, pap1-D showed enhanced tolerance to salt, an important abiotic stress imposed on plants. In pap1-D plants, PAP1 transcript accumulation increased significantly in response to salt stress and abscisic acid. Enhanced anthocyanin levels induced by sucrose treatment increased the chance of survival of pap1-D and wild-type plants exposed to a high salt medium. Taken together, results of the present study indicate that genetic engineering of the flavonoid biosynthetic pathway can be used for successful improvement of plant survival in arid areas.
Similar content being viewed by others
References
Azuma K, Ohyama A, Ippoushi K, Ichiyanagi T, Takeuchi A, Saito T, and Fukuoka H (2008) Structures and antioxidant activity of anthocyanins in many accessions of eggplant and its related species. J Agr Food Chem 56, 10154–10159.
Bechtold N and Pelletier G (1998) In planta Agrobacteriummediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods Mol Biol 82, 259–266.
Borevitz JO, Xia Y, Blount J, Dixon RA, and Lamb C (2000) Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis. Plant Cell 12, 2383–2394.
Dixon RA, Xie DY, and Sharma SB (2005) Proanthocyanidins: a final frontier in flavonoid research? New Phytol 165, 9–28.
Grotewold E (2006) The genetics and biochemistry of floral pigments. Annu Rev Plant Biol 57, 761–780.
Ishitani M, Xiong L, Lee H, Stevenson B, and Zhu JK (1998) HOS1, a genetic locus involved in cold-responsive gene expression in Arabidopsis. Plant Cell 10, 1151–1162.
Jarillo JA, Leyva A, Salinas J, and Martinez-Zapater JM (1993) Low temperature induces the accumulation of alcohol dehydrogenase mRNA in Arabidopsis thaliana, a chillingtolerant plant. Plant Physiol 101, 833–837.
Jefferson RA, Kavanagh TA, and Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6, 3901–3907.
Kurkela S and Franck M (1990) Cloning and characterization of a cold-and ABA-inducible Arabidopsis gene. Plant Mol Biol 15, 137–144.
Lepiniec L, Debeaujon I, Routaboul JM, Baudry A, Pourcel L, Nesi N, and Caboche M (2006) Genetics and biochemistry of seed flavonoids. Annu Rev Plant Biol 57, 405–430.
Lesnick ML and Chandler VL (1998) Activation of the maize anthocyanin gene a2 is mediated by an element conserved in many anthocyanin promoters. Plant Physiol 117, 437–445.
Loreti E, Povero G, Novi G, Solfanelli C, Alpi A, and Perata P (2008) Gibberellins, jasmonate and abscisic acid modulate the sucrose-induced expression of anthocyanin biosynthetic genes in Arabidopsis. New Phytol 179, 1004–1016.
Mancinelli AL, Hoff AM, and Cottrell M (1988) Anthocyanin Production in Chl-Rich and Chl-Poor Seedlings. Plant Physiol 86, 652–654.
Mendoza I, Rubio F, Rodriguez-Navarro A, and Pardo JM (1994) The protein phosphatase calcineurin is essential for NaCl tolerance of Saccharomyces cerevisiae. J Biol Chem 269, 8792–8796.
Nakamura T, Liu Y, Hirata D, Namba H, Harada SI, Hirokawa T, and Miyakawa T (1993) Protein phosphatase type 2B (calcineurin)-mediated, FK506-sensitive regulation of intracellular ions in yeast is an important determinant for adaptation to high salt stress conditions. EMBO J 12, 4063–4071.
Pardo JM, Reddy MP, and Yang S (1998) Stress signaling through Ca2+/calmodulin-dependent protein phosphatase calcineurin mediates salt adaptation in plants. Proc Natl Acad Sci USA 95, 9681–9686.
Ray H, Yu M, Auser P, Blahut-Beatty L, McKersie B, Bowley S, Westcott N, Coulman B, Lloyd A, and Gruber MY (2003) Expression of anthocyanins and proanthocyanidins after transformation of alfalfa with maize Lc. Plant Physiol 132, 1448–1463.
Sainz MB, Grotewold E, and Chandler VL (1997) Evidence for direct activation of an anthocyanin promoter by the maize C1 protein and comparison of DNA binding by related myb domain proteins. Plant Cell 9, 611–625.
Shinozaki K and Yamaguchi-Shinozaki K (1997) Gene expression and signal transduction in water stress response. Plant Physiol 115, 327–334.
Shirley BW, Kubasek WL, Storz G, Bruggemann E, Koornneef M, Ausubel FM, and Goodman HM (1995) Analysis of Arabidopsis mutants deficient in flavonoid biosynthesis. Plant J 8, 659–671.
Solano R, Nieto C, Avila J, Canas L, Diaz I, and Paz-Ares J (1995) Dual DNA binding specificity of a petal epidermisspecific MYB transcription factor (MYB.Ph3) from Petunia hybrida. EMBO J 14, 1773–1784.
Solfanelli C, Poggi A, Loreti E, Alpi A, and Perata P (2006) Sucrose-Specific Induction of the Anthocyanin Biosynthetic Pathway in Arabidopsis. Plant Physiol 140, 637–646.
Song H, Zhao R, Fan P, Wang X, Chen X, and Li Y (2009) Overexpression of AtHsp90.2, AtHsp90.5 and AtHsp90.7 in Arabidopsis thaliana enhances plant sensitivity to salt and drought stresses. Planta 229, 955–964.
Spelt C, Quattrocchio F, Mol J, and Koes R (2002) ANTHOCYANIN1 of petunia controls pigment synthesis, vacuolar pH, and seed development by genetically distinct mechanisms. Plant Cell 14, 2121–2135.
Stockinger EJ, Gilmour SJ, and Thomashow MF (1997) Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/ DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc Natl Acad Sci USA 94, 1035–1040.
Teng S, Keurentjes J, Bentsink L, Koornneef M, and Smeekens S (2005) Sucrose-specific induction of anthocyanin biosynthesis in Arabidopsis requires the MYB75/PAP1 gene. Plant Physiol 139, 1840–1852.
Tohge T, Matsui K, Ohme-Takagi M, Yamazaki M, and Saito K (2005) Enhanced radical scavenging activity of genetically modified Arabidopsis seeds. Biotech Lett 27, 297–303.
van Acker FA, Schouten O, Haenen GR, van der Vijgh WJ, and Bast A (2000) Flavonoids can replace alpha-tocopherol as an antioxidant. FEBS Lett 473, 145–148.
Wang Q, Guan Y, Wu Y, Chen H, Chen F, and Chu C (2008) Overexpression of a rice OsDREB1F gene increases salt, drought, and low temperature tolerance in both Arabidopsis and rice. Plant Mol Biol 67, 589–602.
Wieland J, Nitsche AM, Strayle J, Steiner H, and Rudolph HK (1995) The PMR2 gene cluster encodes functionally distinct isoforms of a putative Na+ pump in the yeast plasma membrane. EMBO J 14, 3870–3882.
Winkel-Shirley B (2002) Biosynthesis of flavonoids and effects of stress. Curr Opin Plant Biol 5, 218–223.
Yamaguchi-Shinozaki K and Shinozaki K (1993) Characterization of the expression of a desiccation-responsive RD29 gene of Arabidopsis thaliana and analysis of its promoter in transgenic plants. Mol Gen Genet 236, 331–340.
Yamaguchi-Shinozaki K and Shinozaki K (1994) A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 6, 251–264.
Zhu JK (2001) Cell signaling under salt, water and cold stresses. Curr Opin Plant Biol 4, 401–406.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oh, J.E., Kim, Y.H., Kim, J.H. et al. Enhanced level of anthocyanin leads to increased salt tolerance in arabidopsis PAP1-D plants upon sucrose treatment. J. Korean Soc. Appl. Biol. Chem. 54, 79–88 (2011). https://doi.org/10.3839/jksabc.2011.011
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.3839/jksabc.2011.011