Abstract
We examined the efficacy and feasibility of an iPad® app used at-home in identifying a postexercise benefit to executive function. The iPad® app required simple reaching movements mirror-symmetrical to an exogenously presented target (i.e., antipointing) and is a task that lab-based behavioral and neuroimaging work has shown to provide a valid measure of the response inhibition component of executive function. Fifty English-speaking individuals (18 female, age range 18–26 years of age) completed the iPad® app before and immediately after a 20-min session of heavy-intensity aerobic exercise, and on a separate day completed the app prior to and following a 20-min non-exercise control condition. Results showed antipointing reaction times (RTs) in the exercise condition decreased by an average of 18 ms postexercise (p < 0.001) with an observed large effect size (dz = 0.90), whereas control condition pre- and post-assessment RTs did not reliably differ (p = 0.12, dz = 0.22) and were within an equivalence boundary (p < 0.005). Further, pre-assessment exercise and control condition antipointing RTs were within an equivalence boundary (p < 0.05). Accordingly, a simple iPad® app provides the requisite resolution to detect subtle executive function benefits derived from a single bout of exercise.
Similar content being viewed by others
Introduction
A myriad of research and long-standing public recognition supports the view that exercise promotes cardiovascular and metabolic health and reduces the incidence of chronic disease. It is, however, less widely recognized that regular exercise benefits the spectrum of cognition (e.g., attention, executive function, memory, verbal, and numerical ability) (for meta-analyses see, Colcombe & Kramer, 2003). For example, Colcombe et al.’s (2004) influential randomized control trial had older adults (58–77 years of age) participate in either a 6-month aerobic exercise intervention (i.e., walking at progressive work rates and durations 3 times/week) or a non-exercise control (i.e., stretching and toning) wherein pre- and postintervention cognition was examined via the Eriksen flanker task (i.e., a measure of executive function) and concurrent functional magnetic resonance imaging. The authors reported that the exercise – but not control – group showed a postintervention behavioral improvement (i.e., 11%) and a task-based increase in frontoparietal activity. Subsequent work has shown that aerobic and resistance training improves cognition across the continuum of healthy young and older adults and delays normative and disease-related (e.g., Alzheimer’s disease) cognitive decline (for review see, Mandolesi et al., 2018). The improvement has been linked to increased cerebral blood flow (Lucas et al., 2012) and biomolecule concentration (i.e., brain-derived neurotrophic factor, catecholamines) (Knaepen et al., 2010; Zouhal et al., 2008) as well as increased functional connectivity (Chirles et al., 2017) and cortical density (Weinstein et al., 2012).
Although chronic exercise reliably benefits cognition, there is mixed evidence as to whether a single bout of aerobic and/or resistance exercise provides a transient benefit to cognition. Moreover, when a benefit has been observed it has been reported to be influenced by several moderators including exercise duration, intensity and the time of executive assessment (for meta-analyses see, Chang et al., 2012; Lambourne & Tomporowski, 2010; Ludyga et al., 2016). Indeed, Chang et al. concluded that the largest positive benefit to cognition occurs 11 to 20 min (see also Lambourne & Tomporowski, 2010) following cessation of a 20-min bout of moderate-intensity exercise. It is, however, important to recognize that work has shown that young and older adults exhibit a benefit up to 60-min postexercise (Hung et al., 2013; Joyce et al., 2009; Shukla & Heath, 2021) for an exercise duration as brief as 10-min (Johnson et al., 2016; Samani & Heath, 2018; Tari et al., 2020) and across the continuum of metabolically sustainable work rates (i.e., from light to very heavy-intensity) (Heath et al., 2018; Petrella et al., 2019; Tari et al. 2021). A fourth, and perhaps most salient moderator, is the cognitive domain that is assessed. Indeed, cognitive domains such as attentional control, general intelligence, numerical and verbal skills as well as information processing speed are generally refractory to a postexercise benefit. (see Tables 1 and 2 of Ludyga et al., 2016 and Chang et al., 2012, respectively). In contrast, executive function has been reported to produce a small (Chang et al., 2012) to moderate (Verburgh et al., 2014) positive benefit postexercise. Executive function includes the core components of inhibitory control, working memory and set-shifting and are control processes mediated via the same frontoparietal networks (Miyake et al., 2000; see also Diamond, 2013) that demonstrate improved task-based activity following chronic and single bouts of exercise (for recent review see, Yu et al., 2021). Notably, many tasks used to assess postexercise executive function (e.g., Ericksen flanker, Stroop task, Tower of London, oddball paradigm) require not only executive control but also non-executive functions such as receptive language, color processing, sequential memory, and top-down perceptual judgments. As a result, tasks involving conjoint executive and non-executive components may – at times – be insufficient for detecting postexercise executive function benefits. In support of this view, recent studies employing the antisaccade task demonstrated that a single bout of exercise elicits a robust executive function benefit (Dirk et al., 2020; Heath et al., 2018; Petrella et al., 2019; Samani & Heath, 2018; Tari et al., 2020). Antisaccades require a goal-directed eye movement mirror-symmetrical to a target and the non-standard nature of the task (i.e., decoupling the spatial relations between stimulus and response) results in longer reaction times (RT) and less accurate and more variable endpoints than their prosaccade (i.e., saccade toward a target) counterparts (for review see, Munoz & Everling, 2004). Extensive neuroimaging in humans and single-cell recordings in non-human primates report that the antisaccade behavioral ‘costs’ reflect the two-component executive demands of response suppression (i.e., inhibition) and the 180° spatial transposition of target coordinates (i.e., vector inversion) mediated via frontoparietal executive networks (Everling & Johnston, 2013). The five independent studies cited above have shown that antisaccades – but not prosaccades – produce an average 21 ms (SD = 5) pre- to postexercise reduction in RT when assessed within the first 20-min postexercise with a ‘large’ pooled effect size (i.e., Cohen’s dz =1.01 [CI95%= 0.74–1.43]). The large effect size in conjunction with their mediation via frontoparietal networks permits the antisaccade task to serve as a directed tool for assessing postexercise executive function benefits.
One limitation of the antisaccade task is that the ability to accurately detect saccade onset requires electrooculography, retroreflective or magnetic scleral search-coil technologies (Brooks et al., 2019). Although these technologies offer exquisite temporal resolution (e.g., 1000 Hz) they are expensive, not available to all labs, and have narrow portability for research outside of a lab environment. As a result, antisaccades are not always feasible for assessing putative exercise-mediated executive function benefits (e.g., rural/remote populations and individuals in assisted care environments) and this limitation has been exacerbated by COVID-19 restrictions. Accordingly, the present work developed a variant of the antisaccade task deployed on an iPad® for at-home assessments of the response inhibition component of executive function. In particular, we developed an iPad® app requiring reaching movements to veridical (i.e., propointing) and mirror-symmetrical (i.e., antipointing) target locations. The antipointing task – as opposed to a home-based measure of antisaccades – was used because: (1) the native resolution of the iPad® provides a valid RT measure for exogenous stimulus presentation (Schatz et al., 2015), and (2) it does not require the complexity of participants using the iPad® camera to self-calibrate their viewing space (i.e., an absolute requirement for any saccade study). What is more, antipointing elicits the same behavioral costs (i.e., increased RT and endpoint variability) (Chua et al., 1992; Carey et al., 1996; Heath et al., 2009; Maraj & Heath, 2010) and recruits the same frontoparietal structures as antisaccades (Connolly et al., 2000; Heath et al., 2010).
Here, participants completed separate blocks of pro- and antipointing trials prior to and immediately following a 20-min single bout of heavy-intensity (i.e., 80% of maximum predicted heart rate: HRmax) aerobic exercise. In addition, a non-exercise control condition was used to determine whether a putative pre- to postassessment change reflects a practice-related performance benefit or an exercise-related benefit. In light of COVID-19 restrictions, participants completed the exercise and control conditions in their own residence using their personal iPad®. In terms of research predictions, if antipointing provides the resolution to detect a postexercise executive function benefit then RTs should be decreased from pre- to postexercise assessments, whereas propointing (exercise and control conditions) and control condition antipointing RTs are expected to not vary with time of assessment (e.g., see Dirk et al., 2020; Heath et al., 2018; Petrella et al., 2019; Samani & Heath, 2018).
Methods
Participants
Fifty English-speaking individuals (18 female, age range 18–26 years) volunteered for this study over a 3-month period with advertising for the study completed via social media (i.e., Facebook, Instagram, Twitter). This sample size was determined a priori using G*Power (Faul et al., 2007, 2009) based on an effect size derived from a previous paired-samples t test contrasting pre- and postexercise antisaccade RTs via a high-resolution eye tracker (α = 0.05, power = 0.99) (dz=1.14; Samani & Heath, 2018). Inclusion criteria included self-reported: normal or corrected-to-normal vision; right-hand dominant (i.e., “I write with my right hand”); no history of smoking, cardiorespiratory, metabolic or musculoskeletal conditions; no history of neuropsychiatric/neurological disorders (e.g., schizophrenia, dementia, concussion), eye and arm injury or SARS-CoV-2. As well, and given the nature of this investigation, study inclusion required access to a personal iPad® and heart rate (HR) monitor (see details below). Whether participants satisfied all of the above inclusion criteria was assessed and confirmed via video call screening prior to study onset. Participants read a letter of information approved by the Health Sciences Research Ethics Board, University of Western Ontario, and provided informed consent. This study was conducted according to the Declaration of Helsinki with the exception that participants were not entered into a database. Participants obtained a full score on the 2020 Physical Activity Readiness Questionnaire (PAR-Q+) (Warburton et al., 2011) and completed the Godin Leisure-Time Exercise Questionnaire (GLTEQ) (Godin, 2011). The PAR-Q+ includes a series of questions endorsed by the Canadian Society for Exercise Physiology that provide a valid metric for determining an individual’s “readiness” to participate in an exercise test. The GLTEQ requires potential participants to report how frequently they engage in strenuous, moderate and mild exercise in a 7-day period (i.e., requiring a score ≥ 14). The average GLETQ was 52 (SD = 24; range, 18–110) and thus indicated participants were recreationally active and physically able to complete an exercise study. Notably, the PAR-Q+ and GLTEQ were completed in-app and if individuals did not satisfy the above criteria (of which they were not previously informed), they were redirected to the application home screen and subsequently excluded from participation.
Seventy-three individuals expressed initial interest in participating. Of that number, 23 did not meet the inclusion criteria (i.e., three reported history of concussion, two reported being left-handed, ten did not have access to an iPad® and/or HR monitor, eight were not eligible for participation due to PAR-Q+ screening).
Experimental overview
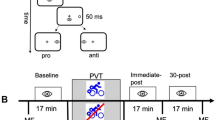
Two experimental sessions separated by at least 24 h were completed. The sessions were performed at the same time of day and in a hydrated state. One session entailed a 20-min single bout of aerobic exercise (i.e., exercise condition) at a heavy-intensity (i.e., 80% of participants’ maximum predicted heart rate; HRmax: 220 minus age in years) (see Robergs & Landwehr, 2002). The exercise duration and intensity were chosen based on work showing that 20-min moderate through very heavy-intensity work rates exhibit an equivalent magnitude postexercise executive function benefit (Heath et al., 2018; Petrella et al., 2019). The second session entailed a non-exercise control condition wherein participants sat and watched a sitcom on their iPad® for an equivalent duration to the exercise condition. Participants completed an assessment of executive function (see details below) prior to and after the exercise and control sessions. The order in which the exercise and control conditions were performed was counterbalanced, and participants were informed – via e-mail – of the order after an initial screening.
Apparatus and procedures
Participants who consented to take part in this study had access to an iPad® equipped with the iOS v.13.0 operating system or later (Apple Inc., Cupertino, CA) and a commercial HR monitor (e.g., Apple Watch®, Apple Inc., Cupertino, CA; or FitBit, Vector Watch Limited, London, UK). Following initial screening, and prior to data collection, participants downloaded the NeuroBehavioural Lab PRO-ANTI-POINT app via the Apple App Store (https://apps.apple.com/us/app/id1527881594) and were provided a study-specific passcode to access the platform. The app included integrated PAR-Q+ and GLETQ questions completed prior to any data collection. An HR monitor was worn for each session and participants reported their HRs at 2.5-, 12.5-, and 22.5-min intervals during each session. Participants recorded their own heart rate and were asked to e-mail their values to the experimenters following the completion of an individual experimental session (i.e., following the postcontrol and postexercise executive assessments; see below).
Exercise Condition
A 2.5-min warmup comprising walking at normal pace was completed and participants were instructed their HR should not exceed 50% of their predicted HRmax. Following the warmup, participants exercised at a heavy-intensity (i.e., 80% HRmax) via fast walking and/or running for 20 min while ensuring their HR was in the targeted intensity. Subsequently, a 2.5-min cool-down was performed at the same intensity as the warmup. Participants were allowed to exercise indoors via treadmill (N = 9) or outdoors (N = 41).
Control Condition
Participants completed a non-exercise control session wherein they sat for a time equivalent to the exercise session (i.e., 25 min) and were instructed to watch a sitcom on their iPad®.
Executive function assessment
Pre- and postassessments of executive function were completed via pro- and antipointing trials presented on a custom-built iPad® app (XCode developed via Swift; v. 5.3 Apple Inc, Cupertino CA) operating at a native screen and touch resolution of 60 Hz. Prior to data collection, participants were familiarized with pro- and antipointing trials via tutorials integrated into the iPad® app. For pre- and postsession assessments, participants sat in a chair in front of a table (i.e., recommended configuration included an office desk/chair or kitchen chair/table) with their iPad® centered on their midline and placed flat and lengthwise (i.e., landscape mode) on the tabletop. Visual stimuli were presented on a grey (RGB code: 125, 125, 125) background and included a centrally located white (RGB code: 255, 255, 255) home location (i.e., 1 by 1 cm cross) and targets (i.e., open white circle; 1 cm in diameter) presented 6 cm (i.e., proximal target) and 9 cm (i.e., distal target) to the left and right of the home location and in the same horizontal plane. The onset of an individual trial was initiated by presentation of the home location which signaled participants to place their right index finger on its location. Following contact with the home location, a uniformly distributed randomized foreperiod between 1000 and 2000 ms was introduced after which a target appeared for 50 ms in one of four locations (i.e., left 6 cm or 9 cm; right 6 cm or 9 cm) and cued participants to either pro- (i.e., point to veridical target location) or antipoint (i.e., point mirror-symmetrical to target location). Participants were asked to complete their response “quickly and accurately” and the tutorials indicated that participants were not to slide their finger from the home location to the target; rather, the instruction was to lift and point to the target. Pro- and antipointing trials were completed in separate and randomly ordered blocks wherein 20 trials were pseudorandomly presented at each target location (i.e., left and right field) and eccentricity (i.e., proximal and distal) for a total of 160 trials. In advance of each block an instruction screen indicated the task (i.e., pro- vs. antipointing) to be performed. Once participants completed their pre-assessment of pro- and antipointing trials they were instructed to immediately begin their exercise or control session. Post-assessment pro- and antipointing trials were completed ~ 4-min after the end of the exercise and control sessions. The basis for the 4-min delay was to allow HR in the exercise session to fall below 100 bpm and is the same protocol used in previous lab-based and continuous HR monitoring studies by our group (Dirk et al., 2020; Heath et al., 2018; Petrella et al., 2019; Shukla & Heath, 2021; Tari et al., 2020). Each pro- and antipointing assessment required approximately 12-min to complete. Notably, the timing of onset of the postexercise assessment and the time required to complete the task is well within the window that has been shown to provide a reliable postexercise benefit for response inhibition (Hung et al., 2013; Joyce et al., 2009; Shukla & Heath, 2021). Upon completion of each executive assessment (i.e., precontrol, postcontrol, preexercise, postexercise), .txt files were generated and uploaded to a secure File Transfer Protocol site run by our lab group. We include the participant-specific data in the Open Science Framework (https://osf.io/9n6by/?view_only=109a7c36be634894b14fc6a1036dc797).
Data reduction, dependent variables, and statistical analyses
As in previous pro- and antipointing studies (e.g., Maraj & Heath, 2010), RTs less than 150 ms (i.e., anticipatory response) or greater than 2.5 SD of a participant- and task-specific mean were excluded from data analysis as were movement times (MT) less than 100 ms or greater than 2.5 SD of a participant- and task-specific mean (Maraj & Heath, 2010). Trials involving a directional error (i.e., propointing instead of an instructed antipointing or vice versa) were excluded from RT and MT analyses because antipointing trials with a directional error are mediated via planning mechanisms distinct from their directionally correct counterparts (Heath et al., 2012). Less than 8% of trials for any participant were omitted.
HR was examined via 2 (condition: control, exercise) by 3 (time: 2.5-, 12.-5, 22.5-min) fully repeated measures ANOVA (α = 0.05). For the exercise session, the 2.5-, 12.5-, and 22.5-min HR intervals represented the end of warmup, the midway point and end of the exercise manipulation, respectively. Pro- and antipointing dependent variables included RT (i.e., time from response cueing to release of pressure from the home location [i.e., movement onset]), movement time (MT: time from movement onset to offset), and horizontal endpoint gain variability (i.e., within-participant standard deviation of movement amplitude/veridical target amplitude). RT data were positively skewed (RT: 0.83 < g1 < 1.30, average g1 = 1.04), we therefore use median values for comparison, whereas MT and gain variability are reported as means given their normal distribution (g1 < 1.00). RT, MT and gain variability were examined via 2 (condition: control, exercise) by 2 (time: pre-, postassessment) by 2 (task: pro-, antipointing) fully repeated measures ANOVA (α = 0.05) and significant interactions were decomposed via simple effects (i.e., time by task ANOVAs and/or paired-samples t test). Data met underlying assumptions for sphericity (i.e., Mauchly’s ps > 0.05)Footnote 1. Where appropriate, dependent samples two one-sided tests (TOST) were used to determine whether means and/or medians were within equivalence bounds determined by Cohen’s d. Due to the novel nature of this study, we used a standard effect size dz = 0.50 for all TOST statistics (Lakens, 2017).
Results
Participant heart rates
HR produced main effects of condition, F(1,49) = 1178.73, p < 0.001, ηp2 = 0.96, time, F(2,98) = 149.78, p < 0.001, ηp2 = 0.75, and their interaction, F(2,98) = 175.60, p < 0.001, ηp2 = 0.78. HR for the control condition did not vary across 2.5- (73 bpm, SD = 11), 12.5- (72 bpm, SD = 11) or 22.5-min (72 bpm, SD = 10) intervals (all t(49) <1.65, ps > 0.10, all dz < 0.23), whereas exercise condition HR at the 2.5-min (107 bpm, SD = 25) interval was less than the 12.5- (159 bpm, SD = 11) and 22.5-min (160 bpm, SD = 10) intervals (t(49) > 13.42, ps < .001, all dz > 1.90) and the latter two did not reliably differ (t(49) = 1.09, p = 0.28, dz = 0.15) (Fig. 1a). Figure 1b presents exercise condition group mean HR difference scores (i.e., reported intensity minus prescribed intensity) and associated 95% confidence intervals, and shows that reported and prescribed intensity did not reliably differ from zero at the 12.5- and 22.5-min intervals, respectively (all t(49) = 0.43 and 1.43, ps = 0.67 and 0.16, all dz = 0.06 and 0.20). In other words, participants exercised within the prescribed work rate.
The large panel (A) presents participant-specific self-reported heart rates (HR) at different intervals in the control and exercise conditions. The smaller panel (B) presents group mean HR and prescribed intensity difference scores (i.e., reported heart rate minus prescribed HRmax) at the 12.5- and 22.5-min intervals. Error bars represent 95% between-participant confidence intervals
Pro- and antipointing performance
Reaction time
Results yielded main effects for time, F(1,49) = 27.08, p < 0.001, ηp2 = 0.36, and task F(1,49) = 141.47, p < 0.001, ηp2 = 0.74, and interactions involving condition by time, F(1,49) = 14.07, p < 0.001, ηp2 = 0.22, time by task, F(1,49) = 6.28, p = 0.02, ηp2 = 0.11, and condition by time by task, F(1,49) = 4.26, p = 0.04, ηp2 = 0.08. The highest-order interaction was decomposed via time by task ANOVAs separately for control and exercise conditions (Fig. 2a). The control condition yielded a main effect of task, F(1,49) = 121.61, p < 0.001, ηp2 = 0.71: propointing RTs were shorter than antipointing. Notably, however, we did not observe a reliable time by task interaction, F(1,49) = 0.32, p = 0.58, ηp2 = 0.006, and TOST statistics indicated that preassessment pro- and antipointing RTs were within an equivalence boundary of their postassessment counterparts (all t(49) > 2.64, ps < 0.005). For the exercise condition, we observed main effects of time, F(1,49) = 42.20, p < 0.001, ηp2 = 0.46, task, F(1,49) = 121.38, p < 0.001, ηp2 = 0.71, and their interaction, F(1,49) = 8.31, p = 0.006, ηp2 = 0.15. As with the control condition, propointing RTs were less than antipointing, and preassessment values for pro- and antipointing were longer than their postassessment counterparts (all t(49) = 3.17 and 6.39, for pro- and antipointing, respectively, ps = 0.003 and < 0.001, dz = 0.45 and 0.90). Importantly, preassessment pro- and antipointing RTs did not reliably differ between conditions (all t(49) = 0.01 and – 0.68, for pro- and antipointing, ps = 0.99 and 0.50, all dz = 0.002 and -0.10) and were within an equivalence boundary (all t(49) > 4.03, ps < 0.001). To further uncover the nature of the time by task interaction, the unshaded region of Fig. 2b presents participant-specific pro- and antipointing RT difference scores (i.e., postassessment minus preassessment) with group means and 95% between-participant confidence intervals. For comparative purposes, Fig. 2b also includes control condition difference scores. The figure demonstrates that exercise condition pro- and antipointing difference scores for 33 and 43 of the 50 participants, respectively, had a negative valence; that is, the majority of participants showed a postexercise reduction in RT. As well, we contrasted pro- and antipointing difference scores to determine if the magnitude of the postexercise RT reduction differed between tasks. The shaded region of Fig. 2b presents the group mean difference score (with double-lined error bars representing 95% and 99% between-participant confidence intervals) and demonstrates that the exercise condition produced a larger magnitude reduction for antipointing (t(49) = 2.88, p = 0.006, dz = 0.41). Thus, although pro- and antipointing RTs decreased postexercise, the magnitude of the benefit was larger in the latter task. By way of comparison, the unshaded region of Fig. 2b shows that the control condition yielded 27 and 30 (out of 50) negative valence participant-specific difference scores for pro- and antipointing, respectively, and overlap between the 95% between-participant confidence intervals and zero for pro- and antipointing indicates that the group means did not reliably differ from zero.
Panel A presents control and exercise condition participant-specific reaction time (RT) for pro- (P: shaded grey area) and antipointing (A) as a function of pre- and postassessments. The group mean RT and 95% between-participant confidence intervals are presented as black lines. Panel B presents control and exercise condition pro- (P) and antipointing (A) participant-specific RT difference scores (postassessment minus preassessment) with associated group means and 95% between-participant confidence intervals presented via black lines. Note: a negative valence indicates a postassessment reduction in RT. The shaded grey region in panel B shows control and exercise condition group means – and associated 95% and 99% between-participant confidence intervals – computed by subtracting each participants’ antipointing difference score from their propointing difference score. The negative valence in the exercise condition and the absence of error bar overlap with zero demonstrates a larger magnitude postexercise RT benefit for antipointing than propointing
Movement time
Results indicated main effects of time, F(1,49) = 10.03, p = 0.003, ηp2 = 0.17, task, F(1,49) = 21.24, p < 0.001, ηp2 = 0.30, and a condition by time interaction, F(1,49) = 4.78, p = 0.03, ηp2 = 0.09. Propointing MTs were shorter than antipointing and values (for pro- and antipointing) decreased from pre- to postassessment in the exercise but not the control condition (all t(49) > 2.97 and < 0.94 for exercise and control conditions, respectively, ps < 0.005 and > 0.35, dz > 0.42 and < 0.13). Further, MT did not yield higher-order interactions involving task, all F(1,49) < 2.81, ps>0.10, ηp2 < 0.05 (Fig. 3a). As with RT, preassessment pro- and antipoint MTs did not reliably differ between conditions (all t(49) = 1.12 and – 0.45, for pro- and antipointing, ps = 0.27 and 0.66, all dz = 0.16 and – 0.06) and were within an equivalence boundary (all t(49)>3.43, ps < 0.001) (Fig. 3a).
Gain variability
A main effect of task, F(1,49) = 101.62, p < 0.001, ηp2 = 0.68, indicated that endpoint variability for propointing (0.12, SD = 0.02) was less than antipointing (0.17, SD = 0.03), and this variable did not produce higher-order interaction involving task, all F(1,49) < 3.80, ps>0.06, η2 < 0.07 (Fig. 3b).
Directional errors
The absolute percentage of trials involving a directional error was less than 1% and 24 and 15 participants did not elicit a single pro- or antipointing error, respectively. The low error rate is attributed to the blocked trial presentation.
Discussion
We examined whether an iPad® app reliably measures a postexercise executive function benefit. As such, participants completed pro- and antipointing trials prior to and immediately following 20-min of heavy-intensity aerobic exercise and completed a non-exercise control condition. In outlining our results, we first summarize the general differences between pro- and antipointing before discussing how each was influenced by control and exercise conditions (Table 1).
Antipointing executive demands increase reaction times
Antipointing RTs were longer than propointing. One possible explanation for this result is that antipointing rendered an implicit – or explicit – control strategy designed to increase RT to maintain endpoint stability (i.e., speed-accuracy trade-off) (Fitts, 1954). This explanation is not supported by our results given that antipointing MTs and endpoints were longer and more variable, respectively, than propointing. Indeed, the longer antipointing MTs likely reflects increased visuomotor uncertainty (Edelman & Goldberg, 2001) and reduced corticomotor excitability associated with inhibiting (or cancelling) a prepotent response (Heath et al., 2012; MacDonald et al., 2014). In turn, the increased endpoint variability is attributed to antipointing mediation via visual information (i.e., relative) fundamentally distinct from the absolute visual information supporting propointing (Heath et al., 2009; for review of duplex vision see, Goodale, 2011). Accordingly, and in line with previous studies, we propose the longer antipointing RTs reflect the executive demands of suppressing a prepotent propointing response (i.e., inhibitory control) and transforming a target’s coordinates to mirror-symmetrical space (i.e., vector inversion) (Chua et al., 1992; Heath et al., 2009; Maraj & Heath, 2010). Moreover, the average 41 ms (SD = 24) difference between pro- and antipointing is comparable to the 50 ms difference reported in the more extensively studied pro- and antisaccade literature (e.g., see Evdokimidis et al., 1996; for review see, Munoz & Everling, 2004). In other words, the time-consuming executive demands of antipointing are broadly comparable to antisaccades. Most notably, our results demonstrate that the antipointing iPad® app used here provides a framework to evaluate pre- to postexercise changes in executive function.
Heart rate (HR) during exercise and control conditions
The self-report of HR at the midway and end of the 20-min aerobic exercise session indicated adherence to the prescribed heavy-intensity work rate. In turn, HR during the control condition did not vary across the different time points. Participants therefore exercised at an intensity known to elicit a postexercise executive function benefit (Heath et al., 2018; Verburgh et al., 2014). Moreover, the null time-dependent modulation of HR in the control condition indirectly demonstrates that the activity performed during this time (i.e., sitting while watching a sitcom on the iPad®) did not alter participants’ physiological or psychological arousal (Wang et al., 2018).
A single bout of exercise benefits pro- and antipointing reaction times
Antipointing RTs reliably decreased from pre- to postexercise by an average of 18 ms (SD = 20) and is a result unrelated to a practice-related performance benefit given that the 5 ms (SD = 21) difference in the control condition was within an equivalence boundary. Instead, our findings add to convergent literature asserting that a single bout of exercise improves executive function (Chang et al., 2012; Lambourne & Tomporowski, 2010; Ludyga et al., 2016). As indicated in the Introduction, the improvement may reflect an exercise-mediated increase in cerebral blood flow (Lucas et al., 2012; Tari et al., 2020), biomolecule concentration (Knaepen et al., 2010; Zouhal et al., 2008) and/or resting state functional connectivity that enhances the efficiency and effectiveness of the local neural circuitry supporting executive function (i.e., the hemo-neural hypothesis; see Moore & Cao, 2008). Regardless of the mechanism, or mechanisms, the magnitude of the pre- to postexercise decrease in antipointing RT and reported effect size (dz = 0.90) is markedly similar to five previous studies employing a lab-based assessment of antisaccade performance (i.e., average postexercise RT reduction of 21 ms with a pooled effect size of 1.01) (Dirk et al., 2020; Heath et al., 2018; Petrella et al., 2019; Samani & Heath, 2018; Tari et al., 2020). Such a comparison evinces that the antipointing app provides a simple and cost-effective tool for evaluating postexercise executive function.
Propointing RTs reliably decreased from pre- to postexercise by an average of 8 ms (SD = 18) and this difference was not observed in the control condition (3 ms, SD = 13). This represents an unexpected finding given work showing that prosaccades are refractory to a single bout of exercise (Dirk et al., 2020; Heath et al., 2018; Petrella et al., 2019; Samani & Heath, 2018). Indeed, from previous work it was argued that prosaccades are immutable to an exercise intervention because they are mediated via direct retinotopic projections in the superior colliculus (Munoz & Everling, 2004); that is, the response is largely governed via a central nervous system structure that does not demonstrate exercise-mediated neuroplasticity. Accordingly, a possible explanation for the propointing RT findings observed here is that the single bout of exercise increased physiological and/or psychological arousal (Dietrich & Audiffren, 2011) and improved information processing speed. This explanation is tempered by two factors. First, the extant literature reports that a single bout of exercise does not elicit a general improvement to information processing speeds (Chang et al., 2012; Lambourne & Tomporowski, 2010). Second, an arousal explanation would assert a comparable postexercise reduction for pro- and antipointing; however, our results showed that antipointing produced a larger magnitude benefit. An alternative, and more parsimonious explanation is that propointing requires top-down executive control. This contention is supported by numerous studies showing that the frontoparietal networks mediating the visuomotor transformations necessary for propointing overlap with executive function networks (Gallivan & Culham, 2015). Thus, pro- and antipointing quantify a postexercise executive function benefit; albeit the increased executive demands in the latter may provide greater resolution.
Limitations and future directions
We acknowledge that our work may be limited by several methodological aspects. First, only a single intensity and postexercise assessment was employed. It is, therefore, unclear whether the current task provides the sensitivity to detect a postexercise executive function benefit across the continuum of exercise intensities and whether the task is able to detect a benefit beyond the postexercise window used here (i.e., > 16-min). What is more, the at-home nature of this work precluded the precise monitoring of the timeframe of postexercise assessments. As previously indicated, the time of assessment can be a moderator of the magnitude of a putative postexercise executive benefit (Chang et al., 2012). However, based on participant reports, and the nature of our in-app task, we are confident that participants were able to complete the postexercise assessment approximately 16 min following exercise cessation, and well within the ~ 47-min window for which an executive function benefits persist (see Hung et al., 2013; Joyce et al., 2009). Second, only healthy and recreationally active young adults were examined. This is a limitation because reactivity to a single bout of exercise can vary with fitness level (Chang et al., 2012; cf. Ludyga et al., 2016), age and health status (Ludyga et al., 2016). Third, we would like to comment on the most significant barrier we encountered when recruiting participants for this study; i.e., iPad® availability. We primarily enlisted participants via social media (i.e., Facebook, Instagram, Twitter), and responses were generally positive; perhaps due to the eagerness of individuals to participate in research during a period of COVID-19 pandemic closure in the province of Ontario; however, subsequent screening for iPad® availability reduced the number of eligible individuals. Indeed, ten of the 73 individuals that expressed interest in this study were excluded because they did not have access to an iPad® with an appropriate iOS. That said, this was a proof-of-concept study and it is our goal to deploy the task to examine whether single bout and chronic exercise influence executive function across a number of moderator variables (i.e., intensity, duration, time of assessment) in hard-to-access populations (e.g., persons in rural environments or assisted care facilities). Notably, the difficulty we experienced in recruiting healthy university-aged individuals may make this goal seem unattainable; however, we acknowledge that in future work we will aim to provide iPads® and heart-rate monitors for shared use in hard-to-access populations (i.e., community living and retirement homes in rural midwestern Ontario). At the time this study was completed, the COVID-19 pandemic did not permit for shared use of technology; however, with greater understanding of COVID transmission, it is likely that shared technology use will benefit the efficiency of data collection in future studies. In addition, the cost-effectiveness, transportability, and user-friendliness of the iPad® app developed here may be integrated within existing exercise reward apps (Mitchell et al., 2020) and – in part – serve as a motivation tool to demonstrate exercise benefits to brain health.
Notes
Participant-specific median (and mean) RT distributions were positively skewed for antipointing control (pre- and postassessments: g1 = 1.045 and 1.044) and exercise (preassessment only: g1 < 1.300), whereas all other conditions produced normal distributions (all g1 < 0.866). Given that ANOVA and t-test approaches are robust to violations of non-normality for the sample size used here (Glass et al., 1972; Harwell et al., 1992; Lix et al., 1996) we elected to employ both statistics in our main Results. That said, here we provide Bayesian paired-samples t tests (JASP Team, 2020) for RT because the method robustly handles data that depart from normality (Kruschke, 2013). Exercise condition pro- and antipointing Bayes factors (BF10) were 11.71 and 215191.32, respectively, times more likely under a model including time (i.e., pre- and postassessment) compared to the null model. In contrast, control condition pro- and antipointing RTs produced BF10 that were 0.56 and 0.48, respectively, and these contrasts produced larger factors in favor of the null hypothesis (i.e., BF01 = 1.80, 2.08). Accordingly, the frequentist statistics reported in the main Results and the Bayesian approach used here demonstrate that the exercise – but not control condition – reliably decreased pro- and antipointing RTs. What is more, employing the BF10 nomenclature developed by van Doorn et al. (2021) indicates that the magnitude of the postexercise RT benefit for antipointing (i.e., “very strong”) was larger than propointing (i.e., “strong”).
References
Brooks, J. S., Smith, W. J., Webb, B. M., Heath, M. D., & Dickey, J. P. (2019). Development and validation of a high-speed video system for measuring saccadic eye movement. Behavior Research Methods, 51(5), 2302–2309. https://doi.org/10.3758/s13428-019-01197-2
Chang, Y. K., Labban, J. D., Gapin, J. I., & Etnier, J. L. (2012). The effects of acute exercise on cognitive performance: a meta-analysis. Brain Research, 1453, 87–101. https://doi.org/10.1016/j.brainres.2012.02.068
Chirles, T. J., Reiter, K., Weiss, L. R., Alfini, A. J., Nielson, K. A., & Smith, J. C. (2017). Exercise training and functional connectivity changes in mild cognitive impairment and healthy elders. Journal of Alzheimer's Disease, 57(3), 845–856. https://doi.org/10.3233/JAD-161151
Chua, R., Carson, R. G., Goodman, D., & Elliott, D. (1992). Asymmetries in the spatial localization of transformed targets. Brain and Cognition, 20(2), 227–235. https://doi.org/10.1016/0278-2626(92)90017-g
Colcombe, S., & Kramer, A. F. (2003). Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychological Science, 14(2), 125–130. https://doi.org/10.1111/1467-9280.t01-1-01430
Colcombe, S. J., Kramer, A. F., Erickson, K. I., Scalf, P., McAuley, E., Cohen, N. J., Webb, A., Jerome, G. J., Marquez, D. X., & Elavsky, S. (2004). Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences of the United States of America, 101(9), 3316–3321. https://doi.org/10.1073/pnas.0400266101
Connolly, J. D., Goodale, M. A., DeSouza, J. F., Menon, R. S., & Vilis, T. (2000). A comparison of frontoparietal fMRI activation during anti-saccades and anti-pointing. Journal of Neurophysiology, 84(3), 1645–1655. https://doi.org/10.1152/jn.2000.84.3.1645
Diamond, A. (2013). Executive functions. Annual Review of Psychology, 64, 135–168. https://doi.org/10.1146/annurev-psych-113011-143750
Dietrich, A., & Audiffren, M. (2011). The reticular-activating hypofrontality (RAH) model of acute exercise. Neuroscience and Biobehavioral Reviews, 35(6), 1305–1325. https://doi.org/10.1016/j.neubiorev.2011.02.001
Dirk, K. L., Belfry, G. R., & Heath, M. (2020). Exercise and executive function during follicular and luteal menstrual cycle phases. Medicine and Science in Sports and Exercise, 52(4), 919–927. https://doi.org/10.1249/MSS.0000000000002192
Edelman, J. A., & Goldberg, M. E. (2001). Dependence of saccade-related activity in the primate superior colliculus on visual target presence. Journal of Neurophysiology, 86(2), 676–691. https://doi.org/10.1152/jn.2001.86.2.676
Evdokimidis, I., Liakopoulos, D., Constantinidis, T. S., & Papageorgiou, C. (1996). Cortical potentials with antisaccades. Electroencephalography and Clinical Neurophysiology, 98(5), 377–384. https://doi.org/10.1016/0013-4694(96)94699-4
Everling, S., & Johnston, K. (2013). Control of the superior colliculus by the lateral prefrontal cortex. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 368(1628), Article 20130068. https://doi.org/10.1098/rstb.2013.0068
Faul, F., Erdfelder, E., Lang, A. G., & Buchner, A. (2007). G * Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175-191. https://doi.org/10.3758/bf03193146
Faul, F., Erdfelder, E., Buchner, A., & Lang, A. G. (2009). Statistical power analyzes using G * Power 3.1: Tests for correlation and regression analyzes. Behavior Research Methods, 41(4), 1149-1160. https://doi.org/10.3758/brm.41.4.1149
Fitts, P. M. (1954). The information capacity of the human motor system in controlling the amplitude of movement. Journal of Experimental Psychology, 47(6), 381–391.
Gallivan, J. P., & Culham, J. C. (2015). Neural coding within human brain areas involved in actions. Current Opinion in Neurobiology, 33, 141–149. https://doi.org/10.1016/j.conb.2015.03.012
Glass, G. V., Peckham, P. D., & Sanders, J. R. (1972). Consequences of failure to meet assumptions underlying the fixed effects analyses of variance and covariance. Review of Educational Research, 42(3), 237–288. https://doi.org/10.2307/1169991
Godin, G. (2011). The Godin-Shephard Leisure-Time Physical Activity Questionnaire. The Health & Fitness Journal of Canada, 4(1), 18–22. https://doi.org/10.14288/hfjc.v4i1.82
Goodale, M. A. (2011). Transforming vision into action. Vision Research, 51(13), 1567–1587. https://doi.org/10.1016/j.visres.2010.07.027
Harwell, M. R., Rubinstein, E. N., Hayes, W. S., & Olds, C. C. (1992). Summarizing Monte Carlo results in methodological research: the one- and two-factor fixed effects ANOVA cases. Journal of Educational Statistics, 17(4), 315–339. https://doi.org/10.2307/1165127
Heath, M., Maraj, A., Maddigan, M., & Binsted, G. (2009). The antipointing task: vector inversion is supported by a perceptual estimate of visual space. Journal of Motor Behavior, 41(5), 383–392. https://doi.org/10.3200/35-08-016
Heath, M., Dunham, K., Binsted, G., & Godbolt, B. (2010). Antisaccades exhibit diminished online control relative to prosaccades. Experimental Brain Research, 203(4), 743–752. https://doi.org/10.1007/s00221-010-2290-7
Heath, M., Bell, J., Holroyd, C.B., & Krigolson, O.E. (2012). Electroencephalographic evidence of vector inversion in antipointing. Experimental Brain Research, 221, 19-26. https://doi.org/10.1007/s00221-012-3141-5
Heath, M., Petrella, A., Blazevic, J., Lim, D., Pelletier, A., & Belfry, G. R. (2018). A post-exercise facilitation of executive function is independent of aerobically supported metabolic costs. Neuropsychologia, 120, 65–74. https://doi.org/10.1016/j.neuropsychologia.2018.10.002
Hung, T. M., Tsai, C. L., Chen, F. T., Wang, C. C., & Chang, Y. K. (2013). The immediate and sustained effects of acute exercise on planning aspect of executive function. Psychology of Sport and Exercise, 14(5), 728-736. https://doi.org/10.1016/j.psychsport.2013.05.004
Johnson, L., Addamo, P. K., Selva Raj, I., Borkoles, E., Wyckelsma, V., Cyarto, E., & Polman, R. C. (2016). An acute bout of exercise improves the cognitive performance of older adults. Journal of Aging and Physical Activity, 24(4), 591–598. https://doi.org/10.1123/japa.2015-0097
Joyce, J., Graydon, J., McMorris, T., & Davranche, K. (2009). The time course effect of moderate intensity exercise on response execution and response inhibition. Brain and Cognition, 71(1), 14–19. https://doi.org/10.1016/j.bandc.2009.03.004
Knaepen, K., Goekint, M., Heyman, E. M., & Meeusen, R. (2010). Neuroplasticity - exercise-induced response of peripheral brain-derived neurotrophic factor: a systematic review of experimental studies in human subjects. Sports Medicine, 40(9), 765–801. https://doi.org/10.2165/11534530-000000000-00000
Kruschke J. K. (2013). Bayesian estimation supersedes the t test. Journal of Experimental Psychology. General, 142(2), 573–603. https://doi.org/10.1037/a0029146
Lakens, D. (2017). Equivalence tests: A practical primer for t tests, correlations, and meta-analyses. Social Psychological and Personality Science, 8(4), 355–362. https://doi.org/10.1177/194855061769717
Lambourne, K., & Tomporowski, P. (2010). The effect of exercise-induced arousal on cognitive task performance: a meta-regression analysis. Brain Research, 1341, 12–24. https://doi.org/10.1016/j.brainres.2010.03.091
Lix, L. M., Keselman, J. C., & Keselman, H. J. (1996). Consequences of assumption violations revisited: A quantitative review of alternatives to the one-way analysis of variance F Test. Review of Educational Research, 66(4), 579–619. https://doi.org/10.3102/00346543066004579
Lucas, S. J., Ainslie, P. N., Murrell, C. J., Thomas, K. N., Franz, E. A., & Cotter, J. D. (2012). Effect of age on exercise-induced alterations in cognitive executive function: relationship to cerebral perfusion. Experimental Gerontology, 47(8), 541–551. https://doi.org/10.1016/j.exger.2011.12.002
Ludyga, S., Gerber, M., Brand, S., Holsboer-Trachsler, E., & Pühse, U. (2016). Acute effects of moderate aerobic exercise on specific aspects of executive function in different age and fitness groups: A meta-analysis. Psychophysiology, 53(11), 1611–1626. https://doi.org/10.1111/psyp.12736
MacDonald, H. J., Coxon, J. P., Stinear, C. M., & Byblow, W. D. (2014). The fall and rise of corticomotor excitability with cancellation and reinitiation of prepared action. Journal of Neurophysiology, 112(11), 2707–2717. https://doi.org/10.1152/jn.00366.2014
Mandolesi, L., Polverino, A., Montuori, S., Foti, F., Ferraioli, G., Sorrentino, P., & Sorrentino, G. (2018). Effects of physical exercise on cognitive functioning and wellbeing: biological and psychological benefits. Frontiers in Psychology, 9, Article 509. https://doi.org/10.3389/fpsyg.2018.00509
Maraj, A., & Heath, M. (2010). Antipointing: perception-based visual information renders an offline mode of control. Experimental Brain Research, 202(1), 55–64. https://doi.org/10.1007/s00221-009-2111-z
Mitchell, M., Lau, E., White, L., & Faulkner, G. (2020). Commercial app use linked with sustained physical activity in two Canadian provinces: A 12-month quasi-experimental study. The International Journal of Behavioral Nutrition and Physical Activity, 17(1), Article 24. https://doi.org/10.1186/s12966-020-00926-7
Miyake, A., Friedman, N. P., Emerson, M. J., Witzki, A. H., Howerter, A., & Wager, T. D. (2000). The unity and diversity of executive functions and their contributions to complex "Frontal Lobe" tasks: a latent variable analysis. Cognitive Psychology, 41(1), 49–100. https://doi.org/10.1006/cogp.1999.0734
Moore, C. I., & Cao, R. (2008). The hemo-neural hypothesis: on the role of blood flow in information processing. Journal of Neurophysiology, 99(5), 2035–2047. https://doi.org/10.1152/jn.01366.2006
Munoz, D. P., & Everling, S. (2004). Look away: The anti-saccade task and the voluntary control of eye movement. Nature Reviews. Neuroscience, 5(3), 218–228. https://doi.org/10.1038/nrn1345
Petrella, A., Belfry, G., & Heath, M. (2019). Older adults elicit a single-bout post-exercise executive benefit across a continuum of aerobically supported metabolic intensities. Brain Research, 1712, 197–206. https://doi.org/10.1016/j.brainres.2019.02.009
Robergs, R. A., & Landwehr, R. (2002). The surprising history of the "HRmax=220-age" equation. Journal of Exercise Physiology Online, 5(2), 1-10.
Samani, A., & Heath, M. (2018). Executive-related oculomotor control is improved following a 10-min single-bout of aerobic exercise: Evidence from the antisaccade task. Neuropsychologia, 108, 73–81. https://doi.org/10.1016/j.neuropsychologia.2017.11.029
Schatz, P., Ybarra, V., & Leitner, D. (2015). Validating the accuracy of reaction time assessment on computer-based tablet devices. Assessment, 22(4), 405–410. https://doi.org/10.1177/107319111456662
Shukla, D., & Heath, M., (2021). A single bout of exercise provides a persistent benefit to cognitive flexibility. Research Quarterly for Exercise and Sport. Manuscript accepted for publication.
Tari, B., Vanhie, J. J., Belfry, G. R., Shoemaker, J. K., & Heath, M. (2020). Increased cerebral blood flow supports a single-bout postexercise benefit to executive function: evidence from hypercapnia. Journal of Neurophysiology, 124(3), 930–940. https://doi.org/10.1152/jn.00240.2020
van Doorn, J., van den Bergh, D., Böhm, U., Dablander, F., Derks, K., Draws, T., Etz, A., Evans, N. J., Gronau, Q. F., Haaf, J. M., Hinne, M., Kucharský, Š., Ly, A., Marsman, M., Matzke, D., Gupta, A., Sarafoglou, A., Stefan, A., Voelkel, J. G., & Wagenmakers, E. J. (2021). The JASP guidelines for conducting and reporting a Bayesian analysis. Psychonomic Bulletin & Review, 28, 813–826. https://doi.org/10.3758/s13423-020-01798-5
Verburgh, L., Königs, M., Scherder, E. J., & Oosterlaan, J. (2014). Physical exercise and executive functions in preadolescent children, adolescents and young adults: a meta-analysis. British Journal of Sports Medicine, 48(12), 973–979. https://doi.org/10.1136/bjsports-2012-091441
Wang, C. A., Baird, T., Huang, J., Coutinho, J. D., Brien, D. C., & Munoz, D. P. (2018). Arousal effects on pupil size, heart rate, and skin conductance in an emotional face task. Frontiers in Neurology, 9, Article 1029. https://doi.org/10.3389/fneur.2018.01029
Warburton, D. E. R., Jamnik, V. K., Bredin, S. S. D., & Gledhill, N. (2011). The Physical Activity Readiness Questionnaire for Everyone (PAR-Q+) and Electronic Physical Activity Readiness Medical Examination (ePARmed-X+). The Health & Fitness Journal of Canada, 4(2), 3–17. https://doi.org/10.14288/hfjc.v4i2.103
Weinstein, A. M., Voss, M. W., Prakash, R. S., Chaddock, L., Szabo, A., White, S. M., Wojcicki, T. R., Mailey, E., McAuley, E., Kramer, A. F., & Erickson, K. I. (2012). The association between aerobic fitness and executive function is mediated by prefrontal cortex volume. Brain, Behavior, and Immunity, 26(5), 811–819. https://doi.org/10.1016/j.bbi.2011.11.008
Yu, Q., Herold, F., Becker, B., Klugah-Brown, B., Zhang, Y., Perrey, S., Veronese, N., Müller, N. G., Kramer, A. F., & Zou, L. (2021). Cognitive benefits of exercise interventions: an fMRI activation likelihood estimation meta-analysis. Brain Structure & Function, 226(3), 601–619. https://doi.org/10.1007/s00429-021-02247-2
Zouhal, H., Jacob, C., Delamarche, P., & Gratas-Delamarche, A. (2008). Catecholamines and the effects of exercise, training and gender. Sports Medicine, 38(5), 401–423. https://doi.org/10.2165/00007256-200838050-00004
Carey, D. P., Hargreaves, E. L., & Goodale, M. A. (1996). Reaching to ipsilateral or contralateral targets: withinhemisphere visuomotor processing cannot explain hemispatial differences in motor control. Experimental Brain research, 112(3), 496–504. https://doi.org/10.1007/BF00227955
JASP Team (2020). JASP (Version 0.14.1.0) [Computer software].
Tari, B, Shirzad, M., Behboodpour, N., Belfry, G., & Heath, M. (2021). Exercise intensity-specific changes to cerebral blood velocity to no modulate a postexercise executive function benefit. Neuropsychologia, 161, 108018. https://doi.org/10.1016/j.neuropsychologia.2021.108018
Code Availability
NA
Funding
Supported by a Discovery Grant and Postgraduate Doctoral Scholarship from the Natural Sciences and Engineering Research Council (NSERC) of Canada.
Author information
Authors and Affiliations
Contributions
BT and MH conceived and designed the research; BT performed experiments; BT and MH analyzed data; BT and MH interpreted results of experiments; BT and MH prepared figures; BT and MH drafted, edited, revised, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest
The authors have no personal, financial or other conflicts of interest to disclose.
Ethics Approval
This work was approved by the Health Sciences Research Ethics Board, University of Western Ontario (No. 116480) and conducted in accordance with the Declaration of Helsinki.
Consent to Participate
Participants gave informed written consent.
Consent for Publication
NA
Additional information
Open Practices Statement
Data generated and/or analyzed during the current study are available on the Open Science Framework (https://osf.io/9n6by/?view_only=109a7c36be634894b14fc6a1036dc797). The NeuroBehavioural Lab PRO-ANTI-POINT app is available for free on the Apple App Store (https://apps.apple.com/us/app/id1527881594) and a demo version can be accessed with the password “open_access”. This link and password are also available at the Open Science Framework link presented above. The app may also be modified for third-party use upon reasonable request.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tari, B., Heath, M. Evaluating the efficacy of an iPad® app in determining a single bout of exercise benefit to executive function. Behav Res 54, 2398–2408 (2022). https://doi.org/10.3758/s13428-021-01735-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13428-021-01735-x