Abstract
Conditioned gaping occurs through a classically conditioned association between a flavor or a context (CS) and an unconditioned stimulus (US) that produces nausea, such as lithium chloride (LiCl; US). Rats display conditioned gaping to a flavor or context previously associated with nausea; thus, our aim was to investigate whether rats acquire second-order conditioning to a flavor experienced in a nausea-paired context. In Experiment 1, rats were assigned to one of three groups, based upon the contingency of the first order pairing (CS1 context and LiCl) and the contingency of the second-order pairing (CS2 saccharin CS1 context) including: Group Paired/Paired (P/P), Group Paired/Unpaired (P/U) and Group Unpaired/Paired (U/P). In the initial context conditioning, rats were injected with LiCl (Paired) or Saline (Unpaired) prior to a 30 min confinement in a distinctive context (CS1). Drug-free second-order conditioning training among Groups P/P and U/P then consisted of a 5 min intraoral infusion of 0.1 % saccharin (CS2) in the context (CS1), while Group P/U received saccharin in the home cage 24 hr prior to the CS1 exposure. Twenty four hr later, the rats were tested for second-order conditioning during a 2 min taste reactivity (TR) test. Saccharin (CS2) elicited gaping in Group P/P, but not Groups P/U or U/P. Experiment 2 revealed that second-order conditioning was produced in rats given 4 or 8 first-order conditioning trials, but not 2 trials. These results demonstrate that an excitatory contextual CS+ has the potential to confer second-order conditioning to a novel flavor in the absence of any direct pairing with LiCl.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
It is well documented that flavors paired with an emetic drug are not only avoided by rats but are also conditionally rejected in the taste reactivity (TR) test (Grill and Norgren, 1978), reflected by the distinct conditioned response (CR) of gaping (wide open mouth with lower incisors displayed). Although most drugs, even those with rewarding properties (see Parker, 2014 for review), produce conditioned flavor avoidance, as measured using a fluid consumption test, only drugs which produce vomiting in emetic species produce a CR of gaping when paired with a distinct flavor conditioned stimulus (CS). As well, conventional anti-emetic drugs, such as 5-hydroxytryptamine (5-HT3) antagonists and cannabinoid compounds, selectively prevent the establishment the CR of gaping, but not conditioned flavor avoidance (Parker, 2014). Therefore, the gape CR is a more selective reflection of nausea in rats than is conditioned flavor avoidance.
Recently, it has been demonstrated that not only nausea-paired flavors, but also nausea-paired contexts, can elicit conditioned gaping reactions in rats (Limebeer et al., 2014; Limebeer, Krohn, Cross-Mellor, Ossenkopp, & Parker, 2008; Limebeer, Hall, & Parker, 2006; Rock, Limebeer, Mechoulam, Piomelli, & Parker LA, 2008). Initially, Rodriguez, Lopez, Symonds and Hall (2000) found that when rats were re-exposed to a context previously paired with LiCl, they displayed suppressed consumption of a sucrose solution, suggesting that this reduced consumption is the result of conditioned nausea elicited by the illness-paired context. Indeed, it was subsequently found that while in a LiCl-paired context, rats displayed gaping reactions both during an infusion of a novel sucrose solution (1 min infusion every 5 min during a 30 min test) and during inter-infusion intervals (Limebeer et al., 2006). Similarly, Limebeer et al. (2008) demonstrated that rats display conditioned gaping to the context alone, even in the absence of a flavored solution or a distinctive odor. Moreover, escalating doses of LiCl have been shown to dose-dependently increase the overall number gapes, presumably because higher doses are associated with greater (more severe) nausea (Ossenkopp, et al., 2011).
In the present study we evaluated the potential of a flavor to acquire secondary conditioning when paired with a context that has previously been associated with LiCl-induced nausea. Second-order conditioning can be produced if a novel CS2 is paired with an excitatory CS1 that has previously been paired with the Unconditioned Stimulus (US); thus, second-order conditioning is evident if CS2 elicits the same CR elicited by CS1 (Pavlov, 1927). Although second-order conditioning is a well-documented phenomenon, it is unclear whether excitatory conditioning can be produced between a nausea-paired context and a novel flavor cue. Such a demonstration is important, because it would confirm that the gaping produced by the LiCl-paired context reflects conditioned nausea that is capable of becoming associated with a novel flavor. As measured by conditioned gaping reactions, the present study sought to determine whether a novel flavor (CS2) would gain excitatory properties when it is delivered in a context (CS1) previously paired with LiCl.
Experiment 1
Following four contextual conditioning trials (separated 72 hr apart), the rats received a single flavor (CS2)-context (CS1) pairing in the absence of LiCl. Second-order conditioning to saccharin (CS2) was subsequently assessed in the TR test, which occurred in a different context. Since rats do not gape to unpaired saccharin solution (e.g. Berridge, Grill, & Norgren, 1981; Grill & Norgren, 1978), if the flavor (CS2) elicits conditioned gaping following CS2-CS1 pairings it can be assumed to have acquired secondary conditioning properties. To assess this, we employed the following three experimental groups (n = 8/group): Group Paired/Paired (P/P) initially acquired an association between the CS1 contextual cue and LiCl during four first order conditioning trials, then received a 5 min drug-free second order pairing of the CS2 saccharin in the CS1 context; Group Paired/Unpaired (P/U) initially acquired the CS1-LiCl association, then the rats received unpaired exposure to the CS2 saccharin solution in their home cage 24 hr prior to a drug-free exposure to the CS1 context; Group Unpaired/Paired (U/P) was injected with saline prior to placement in the CS1 context on each of four trials (and injected 24 hr later with LiCl in the home cage), then received a 5 min exposure to CS2 saccharin in the CS1 context.
Methods
All experiments were approved by the Animal Care Committee of the University of Guelph and were carried out in accordance with the recommendations of the Canadian Council on Animal Care.
Subjects
The subjects were 24 experimentally naïve male Sprague–Dawley rats weighing between 200–250 g at the start of experiments (Charles River Lab, St Constant, Quebec). The rats were single-housed in Plastic cages (10.25”W × 18.75”D × 8”H) at 22 °C and maintained on a reverse light–dark cycle (7:00 lights off; 19:00 lights on) and were conditioned and tested 2–6 hr into their active cycle. They had free access to food (Iams rodent chow, 18 % protein) and tap water except during testing. After all behavioral testing, the animals were euthanized by CO2.
Intraoral cannulation surgery
In order to present the flavor (CS2) paired with the excitatory context (CS1) during a second order conditioning trial, 48 hr prior to the final contextual conditioning trial (to prevent loss of cannulae), the rats were anaesthetized with isoflurane gas, administered the antibiotic Depocillin (0.33 mg/kg, sc; Pen Aqueous) and the nonsteroidal anti-inflammatory/analgesic drug, Carprofen (0.1 mg/kg, ip; Pfizer). A 15-gauge stainless steel needle was inserted at the mid-area on the back of the neck, and guided subcutaneously below the ear and across the cheek, where it exited into the oral cavity behind the first molar. A 10-cm section of polyethylene tubing (PE 90, I.D. 0.86 mm, O.D. 1.27 mm) was inserted into the needle, which was then removed from the animal, allowing only the tubing to remain in place. Three elastomer squares (8x8 mm) were threaded onto the tubing and drawn all the way to the neck, securing the cannula firmly in place. The intraoral section of the cannula was held in place by a flanged-end of the tubing over a section of surgical mesh that rested flush against the skin. Twenty-four hours after surgery, rats were administered a second dose of Carprofen (0.1 mg/kg) and their health was monitored for three days following surgery. Intraoral cannulae were also flushed once a day, for three days, with Chlorhexidine.
Procedures
The rats were randomly assigned to one of three groups based upon the contingency during the initial CS1 first-order conditioning trials and the contingency during the CS2-CS1 second-order conditioning trials (n = 8/group): Group P/P, Group P/U and Group U/P. During the initial CS1 context conditioning trials, the rats in Group P/P and Group P/U were intraperitoneally (ip) injected with LiCl (0.15 M, 20 ml/kg) while the rats in Group U/P were injected with physiological saline (20 ml/kg) immediately prior to confinement in the distinctive context (CS1) for 30 min on each of the conditioning sessions spaced 72–96 hr apart. The distinctive context consisted of a black Plexiglas chamber (29 × 29 × 10 cm) resting atop a stainless steel grid floor that was placed on a clear glass surface, and illuminated from below by two 60 W bulbs. Twenty four hr following the context conditioning trial, rats in Group U/P were injected with LiCl in their home cage and those in Group P/P and Group P/U were injected with an equal volume of saline solution.
Four days after the final CS1 context conditioning trial, the rats in Group P/P and Group U/P received a 5-min drug-free CS2-CS1 second-order conditioning trial, in which the rats’ cannulae were attached to an infusion pump (KD Scientific; model # KDS220) with tubing directed through the top of the chamber. The animals were placed in the CS1 context for 5-min while receiving an intraoral infusion (1 ml/min) of 0.1 % saccharin solution (CS2). Rats in Group P/U were placed in the CS1 context but were not infused with saccharin; instead, they received a 5 min (CS2) saccharin infusion (1 ml/min) in their home cage 24 hr prior to the CS1 context exposure.
Twenty four hr following the second order conditioning trial, responding to the CS2 saccharin solution was assessed in a two min TR test. The TR test was conducted in a different testing room and in a different apparatus than that used for contextual conditioning. The rats were placed in a clear Plexiglas chamber (without stainless steel grid floor) that rested atop a clear glass surface. Two 60 W lights suspended from the apparatus illuminated the chamber. To allow optimal viewing of orofacial responses, a mirror was placed at a 45-degree angle below the glass surface. The rats’ intraoral cannulae were attached to an infusion pump for delivery of saccharin (1 ml/min) for two min while in the chamber. During the course of the session, orofacial and somatic responses were recorded using a Sony video camera (Handy Cam) that was placed directly in front of the mirror. The videotapes were scored for the behavior of gaping (wide open mouth with lower incisors exposed).
Results and Discussion
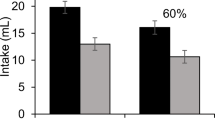
Rats in Group P/P, but not P/U or U/P, displayed second-order conditioned gaping during the TR test, as is evident in Fig. 1. A single factor ANOVA revealed a significant effect of group, F(2, 21) = 13.8; p < 0.001. Subsequent Bonferroni post hoc comparison tests showed that rats in Group P/P displayed more gaping (ps < 0.001) than either group P/U or U/P, which did not gape during the test.
Mean (±sem) number of gapes elicited by 0.1 % saccharin (CS2) solution during the TR test of second-order conditioning for Groups P/P, P/U and U/P in Experiment 1. The asterisk (*** p < 0.001) denotes a significant difference between Groups P/P, P/U and U/P
Although the novel saccharin solution (CS2) was never explicitly paired with LiCl, its association with an excitatory LiCl-paired context (CS1) resulted in subsequent conditioned rejection of the taste in the TR test as measured by the frequency of conditioned gaping. That is, a single five min exposure to an intraoral infusion of novel saccharin (CS2) in a nausea-associated context (CS1) produced second-order conditioning to the saccharin (CS2). The fact that group PU did not display conditioned gaping during the TR test provides evidence that the gaping displayed by group PP was the result of second order conditioning and not simply generalization between the two contexts.
Experiment 2
Since the strength of first-order conditioning would be expected to influence the strength of second-order conditioning, Experiment 2 evaluated the potential of various numbers of first-order trials (2, 4 or 8 pairings) to support second-order conditioning of the CS2 saccharin solution. Since Group P/U showed no second order conditioned gaping in Experiment 1, and because rats do not unconditionally gape to saccharin, this group was not included in Experiment 2.
Method
A total of 48 experimentally naïve male Sprague–Dawley rats weighing between 200–250 g at the start of experiments (Charles River Lab, St Constant, Quebec) were maintained as in Experiment 1. They were assigned to one of six groups (n = 8/group) on the basis of the first order conditioning contingency (Paired or Unpaired) and number of first order conditioning trials (2, 4 or 8); Otherwise, all experimental procedures were identical to those of Experiment 1.
Results and Discussion
Contextual conditioning
The frequency of gapes during the first two min (to equate test duration with the test of second order conditioning) of contextual conditioning for Group Paired and Group Unpaired among the rats given two, four, or eight pairings is presented in Table 1. Separate analyses were used for rats given two, four, or eight contextual conditioning trials. To assess the progression of initial contextual conditioning, the mean number of gapes during the first two min of each trial was analyzed using contingency by trials mixed-factors ANOVAs. Group Paired displayed significantly more conditioned gaping than Group Unpaired among rats that received four pairings, F(1,14) = 10.43, p < 0.01, and eight pairings F(1,14) = 37.87, p < 0.001, but not among rats that received only two pairings. Among the four and eight pairing groups, there was a significant contingency by trial interaction (four trial group, F(3,42) = 8.60, p < 0.001, and eight trial group, F(7,98) = 6.68, p < 0.001). Among the rats given four pairings, Group Paired displayed significantly more gapes on trials three and four compared to trials one and two (ps < 0.05). Among the rats given eight pairings, Group Paired displayed significantly more gapes on trials three (ps <0.05) and four (ps < 0.01) through eight than on trials one and two; they did not significantly differ on trials four through eight, suggesting an asymptotic effect on gaping after four trials. Altogether, these findings are consistent with the contextually elicited gaping literature indicating that gaping is not evident on the second conditioning trial (Chan, Cross-Mellor, Kavaliers, & Ossenkopp, 2013; Limebeer et al., 2008).
TR test of second-order conditioning
Following four or eight contextual conditioning trials, but not two pairings, the rats in Group P/P demonstrated second-order conditioning to the flavor CS2 that had never been explicitly paired with LiCl. Figure 2 presents the mean (+SEM) number of gapes displayed by rats in Group P/P and by rats in Group U/P during the TR test of second-order conditioning. To determine if the CS2-CS1 pairing resulted in second-order conditioning, the mean number of gapes during the TR test of secondary conditioning was analyzed as a contingency (Group P/P, Group U/P) by number of initial conditioning trials (two, four, or eight contextual conditioning trials) between groups ANOVA. The ANOVA revealed a significant effect of contingency, F(1,42) = 21.20, p < 0.001, number of conditioning trials, F(1,42) = 5.33, p < 0.01, and a significant contingency by number of trials interaction, F(2,42) = 5.33; p < 0.01. Subsequent planned comparison tests of our predicted effects revealed that Group P/P displayed significantly more gaping during the TR test than Group U/P only among the groups that received four (p < 0.001) or eight contextual conditioning trials (p =0.012), but not among rats that received two conditioning trials.
General Discussion
After 4 first-order context conditioning pairings with LiCl, the CS1 context produced asymptotic second-order conditioning to a novel saccharin (CS2) solution with which it was paired. The finding that a context (CS1) with sufficiently strong excitatory strength (following 4 or 8 prior pairings with LiCl) can produce second-order conditioning to saccharin (CS2) is particularly interesting as it suggests that a context previously associated with illness elicits nausea even in the absence of any pharmacologically induced illness. Thus, following four or eight context-illness pairings, a context itself acquires properties similar to the US, which can subsequently support new conditioning. The results of the current study provide additional evidence that conditioned gaping elicited by a LiCl-paired context is mediated by conditioned nausea that, in turn, can produce secondary conditioning to a distinct flavor with which it is paired. Rodriguez et al. (2000) similarly argued that a LiCl-paired context elicits conditioned nausea based on their finding that rats consumed less of a palatable solution when in the presence of LiCl-paired contextual cues as opposed to a neutral environment. Interestingly, this effect, like contextually elicited conditioned gaping (Limebeer et al., 2008; Rock et al., 2014), is not ameliorated by pre-treatment with the classic anti-emetic, ondansetron (Symonds and Hall, 2000), as is also evident in human chemotherapy patients that experience anticipatory nausea when returning to the treatment context (e.g. Morrow et al., 1998.
The finding that second-order conditioning occurs to a LiCl-paired context is particularly important with respect to illness conditioning and treatments that induce nausea, as it suggests that after repeated pairing with nausea-inducing stimuli, the conditioned stimulus, in effect, functions as a US to elicit some sensation of nausea or illness. This CR, the magnitude of which may be comparable in some respect to the UR, becomes so prevalent following several conditioning trials that it supports conditioning to a novel flavored solution (CS2). However, this effect was only apparent in the rats that received four or eight contextual conditioning trials, as rats that received only two pairings did not display second-order conditioning to the CS2. Presumably, the excitatory strength of CS1 was not strong enough to support second-order conditioning among rats receiving only two prior pairings of the context with LiCl, which is consistent with reports that two CS1 conditioning trials only produces minimal contextually elicited conditioned gaping (Chan et al., 2013; Limebeer et al., 2008). It is also interesting that the four and eight trial groups did not significantly differ from one another in the number of conditioned gapes displayed during the test of second order conditioning, Indeed, this finding is consistent with the pattern of contextually elicited gaping among animals that received eight CS1 pairings, which was asymptotic after four trials; thus, four LiCl-CS1 pairings resulted in maximal contextual conditioning and second order conditioning.
The contextually-elicited conditioned gaping model has considerable face validity as a model of anticipatory nausea in human chemotherapy patients (Limebeer et al., 2006; Rock et al., 2014). Furthermore, shrews (which vomit in response to toxins) also display contextually-elicited conditioned gaping when returned to a LiCl-paired context in which they previously vomited (Parker & Kemp, 2001; Parker, Kwiatkowska, & Mechoulam, 2006). Interestingly, as is apparent with human chemotherapy patients (eg. Morrow et al., 1998), the expression of contextually-elicited conditioned gaping in both rats and shrews is not modified by pre-treatment with classical anti-emetics such as ondansetron (Limebeer et al., 2006; Parker et al., 2006; Parker & Kemp, 2001; Rock et al., 2014). On the other hand, cannabinoid compounds, including the psychoactive Δ9-tetrahydrocannabinol (THC), but also non-psychoactive cannabidiol and cannabidiolic acid, are highly effective in reducing these behaviors reflective of anticipatory nausea (Parker et al., 2006; Rock et al., 2008, 2014). Although such manipulations were not assessed in the current study, these compounds would be likely to reduce the establishment and/or expression of second order conditioning, as well.
Future experiments will explore the potential for anti-nausea treatments to interfere with second order conditioning of nausea-paired stimuli, as well as the particular conditions that lead to second-order conditioning using the paradigm described here. For instance, it is conceivable that using a more ‘prepared’ or ‘biologically relevant’ (e.g. Garcia, Hankins, & Rusiniak, 1974; Seligman, 1971) first-order association such as a flavor and LiCl-induced nausea would produce more robust second-order conditioning when a contextual cue serves as a CS2 during the CS2-CS1 pairing(s). Classical conditioning readily occurs following presentation of a neutral stimulus and an unconditioned stimulus in close temporal sequence, and by nature of this contingency (and contiguity) the CS comes to serve as an important predictor of some rewarding or aversive US (Pavlov, 1927). Although countless studies have been conducted on various aspects of classically conditioned associations, the present study is the first such investigation to date of second-order nausea-induced conditioned gaping in rats. Here we report that contextual cues associated with nausea acquire the capacity to confer second-order conditioning properties to novel stimuli experienced in their presence. This finding suggests that certain stimuli experienced in the chemotherapy context have the potential of acquiring aversive properties even in the absence of experience with chemotherapy treatment.
References
Berridge, K. C., Grill, H. J., & Norgren, R. (1981). Relation of consummatory responses and preabsorptive insulin release to palatability and learned taste aversions. Journal of Comparative and Physiology Psychology, 95(3), 363–382.
Chan, M. Y. T., Cross-Mellor, S. K., Kavaliers, M., & Ossenkopp, K. P. (2013). Impairment of lithium chloride-induced conditioned gaping responses (anticipatory nausea) following immune system stimulation with lipopolysaccharide (LPS) occurs in both LPS tolerant and LPS non-tolerant rats. Brain, Behavior, and Immunity, 27, 123–132.
Garcia, J., Hankins, W. G., & Rusiniak, K. W. (1974). Behavioral regulation of the milieu interne in man and rat. Science, 185(4154), 824–831.
Grill, H. J., & Norgren, R. (1978). The taste reactivity test. I. mimetic responses to gustatory stimuli in neurologically normal rats. Brain Research, 143(2), 263–279.
Limebeer, C. L., Hall, G., & Parker, L. A. (2006). Exposure to a lithium-paired context elicits gaping in rats: A model of anticipatory nausea. Physiology and Behavior, 88(4–5), 398–403.
Limebeer, C. L., Krohn, J. P., Cross-Mellor, S., Ossenkopp, K. P., & Parker, L. A. (2008). Exposure to a context previously associated with toxin (LiCl)- or motion-induced sickness elicits conditioned gaping in rats: evidence in support of a model of anticipatory nausea. Behavior Brain Research, 187(1), 33–40.
Limebeer, C. L., Abdullah, R., Rock, E. M., Imhof, E., Wang, K., Lichtman, A. H., et al. (2014). Attenuation of anticipatory nausea in a rat model of contextually-elicited conditioned gaping by enhancement of the endocannabinoid system. Psychopharmacology, 231(3), 603–612.
Morrow, G. R., Roscoe, J. A., Kirshner, J. J., Hynes, H. E., & Rosenbluth, R. J. (1998). Anticipatory nausea and vomiting in the era of 5-HT3 antiemetics. Support Care in Cancer, 6(3), 244–247.
Ossenkopp, K. P., Biagi, E., Cloutier, C. J., Chan, M. Y. T., Kavaliers, M., & Cross-Mellor, S. K. (2011). Acute corticosterone increases conditioned spontaneous orofacial behaviors but fails to influence dose related LiCl-induced conditioned “gaping” responses in a rodent model of anticipatory nausea. European Journal of Pharmacology, 660, 358–362.
Parker, L. A. (2014). Conditioned taste avoidance versus conditioned gaping: Rat models of nausea-induced behavior. European Journal of Pharmacology, 722, 122–133.
Parker, L. A., & Kemp, S. (2001). Tetrahydrocannabinol (THC) interferes with conditioned retching in Suncus murinus: An animal model of anticipatory nausea and vomiting (ANV). NeuroReport, 12(4), 749–752.
Parker, L. A., Kwiatkowska, M., & Mechoulam, R. (2006). Delta-9-Tetrahydrocannabinol and cannabidiol, but not ondansetron, interfere with conditioned retching reactions elicited by a lithium-paired context in Suncus murinus: An animal model of anticipatory nausea and vomiting. Physiology & Behavior, 87(1), 66–71.
Pavlov, I. P. (1927). Conditioned Reflexes. NY: Dover Press.
Rock, E. M., Limebeer, C. L., Mechoulam, R., Piomelli, D., & Parker, L. A. (2008). The effect of cannabidiol and URB597 on conditioned gaping (a model of nausea) elicited by a lithium-paired context in the rat. Psychopharmacology, 196(3), 389–395.
Rock, E. M., Limebeer, C. L., Navaratnam, R., Sticht, M. A., Bonner, N., Engeland, K., et al. (2014). A comparison of treatments for anticipatory nausea using a rat model of contextually elicited conditioned gaping. Psychopharmacology, 231(16), 3207–3215.
Rodriguez, M., Lopez, M., Symonds, M., & Hall, G. (2000). Lithium-induced context aversion in rats as a model of anticipatory nausea in humans. Physiology and Behavior, 71(5), 571–579.
Seligman, M. E. (1971). Phobias and preparedness. Behavior Therapy, 2(3), 307–320.
Symonds, M., & Hall, G. (2000). Contextual conditioning with an illness US is attenuated by the antiemetic ondansetron. Psychobiology, 28, 360–366.
Acknowledgments
This research was supported by an operating grant from the Natural Sciences and Engineering Research Council (NSERC-92057) of Canada to Linda A Parker, and an NSERC Doctoral Canada Graduate Scholarship to Martin A Sticht. We would like to thank Dr Cheryl Limebeer with her help throughout the collection of the data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sticht, M.A., Leach, Z.K., Wilson, J.C. et al. Second-order conditioning of LiCl-induced gaping with flavor and contextual cues. Learn Behav 43, 95–100 (2015). https://doi.org/10.3758/s13420-014-0164-8
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13420-014-0164-8