Abstract
The brain is an intricate network, not only structurally but also functionally. On the functional level, connectivity in the brain is organized in separable yet interacting networks that support information processing by maintaining a ready state, even in the absence of external stimulation. It has been hypothesized that an insular-opercular network underlies the processing of emotionally salient information and that individual differences in functional connectivity within this network correspond to individual differences in trait anxiety. Here, we tested this relationship by applying graph analysis to multiple regions of interests delineating the insular-opercular network to estimate the characteristic path length that quantifies the overall information exchange efficiency within a given network. We found that people scoring high on the anxiety-related temperament-dimension harm avoidance had decreased insular-opercular network efficiency in the resting state, as indicated by a higher characteristic path length. Furthermore, people scoring high on harm avoidance showed generally reduced functional connectivity between brain regions; the relationship between harm avoidance and insular-opercular network efficiency remained significant when controlling for mean connectivity within this network. No such results were found for other resting-state networks. The results provide insights into how personality is organized in the human brain and point toward clinically relevant endophenotypes for affective and mood disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Affective disorders and mood disorders represent a considerable burden for inflicted patients and for society because of the tremendous costs for health care (Berger, Edelsberg, Treglia, Alvir, & Oster, 2012; Ilyas & Moncrieff, 2012; Simon, 2003; Smit et al., 2006; Wittchen et al., 2011). Psychiatric disorders are thought to have a dimensional instead of a categorical character, with psychopathology lying at the extreme end of otherwise normal individual variation (Plomin, Haworth, & Davis, 2009; Trull, Tragesser, Solhan, & Schwartz-Mette, 2007). Understanding the normal anxiety response is therefore an important step toward an evidence-based treatment of such disorders (Bateson, Brilot, & Nettle, 2011; Montag, Reuter, Jurkiewicz, Markett, & Panksepp, 2013).
All personality theories, which provide taxonomies to characterize and explain individual differences in behavior, agree on a personality dimension linked to trait anxiety. These theories describe anxiousness as a key personality dimension and define it as responsiveness of an individual to aversive stimuli of punishment or uncertainty (Cloninger, 1986; Costa & McCrae, 1992; Eysenck, 1947; Gray, 1971; Panksepp, 1998). The anxious personality is thought to underlie anxiety disorders, either as a risk factor (Clark, Watson, & Mineka, 1994) or as an endophenotype (Leonardo & Hen, 2006). A thriving question is how trait anxiety is represented in the brain. The application of neuroimaging techniques to study individual differences in anxiety in healthy human research participants has the potential of revealing brain regions and neural circuitry that may serve as biomarkers or drug targets for the treatment of affective disorders (for review on structural imaging and anxiety, see Montag et al., 2013; for a review on a functional magnetic resonance imaging and anxiety, see Etkin & Wager, 2007).
In recent years, the cognitive, affective, and behavioral neurosciences have witnessed a paradigm shift toward an integrative view of brain functioning as an intricate network of highly specialized processing units (Smith, 2013). Several large-scale brain networks are not only conjointly activated during task conditions but also become apparent in the resting brain in the form of spatio-temporal synchronies in the blood oxygen-level dependent (BOLD) time series of intrinsically generated brain activity (Fox & Raichle, 2007; van den Heuvel & Hulshoff Pol, 2010). In the context of fMRI, the resting state is defined as a condition where the research participant does not engage in a behavioral task but is instructed to lie still, with closed eyes, and without thinking of anything in particular. Synchronous fluctuations in resting-state fMRI are interpreted as functional connectivity (Friston, Frith, Liddle, & Frackowiak, 1993), a concept closely related yet not redundant to anatomical links through white matter tracts (Greicius, Supekar, Menon, & Dougherty, 2009; Honey et al., 2009; van den Heuvel, Mandl, Kahn, & Hulshoff Pol, 2009). The architecture of functional networks is considerably stable across time (Shehzad et al., 2009), preserved across different states of consciousness (Fukunaga et al., 2006; Greicius et al., 2008; Vincent et al., 2007), and not only parallels the topography of stimulus-evoked brain activation (Dosenbach et al., 2007) but also is able to predict neural activity during task performance (Gordon, Stollstorff, & Vaidya, 2011). All this evidence suggests that resting-state brain activity is not a neural correlate of ongoing information processing but rather reflects a ready state that maintains responsiveness to external events and facilitates adaptation to changing environmental demands (Raichle, 2010, 2011).
This view on resting-state brain connectivity makes it a promising research target for the neural correlates of personality. Personality traits are conceptualized as behavioral dispositions that explain and predict behavior across different situations. Early on, personality theorists have argued that these traits should be considered as neuropsychological systems with the purpose of triggering consistent behavioral responses to functionally equivalent stimuli (Allport, 1937). Accordingly, individual differences in behavior are thought to stem from variability in these systems (Eysenck, 1947). Large-scale networks, as described in the resting-state literature, might constitute a functional neural basis for these hypothesized neuropsychological systems, and, in turn, individual differences in the networks’ architecture and reactivity to stimulation might underlie individual differences in behavioral dispositions.
In line with the dimensional character of anxiety and anxiety disorders, the insular-opercular network has been hypothesized to be a major contributor to anxiety disorders (Sylvester et al., 2012). The insular-opercular network is an intrinsic connectivity network centering on the anterior insular (Seeley et al., 2007) and comprising anterior cingular, anterior prefrontal, thalamic, and striatal sites, as well as the amygdala and cortical areas along the operculum. Task-based neuroimaging studies have shown that elevated levels of trait anxiety are associated with increased activity within the insular-opercular network (Hajcak, McDonald, & Simons, 2003; Most, Chun, Johnson, & Kiehl, 2006; Paulus, Rogalsky, Simmons, Feinstein, & Stein 2003). This stronger activation has been discussed as a compensatory mechanism for reduced processing efficiency, a claim backed up by the observation that activity increases are paralleled by decreases in within-network connectivity during task processing (Basten, Stelzel, & Fiebach, 2011).
First, evidence from resting-state fMRI points toward a role of the insular-opercular network in anxiety as well: Patients diagnosed with social and generalized anxiety disorders show aberrant within-network connectivity, with either increased or decreased connectivity depending on neural site (Liao et al., 2010; Pannekoek et al., 2013; Roy et al., 2013). But also in the nonpathological range, state and trait anxiety measures correlate with within-network connectivity. Although the majority of studies reports positive relationships between anxiety and connectivity strength on specific within-network routes (Baur, Hänggi, Langer, & Jäncke, 2013; Markett et al., 2013; Seeley et al., 2007), other studies report both increases and decreases, depending on the route under study (Aghajani et al., 2014).
These pioneering studies provide evidence for an important role of the insular-opercular network in trait anxiety and anxiety disorders. By doing this, however, they only describe relationships on the level of activity of single neural sites or connectivity on specific routes within the network, while neglecting the overall architecture of the network as a whole. Although a functional link between two brain areas is the basic entity of a network, brain functioning can be better understood as an interplay between multiple functional connectivities embedded into a network of multiple brain areas. That is, an association between anxiety and single functional connectivities, as reported in previous work, can only be a first (yet important) step toward the discovery of network correlates of anxiety. In this study, we therefore seek to zoom out from single brain areas and their connectivity and take a perspective on anxiety on the basis of network efficiency. Network efficiency is a graph-analytical concept. Graph analyses make use of algorithms to describe network properties based on the representation of a network as a set of nodes that are connected to each other by edges. In the case of resting-state networks, brain regions of interest are defined as network nodes, and the functional connectivity between each pair of nodes as a connecting edge (Fornito, Zalesky, & Breakspear, 2013). This allows for the calculation of network metrics that describe the efficiency of information flow through the network, or the relative importance of single nodes to the network architecture. Compared to the earlier studies in the field that focused merely on bivariate relationship between two network nodes (Aghajani et al., 2014; Baur et al., 2013; Markett et al., 2013; Seeley et al., 2007), our present approach takes the richness of connectivity throughout the entire network into account by characterizing the network as a whole and describing characteristics in the organization of functional connectivity at a level superordinate to single connections.
As outlined earlier, decreased insular-opercular processing efficiency has been put forward as an explanation for increased reactivity of single parts of the network in highly anxious participants (Basten et al., 2011; Sylvester et al., 2012). Here, processing efficiency refers to the metabolic demands of a neural system to create a given output. Reduced processing efficiency would therefore be compensated by an increase in neural activation to produce a similar output. A potential neural basis for a processing deficit could be reflected in the overall information exchange capability between different parts of the network. In graph-analytical terms, this can be described by the network’s characteristic path length. This measure quantifies the average shortest path along functional connections between any pair of nodes, either directly or by traveling through other nodes. Shorter path lengths allow for faster information exchange, hence, a shorter characteristic path length reflects higher network efficiency. Thus, one could expect a positive correlation between anxiety scores and individual insular-opercular characteristic path lengths (i.e., a decreased network efficiency in highly anxious participants). Throughout this article, we will use the term network efficiency to refer to the graph-analytical concept of characteristic path length, and the term processing efficiency to refer to the relationship between metabolism and system output, as outlined earlier.
In the context of this study, we seek to relate the insular-opercular network’s characteristic path length to trait anxiety as conceptualized by the harm-avoidance construct in the Temperament and Character Inventory (TCI; Cloninger, Svrakic, & Przybeck, 1993). Harm avoidance is a temperament dimension that describes the heritable tendency to react overly cautiously, nervously, pessimistically, and passively and to respond intensely to stimuli of punishment and nonreward (Cloninger, 1986). Conceptually, the harm-avoidance temperament resembles the conceptualization of anxiety as a behavioral inhibition system because it taps the same idea that anxiety arises from uncertainty (Gray & McNaughton, 2000). Various self-report measures of anxiety are highly correlated (Montag et al., 2013); the correlation between harm avoidance and trait anxiety from the Spielberger inventory (Spielberger, 1989), for instance, falls in the range of .6 to .8 (Jiang et al., 2003). This large proportion of shared variance between different measures has motivated researchers, including ourselves, to use composite scores of several measures to assess this shared variance (Bijsterbosch, Smith, Forster, John, & Bishop, 2014; Markett, Montag, & Reuter, 2011). In this study, however, we focus on a single questionnaire, the TCI. Because of the high level of aggregation, the findings are more abstract and less easy to interpret when many different measures are used. We choose the TCI because elevated levels of harm avoidance have been described in patients diagnosed with anxiety disorders (Nery et al., 2008; Öngür, Farabaugh, Iosifescu, Perlis, & Fava, 2005), pointing toward the role of harm avoidance as an endophenotype or disposition of anxiety disorders. Also, the TCI offers four subscales of harm avoidance (Anticipatory Worry, Fear of Uncertainty, Shyness, and Fatigability) that allow for a more fine-grained perspective on the different facets of trait anxiety. And also, the TCI has been most extensively applied in many neuroimaging studies on anxiety, which helps to relate our findings to the body of literature in the field (Markett, Montag, & Reuter, 2016).
Method
Participants
Forty-eight healthy female participants (mean age = 22.83, SD = 6.7 years) gave their informed written consent to participate in the study. We chose to invite females only to control for possible gender effects because males could not have been included in sufficient numbers for this study (because we recruited mainly from psychology classes). All participants were free of neurological or psychiatric disorders, as indicated by a screening questionnaire. The study protocol was carried out in accordance with the Declaration of Helsinki and was approved by the local ethics committee at the University Clinics in Bonn.
Personality assessment
All participants filled in the Temperament and Character Inventory (TCI) before the acquisition of imaging data. This was done on a different day than MRI scanning, but not more than 2 weeks before image acquisition. Besides the TCI, participants filled in a battery of other psychometric tests. Based on the extensive use of the TCI in neuroimaging of anxiety (Markett et al., 2016), we choose to focus exclusively on this assessment tool. The TCI consists of 240 items with a dichotomous response format and aims to measure four temperament (novelty seeking, harm avoidance, reward dependence, persistence) and three character dimensions (self-directedness, cooperation, self-transcendence). For psychometric reasons, the complete TCI was administered. Given our clear hypothesis and to circumvent the need to control for multiple testing, we only analyzed the items for the temperament harm avoidance, including its four subscales.
Image acquisition
From each participant, 245 T2*-weighted volumes were obtained on a Siemens Avanto 1.5 T scanner (Siemens, Erlangen, Germany) at the Life & Brain Center in a single 12-min session. Each volume consisted of 38 slices (thickness: 3 mm, interslice gap: 1 mm, in plane resolution: 3 mm × 3 mm) scanned in interleaved order (TR: 3.06 s, TE: 45 ms, flip angle: 90°, field of view: 192 mm). Participants were instructed to lie as still as possible, with their eyes closed, and without thinking about anything in particular and without falling asleep. After the functional scan, a high-resolution structural T1-weighted image with 160 sagittal slices, with 1 mm thickness, was acquired from each participant using a Magnetization Prepared Rapid Gradient Echo (MP-RAGE) sequence (field of view: 256 mm, in-plane resolution: 1 mm × 1 mm). Foam padding was used to constrain head movements during image acquisition.
Image analysis
All analyses were carried out in MATLAB (MathWorks) using the REST toolbox (www.restfmri.net) in SPM8 (www.fil.ion.ucl.ac.uk/spm), the Data Processing Assistant for Resting State fMRI (DPARSF; Yan & Zhang, 2010) and the Brain Connectivity Toolbox (BCT; Rubinov & Sporns, 2010). Preprocessing contained the following steps in the described order: (1) removal of the first 10 volumes; (2) slice timing to the middle slice; (3) realignment; (4) controlling for 12 voxel-specific movement parameters (three voxel-specific translations, three voxel-specific rotations, and six corresponding squared items; Satterthwaite et al., 2013) by means of linear regression; (5) coregistration of the mean functional image with the high-resolution structural scan; (6) additional motion correction by modeling time points with excessive motion as separate regressors and controlling for nuisance signals extracted from white matter (SPM’s tissue probability map thresholded at .9 and warped into native space using the inverse transformation matrix from SPM’s unified segmentation routine), cerebrospinal fluid (thresholded at 70 %), and the global mean signal by means of linear regression; (7) band-pass filtering (.01–.08 Hz); (8) spatial normalization using the T1-unified segmentations of the structural image; and (9) spatial smoothing with a Gaussian kernel with a full width of 6 mm at half maximum.
Preprocessing step six (additional motion correction) was carried out by “scrubbing” bad time points (frame wise displacement > .5), including one time point before and two time points after marked time points by means of linear regression (Power, Barnes, Snyder, Schlaggar, & Petersen, 2012). A mean of 2.79 frames was treated as “bad” time points (range: 0–27). Three participants with excessive motion (classified as outliers according to the Tukey, 1977, criterion, i.e. 1.5* interquartile range above the third quartile of the distribution) were excluded from subsequent analyses.
Graph construction
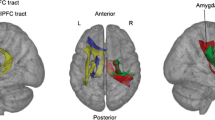
We modeled the whole-brain network as a weighted undirected graph G = (V,E), with nodes (vertices) V based on the 160 regions of interest (ROIs) from the Dosenbach atlas (Dosenbach et al., 2010), which provides a meta-analytically derived whole brain parcellation scheme based on functional activation during task fMRI. For edges E, time courses were extracted as the mean BOLD time series from all regions of interest (spheres with 5-mm radius), and linear correlations were computed between time series. Correlation coefficients were standardized using Fisher’s r-to-z transformation to approximate a Gaussian distribution. To facilitate between-subjects comparisons, the z standardized connectivity matrices were thresholded at different costs (.1 to .25, in increments of .05). Equating the number of network edges across participants by thresholding at a fixed set of costs (i.e., percentage of edges) prevents the biasing of network metrics by individual differences in edge density. Network metrics were calculated for each cost and then averaged for between-subjects comparison (van Wijk, Stam, & Daffertshofer, 2010).
Graph-theoretical metrics
To obtain the characteristic path length of the insular-opercular network for each individual, each thresholded connectivity matrix was first transformed into a connection-length matrix by computing the matrix’s inverse. Then, the shortest paths between each pair of nodes were calculated by the Dijkstra algorithm. In brief, this algorithm finds shortest paths from a source node to all other nodes by constructing shortest-path trees to all other nodes in the network. The calculation of shortest paths was based on the full network, consisting of all 160 vertices in the graph. The characteristic path length of the insular-opercular network was then calculated as the average of all shortest paths between the 32 nodes labeled as “insular-opercular network” by Dosenbach et al. (2010) (for an overview of insular-opercular nodes see (Fig. 1)). By first taking connectivity in the whole brain into account and then narrowing down on the insular-opercular network, we ensured that the shortest paths between two insular-opercular nodes that traverse nodes outside the network were also considered when calculating the characteristic path length. We also computed the global clustering coefficient and mean connectivity as further measures of integration that provide information on network efficiency. The mean clustering coefficient was determined by first computing, for each of the 160 nodes in the whole-brain network, the fraction of each node’s neighbors that are themselves neighbors to each other. Then, these values were averaged across all nodes labeled as insular opercular. Mean connectivity was determined by averaging all edges within the insular-opercular network.
To obtain discriminant validity, we also analyzed the other five networks from the Dosenbach atlas in a similar fashion. These networks included the fronto-parietal (34 nodes), the default mode (21), the sensorimotor (33), the cerebellar (18), and the visual network (22). Characteristic path length, global clustering coefficient, and mean connectivity were also computed for the whole-brain network based on 160 nodes.
Results
Neither the network-efficiency measures nor harm avoidance was correlated with participants’ age (all |r| < .2). The correlation between the harm-avoidance scale and the characteristic path length controlling for mean framewise displacement (FD) was significant (r = .422, p = .002, one-tailed: see Table 1). In line with our prediction, increasing network efficiency, as indexed by a shorter characteristic path length, was accompanied by decreasing levels of harm avoidance. There was also a negative partial correlation between harm avoidance and the global clustering coefficient (r = -.353, p = .029, one-tailed) and between harm avoidance and mean connectivity (r = -.334, p = .013, one-tailed). Because random networks with higher mean connectivity tend to exhibit decreased shortest-path lengths and higher clustering coefficients, we reran the correlation analysis between harm avoidance, characteristic path lengths, and the global clustering coefficient while also controlling for mean within-network connectivity. The correlation between harm avoidance and the characteristic path length remained significant after controlling for mean connectivity (r = .284, p = .032); the correlation between harm avoidance and the global clustering coefficient, however, did not (r = -.126, p = .211).
Table 1 gives partial correlations for the relationships between the characteristic path length and the four subscales. The top row gives coefficients for the relationship between the subscales and insular-opercular characteristic path length controlled for mean FD, and the bottom row gives the same coefficients, while additionally controlling for mean connectivity within the insular-opercular network. When only controlling mean FD, characteristic path length was significantly intercorrelated with the facets Anticipatory Worry (HA1) and Shyness (HA3). After controlling for mean connectivity, only the facet Fatigability (HA4) was significantly intercorrelated with characteristic path length, indicating that this aspect of the harm-avoidance construct contributes to the observed relationship between network efficiency (characteristic path length) in the insular-opercular network and trait anxiety.
We next examined the relationship between harm avoidance and properties of the other five resting-state networks. Partial correlation coefficients are given in Table 2. The coefficients in the top row suggest similar relationships between harm avoidance and other resting-state networks as reported for the insular-opercular network. However, when controlling for mean connectivity, the relationships between harm avoidance and the characteristic path lengths and global clustering coefficients in all five networks fall beneath the statistical threshold, indicating some specificity for the insular-opercular network. A formal comparison of the partial correlation coefficients using Meng’s z test indicated significant differences between the harm-avoidance–insular-opercular path length correlation and the correlations between harm avoidance and the path lengths of the fronto-parietal, cerebellar, and visual networks (all ps < .05). The partial correlations between harm avoidance and characteristic path length in the default mode network (z = .29, p =.38) and in the somato-motor network (z = .594, p =.276) were not significantly smaller than the partial correlation between harm avoidance and path length in the insular-opercular network.
We also analyzed the relationship between harm avoidance and network measures at the whole-brain level. Here, harm avoidance was positively related to characteristic path length (r = .421, p = .004) and negatively to the global clustering coefficient (r = -.351, p = .02) and mean connectivity (r = -.379, p = .011). When additionally controlling for mean connectivity, however, the partial correlations between harm avoidance and characteristic path length and the global clustering coefficient ceased to be significant (see Table 2).
All results are based on graph-theoretical measures averaged across four different cost thresholds (i.e., sparsity levels; see Method). Results, however, were robust with regard to the thresholding procedure: Graph metrics and questionnaire measures were significantly correlated at all cost thresholds, except for the highest threshold of .25, where the correlation coefficients fell short of formal significance for some of the analyses.
Discussion
The rationale of this study was to assess whether trait anxiety—as operationalized by the temperament dimension harm avoidance—modulates the overall behavior of the insular-opercular network in the resting state. This hypothesis was grounded on a recent assumption that activity of the insular-opercular network correlates with trait anxiety (Sylvester et al., 2012). We applied graph analysis to multiple regions of interest to estimate the insular-opercular network’s characteristic path length as a measure for network efficiency. Participants scoring high on harm avoidance showed an increased characteristic path length, indicating less efficient information exchange in the insular-opercular network at rest. Previous studies had shown that functional connectivity on single routes within the network is altered in anxious people. Our study extends this evidence from single connections to the organization of the network as a whole. The relationship between harm avoidance and the characteristic path length remained significant after controlling for mean connectivity, which itself was negatively related to harm avoidance.
Harm avoidance can be split into four facets that allow for a detailed analysis of the present these findings. When controlling for mean within network connectivity, only the correlation between the “fatigability” subscale and insular-opercular characteristic path length reached statistical significance, unlike the correlations between insular-opercular path length and Anticipatory Worry, Fear of Uncertainty, and Shyness that were all not significant. In a previous study on the relationship between harm avoidance and connectivity within the insular-opercular network (Markett et al., 2013), we found less specific associations between the subscales and insular connectivity: All four facets were associated with insular-cingulate connectivity on their own, and the highest correlation was observed for the shyness scale. Of note, the shyness scale also showed the nominally largest correlation with characteristic path length in this study without controlling for mean connectivity. The relationship, however, vanished when mean connectivity was considered as confound.
Within the TCI personality framework, fatigability reflects the idea that highly anxious people tire much quicker, presumably as a result of their heightened status of cautious alertness (Cloninger et al., 1993). According to the questionnaire items, fatigability refers to having less energy, feeling less secure and energetic, needing more time or support to recover from stress as well as minor illness and everyday adversity, and having more difficulties to adapt to new situations because of getting weary and worried. Fatigability is a core symptom of generalized anxiety disorder, according to DSM-5, and strong links to major depression and depressive symptoms have been reported (Nyman et al., 2011). The harm-avoidance facets have not been investigated thoroughly in the context of neuroimaging studies, even though the harm-avoidance scale itself has been widely applied (Markett et al., 2016). We are aware of three neuroimaging studies that report data on the facets, and two of these studies report associations with the fatigability scale: Pujol López, Deus, Cardoner, and Vallejo (2002) reported increased surface volume of the right anterior cingulate in healthy participants scoring high on fatigability. The anterior cingulate is one of the core areas of the insular-opercular network (Seeley et al., 2007). Tuominen et al. (2012) found higher availability of mu-opioid receptors throughout the insular-opercular network in highly fatigable participants. This finding was interpreted as an upregulation of receptors, presumably in consequence of a lower endogenous opioid drive that could result in heightened anxiety and fatigability. These two studies support the present finding that aspects of the cingulo-opercular network contribute to fatigability in the context of harm avoidance. Task fMRI studies are needed to characterize this contribution further. Regarding task fMRI, an interesting perspective comes from studies that suggest that the insular-opercular network might be involved in the maintenance of task sets and task-relevant tonic alertness (Dosenbach et al., 2006; Sadaghiani & D’Esposito, 2014). It would be interesting to assess the possibility that heightened fatigability leads to disruptions in the insular-opercular network’s ability to maintain tonic alertness, maybe by decreasing the networks overall efficiency. This could be probed in a within-subjects design by manipulating fatigability and observing changes in insular-opercular connectivity and network efficiency.
The specificity of these findings with respect to the fatigability facet—when controlling for mean connectivity—raises the question of why the other three subscales produced nonsignificant findings with respect to insular-opercular network efficiency. It is conceivable that these aspects of harm avoidance are not encoded within the network’s overall efficiency, but in other network properties. In our previous study, anticipatory worry, fear of uncertainty, and shyness correlated with insular-cingulate and insular-frontal connectivities, a finding that is in line with this view. Pessimistic worry in the anticipation of problems, on the other hand, does also bear a cognitive component, and cognitive processes are more dominantly associated with prefrontal sites. Studies have shown that anticipatory worry correlates with task-elicited activity and glucose consumption in dorsolateral prefrontal cortex (Tillfors, Furmark, Marteinsdottir, & Fredrikson, 2002). Furthermore, task fMRI studies point toward aberrant functional interactions between prefrontal control regions and subcortical areas in highly anxious participants (Basten et al., 2011; Bishop, Duncan, Brett, & Lawrence, 2004), and evidence from structural tractography suggests that individual differences in prefrontal-subcortical white matter projections represents a neurostructural correlate of anxiety (Montag et al., 2013). Although the insular-opercular network has been most dominantly implicated in anxiety (Vaidya & Gordon, 2013), other resting-state networks such as the fronto-parietal control network comprising the dorsolateral prefrontal cortex, medial anterior cingulate, and the intraparietal lobule (Dosenbach et al., 2008) and the default-mode network have also been hypothesized to play a relevant role (Sylvester et al., 2012). Our findings of reduced network efficiency in highly harm-avoidant participants, however, seems to have some specificity for the insular-opercular network: Even though harm avoidance was intercorrelated with the characteristic path lengths of some other networks, these relationships were no longer significant when mean connectivity was taken into account.
Liao et al. (2010) were able to demonstrate causal interactions between different resting-state networks. Future studies may want to assess the strength of such interactions, especially between the fronto-parietal control and the insular-opercular network in the context of trait anxiety and anxiety disorders. Recent data suggest that some aspects of anxiety are reflected in between network connectivity (Bijsterbosch et al., 2014). Even though our study did not find evidence for a global relationship between harm avoidance and network efficiency at the whole-brain level that could not be better explained by a reduced mean connectivity, we still found reduced mean connectivity across the entire brain in highly harm-avoidant participants. This finding corroborates another recent report of a negative relationship between overall brain-wide functional connectivity and anxiety-related personality traits (Servaas et al., 2015) and is further in line with structural imaging work that reports widespread reductions in white matter microstructure of long association fibers in individuals who score high on neuroticism (Bjørnebekk et al., 2013). Given the correspondence of structural and functional connectivity at the global network level (Mišić et al., 2016), our results support also the idea that the “anxious brain” is characterized by having weaker functional and structural connections that may disrupt information flow and exchange at a global level (Servaas et al., 2015). Future studies may want explore hierarchical network clustering to unravel associations between personality traits and the organization of functional connectivity at the whole-brain, the between-network, and the within-network levels.
For the Fear subscale, it is also likely that subcortical brain regions play a dominant role: Commonly, fear is distinguished from anxiety as an emotion elicited by direct and immediate threat to the individual (Gray, 1971). In contrast, anxiety is the emotion provoked by situations of high uncertainty. Anxiety and fear can be functionally dissociated between prefrontal sites, different nuclei of the amygdala and the periaqueductal gray (Mobbs et al., 2007), and it would be interesting to see whether such dissociation could also be obtained using functional connectivity mapping of the resting brain.
An important point that warrants discussion is the direction of the effect, because it appears to be at odds with previous findings of higher functional connectivity on selected within-network routes in this group of participants (Baur et al., 2013; Markett et al., 2013; Seeley et al., 2007). For the analyses of path lengths, between-node connectivity is transformed into a distance measure where higher functional connectivity reflects a shorter distance. Therefore, the path length between two strongly coupled brain regions is low, reflecting fast and efficient information exchange. Following this logic, an effect in the opposite direction (an inverse relationship between anxiety and path length) would have seemed plausible. On the other hand, some studies have reported not only higher but also lower functional connectivity in the highly anxious, depending on the neural sites under scrutiny (Aghajani et al., 2014; Liao et al., 2010). Characteristic path length is an aggregate measure of all connections in a network. Less efficient network behavior (i.e., a high characteristic path length) in a group of participants is therefore possible, even if some paths in their networks are shorter.
Another thriving question is how activity in resting-state networks relates to the processing of affective stimuli in task situations. Following Raichle (2011), resting-state networks maintain a ready state of brain activity that facilitates responsiveness to environmental demands. If personality is encoded in the properties of resting-state networks as our results suggest, a prediction from individual differences in resting-state network activity on relevant stimulus-evoked brain activity should be possible, just in the same way as personality questionnaires can predict neural responsiveness to emotional stimuli (Canli et al., 2001). In the cognitive domain, it has been shown that brain activity during working memory tasks can be predicted from the activity within resting-state networks (Gordon et al., 2011). Future studies may want to assess to what extent similar results can be obtained for the processing of fear- and anxiety-related stimuli. Graph theory provides tools to describe patterns in connectivity data and can help to characterize properties of networks that are thought to underlie cognitive and affective processing. This might be relevant for the specification of neurobehavioral theories: A prominent psychological theory on attentional control, for instance, discusses the relevance of trait anxiety for processing efficiency, which refers to the relationship between the efforts or resources invested during task performance and the quality of task performance (Eysenck, Derakshan, Santos, & Calvo, 2007). Predictions of this theoretic account have been successfully tested using task fMRI (Basten et al., 2011), yet it remains unclear why trait anxiety would affect processing efficiency. One possible account would be suboptimal information exchange capability between relevant brain areas, which might be reflected in network efficiency as indexed by path lengths in relevant functional networks. Future studies may want to study the relationships between properties of resting-state networks such as network efficiency and task-evoked activation, and to characterize changes in network behavior in different psychological contexts, such as the resting state or during task (Cohen, Gallen, Jacobs, Lee, & D’Esposito, 2014).
A limitation of this study is the restriction of the sample to female participants. Only females were invited to control for possible gender effects. Controlling for gender in the context of research on anxiety is important because females usually report higher levels of trait anxiety (e.g., Montag et al., 2010). Future studies, however, should assess whether similar relationships can also be obtained in a male sample or whether resting-state correlates of personality are dimorphic with respect to gender.
Despite of its great success in the cognitive and affective neurosciences, the effective translation of task fMRI into clinical applications is still pending (Matthews, Honey, & Bullmore, 2006). Hope has been raised that resting-state fMRI will eventually produce valid and reliable biomarkers for psychiatric disorders (Fox & Greicius, 2010), and, indeed, the application of multivariate pattern classification to resting-state scans can predict disease state in major depression (Craddock, Holtzheimer, Hu, & Mayberg, 2009). Research into personality correlates of resting-state fMRI can complement such data-driven approaches to disease state by providing endophenotypes that are grounded in neuropsychological theory. In the context of this study, it would be interesting to examine the organization of functional connectivity within the insular-opercular network in patients suffering from anxiety disorders, and to assess whether abnormalities are contingent on symptom severity and sensitive to symptom improvement after therapeutic interventions.
References
Aghajani, M., Veer, I. M., van Tol, M.-J, Aleman, A., van Buchem, M. A., Veltman, D. J., . . .van der Wee, N. J (2014). Neuroticism and extraversion are associated with amygdala resting-state functional connectivity. Cognitive, Affective, and Behavioral Neuroscience, 14(2), 836–848. doi:10.3758/s13415-013-0224-0
Allport, G. (1937). Personality: A psychological interpretation. New York, NY: Holt, Rinehart & Winston.
Basten, U., Stelzel, C., & Fiebach, C. J. (2011). Trait anxiety modulates the neural efficiency of inhibitory control. Journal of Cognitive Neuroscience, 23(10), 3132–3145. doi:10.1162/jocn_a_00003
Bateson, M., Brilot, B., & Nettle, D. (2011). Anxiety: An evolutionary approach. Canadian Journal of Psychiatry, 56(12), 707–715.
Baur, V., Hänggi, J., Langer, N., & Jäncke, L. (2013). Resting-state functional and structural connectivity within an insula–amygdala route specifically index state and trait anxiety. Biological Psychiatry, 1–8. doi:10.1016/j.biopsych.2012.06.003
Berger, A., Edelsberg, J., Treglia, M., Alvir, J. M. J., & Oster, G. (2012). Change in healthcare utilization and costs following initiation of benzodiazepine therapy for long-term treatment of generalized anxiety disorder: A retrospective cohort study. BMC Psychiatry, 12, 177. doi:10.1186/1471-244X-12-177
Bijsterbosch, J., Smith, S., Forster, S., John, O. P., & Bishop, S. J. (2014). Resting state correlates of subdimensions of anxious affect. Journal of Cognitive Neuroscience, 26(4), 914–926.
Bishop, S., Duncan, J., Brett, M., & Lawrence, A. D. (2004). Prefrontal cortical function and anxiety: Controlling attention to threat-related stimuli. Nature Neuroscience, 7(2), 184–188. doi:10.1038/nn1173
Bjørnebekk, A., Fjell, A. M., Walhovd, K. B., Grydeland, H., Torgersen, S., & Westlye, L. T. (2013). Neuronal correlates of the five factor model (FFM) of human personality: Multimodal imaging in a large healthy sample. NeuroImage, 65, 194–208.
Canli, T., Zhao, Z., Desmond, J. E., Kang, E., Gross, J., & Gabrieli, J. D. (2001). An fMRI study of personality influences on brain reactivity to emotional stimuli. Behavioral Neuroscience, 115(1), 33–42.
Clark, L. A., Watson, D., & Mineka, S. (1994). Temperament, personality, and the mood and anxiety disorders. Journal of Abnormal Psychology, 103(1), 103–116.
Cloninger, C. R. (1986). A unified biosocial theory of personality and its role in the development of anxiety states. Psychiatric Developments, 4(3), 167–226.
Cloninger, C. R., Svrakic, D. M., & Przybeck, T. R. (1993). A psychobiological model of temperament and character. Archives of General Psychiatry, 50(12), 975–990.
Cohen, J. R., Gallen, C. L., Jacobs, E. G., Lee, T. G., D’Esposito, M., & 9. (2014). Quantifying the reconfiguration of intrinsic networks during working memory. PLoS One, 9(9), e106636. doi:10.1371/journal.pone.0106636
Costa, P. T., Jr., & McCrae, R. R. (1992). Revised NEO Personality Inventory (NEO-PI-R) and NEO Five Factor inventory (NEO-FFI) (Professional manual). Odessa, FL: Psychological Assessment Resources.
Craddock, R. C., Holtzheimer, P. E., Hu, X. P., & Mayberg, H. S. (2009). Disease state prediction from resting state functional connectivity. Magnetic Resonance in Medicine, 62(6), 1619–1628. doi:10.1002/mrm.22159
Dosenbach, N. U. F., Fair, D. A., Cohen, A. L., Schlaggar, B. L., & Petersen, S. E. (2008). A dual-networks architecture of top-down control. Trends in Cognitive Sciences, 12(3), 99–105. doi:10.1016/j.tics.2008.01.001
Dosenbach, N. U. F., Fair, D. A., Miezin, F. M., Cohen, A. L., Wenger, K. K., Dosenbach, R. A. T., . . . Petersen, S. E. (2007). Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences of the United States of America, 104(26), 11073–11078. doi:10.1073/pnas.0704320104
Dosenbach, N. U. F., Nardos, B., Cohen, A. L., Fair, D. A., Power, J. D., Church, J. A., . . . Schlaggar, B. L. (2010). Prediction of individual brain maturity using fMRI. Science, 329(5997), 1358–1361. doi:10.1126/science.1194144
Dosenbach, N. U., Visscher, K. M., Palmer, E. D., Miezin, F. M., Wenger, K. K., Kang, H. C., . . . Petersen, S. E. (2006). A core system for the implementation of task sets. Neuron, 50(5), 799–812.
Etkin, A., & Wager, T. D. (2007). Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. The American Journal of Psychiatry, 164(10), 1476.
Eysenck, H. J. (1947). Dimensions of personality. Piscataway, NJ: Transaction.
Eysenck, M. W., Derakshan, N., Santos, R., & Calvo, M. G. (2007). Anxiety and cognitive performance: Attentional control theory. Emotion, 7(2), 336.
Fornito, A., Zalesky, A., & Breakspear, M. (2013). Graph analysis of the human connectome: Promise, progress, and pitfalls. NeuroImage, 80(C), 426–444. doi:10.1016/j.neuroimage.2013.04.087
Fox, M. D., & Greicius, M. (2010). Clinical applications of resting state functional connectivity. Frontiers in System Neuroscience, 4, 19. doi:10.3389/fnsys.2010.00019
Fox, M. D., & Raichle, M. E. (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience, 8(9), 700–711. doi:10.1038/nrn2201
Friston, K. J., Frith, C. D., Liddle, P. F., & Frackowiak, R. S. (1993). Functional connectivity: The principal-component analysis of large (PET) data sets. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 13(1), 5–14. doi:10.1038/jcbfm.1993.4
Fukunaga, M., Horovitz, S. G., van Gelderen, P., de Zwart, J. A., Jansma, J. M., Ikonomidou, V. N., . . . Duyn, J. H. (2006). Large-amplitude, spatially correlated fluctuations in BOLD fMRI signals during extended rest and early sleep stages. Magnetic Resonance Imaging, 24(8), 979–992. doi:10.1016/j.mri.2006.04.018
Gordon, E. M., Stollstorff, M., & Vaidya, C. J. (2011). Using spatial multiple regression to identify intrinsic connectivity networks involved in working memory performance. Human Brain Mapping, 33(7), 1536–1552. doi:10.1002/hbm.21306
Gray, J. A. (1971). The psychology of fear and stress. London, UK: Weidenfeld and Nicolson.
Gray, J. A., & McNaughton, N. (2000). The neuropsychology of anxiety. Oxford, UK: Oxford University Press.
Greicius, M. D., Kiviniemi, V., Tervonen, O., Vainionpää, V., Alahuhta, S., Reiss, A. L., & Menon, V. (2008). Persistent default-mode network connectivity during light sedation. Human Brain Mapping, 29(7), 839–847. doi:10.1002/hbm.20537
Greicius, M. D., Supekar, K., Menon, V., & Dougherty, R. F. (2009). Resting-state functional connectivity reflects structural connectivity in the default mode network. Cerebral Cortex, 19(1), 72–78. doi:10.1093/cercor/bhn059
Hajcak, G., McDonald, N., & Simons, R. F. (2003). Anxiety and error-related brain activity. Biological Psychology, 64(1-2), 77–90. doi:10.1016/S0301-0511(03)00103-0
Honey, C. J., Sporns, O., Cammoun, L., Gigandet, X., Thiran, J. P., Meuli, R., & Hagmann, P. (2009). Predicting human resting-state functional connectivity from structural connectivity. Proceedings of the National Academy of Sciences of the United States of America, 106(6), 2035–2040. doi:10.1073/pnas.0811168106
Ilyas, S., & Moncrieff, J. (2012). Trends in prescriptions and costs of drugs for mental disorders in England, 1998–2010. The British Journal of Psychiatry: the Journal of Mental Science, 200(5), 393–398. doi:10.1192/bjp.bp.111.104257
Jiang, N., Sato, T., Hara, T., Takedomi, Y., Ozaki, I., & Yamada, S. (2003). Correlations between trait anxiety, personality and fatigue: Study based on the Temperament and Character Inventory. Journal of Psychosomatic Research, 55(6), 493–500.
Leonardo, E. D., & Hen, R. (2006). Genetics of affective and anxiety disorders. Annual Review of Psychology, 57, 117–137. doi:10.1146/annurev.psych.57.102904.190118
Markett, S., Montag, C., & Reuter, M. (2011). The nicotinic acetylcholine receptor gene CHRNA4 is associated with negative emotionality. Emotion, 11(2), 450.
Markett, S., Montag, C., & Reuter, M. (2016). Anxiety and harm avoidance. In J. R. Absher & J. Cloutier (Eds.), Neuroimaging personality, social cognition, and character. New York, NY: Academic Press.
Markett, S., Weber, B., Voigt, G., Montag, C., Felten, A., Elger, C., & Reuter, M. (2013). Intrinsic connectivity networks and personality: The temperament dimension harm avoidance moderates functional connectivity in the resting brain. Neuroscience, 240, 98–105. doi:10.1016/j.neuroscience.2013.02.056
Matthews, P. M., Honey, G. D., & Bullmore, E. T. (2006). Applications of fMRI in translational medicine and clinical practice. Nature Reviews Neuroscience, 7(9), 732–744. doi:10.1038/nrn1929
Mišić, B., Betzel, R. F., de Reus, M. A., van den Heuvel, M. P., Berman, M. G., McIntosh, A. R., & Sporns, O. (2016). Network-level structure-function relationships in human neocortex. Cerebral Cortex, 1, 12.
Mobbs, D., Petrovic, P., Marchant, J. L., Hassabis, D., Weiskopf, N., Seymour, B., . . . Frith, C. D. (2007). When fear is near: Threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science , 317(5841), 1079–1083. doi:10.1126/science.1144298
Montag, C., Basten, U., Stelzel, C., Fiebach, C. J., & Reuter, M. (2010). The BDNF Val66Met polymorphism and anxiety: Support for animal knock-in studies from a genetic association study in humans. Psychiatry Research, 179(1), 86–90.
Montag, C., Reuter, M., Jurkiewicz, M., Markett, S., & Panksepp, J. (2013). Imaging the structure of the human anxious brain: A review of findings from neuroscientific personality psychology. Reviews in the Neurosciences, 24(2). doi:10.1515/revneuro-2012-0085
Most, S. B., Chun, M. M., Johnson, M. R., & Kiehl, K. A. (2006). Attentional modulation of the amygdala varies with personality. NeuroImage, 31(2), 934–944. doi:10.1016/j.neuroimage.2005.12.031
Nery, F. G., Hatch, J. P., Glahn, D. C., Nicoletti, M. A., Serap Monkul, E., Najt, P., . . . Soares, J. C. (2008). Temperament and character traits in patients with bipolar disorder and associations with comorbid alcoholism or anxiety disorders. Journal of Psychiatric Research, 42(7), 569–577. doi:10.1016/j.jpsychires.2007.06.004
Nyman, E., Miettunen, J., Freimer, N., Joukamaa, M., Mäki, P., Ekelund, J., … Paunio, T. (2011). Impact of temperament on depression and anxiety symptoms and depressive disorder in a population-based birth cohort. Journal of Affective Disorders, 131(1–3), 393–397. doi:10.1016/j.jad.2010.12.008
Öngür, D., Farabaugh, A., Iosifescu, D. V., Perlis, R., & Fava, M. (2005). Tridimensional personality questionnaire factors in major depressive disorder: Relationship to anxiety disorder comorbidity and age of onset. Psychotherapy and Psychosomatics, 74(3), 173–178. doi:10.1159/000084002
Panksepp, J. (1998). Affective neuroscience. London, UK: Oxford University Press.
Pannekoek, J. N., Veer, I. M., van Tol, M.-J., van der Werff, S. J. A., Demenescu, L. R., Aleman, A., … van der Wee, N. J. A. (2013). Resting-state functional connectivity abnormalities in limbic and salience networks in social anxiety disorder without comorbidity. European Neuropsychopharmacology , 23(3), 186–195. doi:10.1016/j.euroneuro.2012.04.018
Paulus, M. P., Rogalsky, C., Simmons, A., Feinstein, J. S., & Stein, M. B. (2003). Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. NeuroImage, 19(4), 1439–1448. doi:10.1016/S1053-8119(03)00251-9
Plomin, R., Haworth, C. M. A., & Davis, O. S. P. (2009). Common disorders are quantitative traits. Nature Reviews Genetics, 10(12), 872–878. doi:10.1038/nrg2670
Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L., & Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59(3), 2142–2154.
Pujol, J., López, A., Deus, J., Cardoner, N., & Vallejo, J. (2002). Anatomical variability of the anterior cingulate gyrus and basic dimensions of human personality. NeuroImage. Retrieved from http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=11906225&retmode=ref&cmd=prlinks
Raichle, M. E. (2010). Two views of brain function. Trends in Cognitive Sciences, 14(4), 180–190. doi:10.1016/j.tics.2010.01.008
Raichle, M. E. (2011). The restless brain. Brain Connectivity, 1(1), 3–12. doi:10.1089/brain.2011.0019
Roy, A. K., Fudge, J. L., Kelly, C., Perry, J. S. A., Daniele, T., Carlisi, C., . . . Ernst, M. (2013). Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 52(3), 290–299.e2. doi:10.1016/j.jaac.2012.12.010
Rubinov, M., & Sporns, O. (2010). Complex network measures of brain connectivity: Uses and interpretations. NeuroImage, 52(3), 1059–1069. doi:10.1016/j.neuroimage.2009.10.003
Sadaghiani, S., & D’Esposito, M. (2014). Functional characterization of the cingulo-opercular network in the maintenance of tonic alertness. Cerebral Cortex, 25(9), 2763–2773. doi:10.1093/cercor/bhu072
Satterthwaite, T. D., Elliott, M. A., Gerraty, R. T., Ruparel, K., Loughead, J., Calkins, M. E., … Wolf, D. H. (2013). An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. NeuroImage, 64, 240–256. doi:10.1016/j.neuroimage.2012.08.052
Seeley, W. W., Menon, V., Schatzberg, A. F., Keller, J., Glover, G. H., Kenna, H., . . . Greicius, M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–2356. doi:10.1523/JNEUROSCI.5587-06.2007
Servaas, M. N., Geerligs, L., Renken, R. J., Marsman, J.-B. C., Ormel, J., Riese, H., & Aleman, A. (2015). Connectomics and neuroticism: an altered functional network organization. Neuropsychopharmacology, 40(2), 296–304. doi:10.1038/npp.2014.169
Shehzad, Z., Kelly, A. M. C., Reiss, P. T., Gee, D. G., Gotimer, K., Uddin, L. Q., . . . Milham, M. P. (2009). The resting brain: Unconstrained yet reliable. Cerebral Cortex, 19(10), 2209–2229. doi:10.1093/cercor/bhn256
Simon, G. E. (2003). Social and economic burden of mood disorders. Biological Psychiatry, 54(3), 208–215.
Smit, F., Cuijpers, P., Oostenbrink, J., Batelaan, N., de Graaf, R., & Beekman, A. (2006). Costs of nine common mental disorders: Implications for curative and preventive psychiatry. Journal of Mental Health Policy and Economics, 9(4), 193–200.doi.10.1038/npp.2014.169
Smith, S. (2013). Introduction to the NeuroImage special issue “Mapping the Connectome”. NeuroImage, 80(C), 1. doi:10.1016/j.neuroimage.2013.07.012
Spielberger, C. D. (1989). State-trait anxiety inventory: Bibliography (2nd ed.). Palo Alto, CA: Consulting Psychologists Press.
Sylvester, C. M., Corbetta, M., Raichle, M. E., Rodebaugh, T. L., Schlaggar, B. L., Sheline, Y. I., . . . Lenze, E. J. (2012). Functional network dysfunction in anxiety and anxiety disorders. Trends in Neurosciences, 35(9), 527–535. doi:10.1016/j.tins.2012.04.012
Tillfors, M., Furmark, T., Marteinsdottir, I., & Fredrikson, M. (2002). Cerebral blood flow during anticipation of public speaking in social phobia: A PET study. Biological Psychiatry, 52(11), 1113–1119.
Trull, T. J., Tragesser, S. L., Solhan, M., & Schwartz-Mette, R. (2007). Dimensional models of personality disorder: Diagnostic and Statistical Manual of Mental Disorders Fifth Edition and beyond. Current Opinion in Psychiatry, 20, 52–56.
Tukey, W. (1977). Exploratory data analysis. New York, NY: Addison-Wesley.
Tuominen, L., Salo, J., Hirvonen, J., Någren, K., Laine, P., Melartin, T., … Hietala, J. (2012). Temperament trait Harm Avoidance associates with μ-opioid receptor availability in frontal cortex: a PET study using [(11)C]carfentanil. NeuroImage, 61(3), 670–676. doi.10.1016/j.neuroimage.2012.03.063
Vaidya, C. J., & Gordon, E. M. (2013). Phenotypic variability in resting-state functional connectivity: Current status. Brain Connectivity, 3(2), 99–120. doi:10.1089/brain.2012.0110
van den Heuvel, M. P., & Hulshoff Pol, H. E. (2010). Exploring the brain network: A review on resting-state fMRI functional connectivity. European Neuropsychopharmacology, 20(8), 519–534. doi:10.1016/j.euroneuro.2010.03.008
van den Heuvel, M. P., Mandl, R. C. W., Kahn, R. S., & Hulshoff Pol, H. E. (2009). Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Human Brain Mapping, 30(10), 3127–3141. doi:10.1002/hbm.20737
van Wijk, B. C., Stam, C. J., & Daffertshofer, A. (2010). Comparing brain networks of different size and connectivity density using graph theory. PLoS One, 5(10), e13701.
Vincent, J. L., Patel, G. H., Fox, M. D., Snyder, A. Z., Baker, J. T., Van Essen, D. C., . . . Raichle, M. E. (2007). Intrinsic functional architecture in the anaesthetized monkey brain. Nature, 447(7140), 83–86. doi:10.1038/nature05758
Wittchen, H. U., Jacobi, F., Rehm, J., Gustavsson, A., Svensson, M., Jönsson, B., . . . Steinhausen, H.-C. (2011). The size and burden of mental disorders and other disorders of the brain in Europe 2010. European Neuropsychopharmacology, 21(9), 655–679. doi:10.1016/j.euroneuro.2011.07.018
Yan, C. G., & Zhang, Y. F. (2010). DPARSF: A MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Frontiers in System Neuroscience. doi:10.3389/fnsys.2010.00013
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Markett, S., Montag, C., Melchers, M. et al. Anxious personality and functional efficiency of the insular-opercular network: A graph-analytic approach to resting-state fMRI. Cogn Affect Behav Neurosci 16, 1039–1049 (2016). https://doi.org/10.3758/s13415-016-0451-2
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-016-0451-2