Abstract
The aim of this study was to determine factors affecting the vertical distribution of Eurytemora lacustris in mesotrophic lakes (Wigry, Hańcza, Szurpiły; north-eastern Poland) during the summer stagnation. Eurytemora lacustris was found in all of the studied lakes, with the highest abundance (8 ind. L−1) in Lake Wigry. In Lake Szurpiły, E. lacustris has never been recorded before. The results of this study revealed that E. lacustris was most numerous in thermocline zones, suggesting that this species could temporarily tolerate warmer water and lower oxygen concentrations due to better food resources. During the study, it was found that a large part of the E. lacustris population had epibiont ciliates, in contrast to other species of zooplankton that did not have any epibionts. The improvement in the water quality of many deep lakes could lead to an increase in the abundance of E. lacustris. However, epibiont ciliates may be a threat for this species and may play a substantial role in determining the production, distribution, and community dynamics of E. lacustris.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eurytemora lacustris (Poppe, 1887) is considered a ‘classical’ glacial relict species in central Europe (Maier et al. 2011), while the inclusion of this species in the relict element in Norway and Sweden has been discussed (Kinsten 2012; Spikkeland et al. 2016). From the zoogeographical point of view, E. lacustris is a typical so-called Baltic species, with a distribution related to the Ancylus stage of the post-glacial Baltic Sea development (Spikkeland et al. 2016). According to Thienemann (1950), E. lacustris is a cold-adapted calanoid copepod which spread over parts of Europe by glacier lakes during the last ice age, about 8500 years ago. The glaciers “inoculated” the species into the lake basins that had been created in the meantime. During the post-glacial period, when the temperatures gradually increased, E. lacustris was able to survive by retreating to the cold water layers of thermally stratified lakes. Eurytemora lacustris occurs exclusively in freshwater ecosystems located in the area from the Boreal highlands of northern Norway to the Black Sea and from the central European lowlands to the eastern parts of the Caspian region (Illies 1978; Spikkeland et al. 2016). However, this species has become rare and endangered due to eutrophication and global change (Maier et al. 2011). As a result of anthropogenic increases in external nutrient loading in lakes of central Europe over the past few decades (Gulati and Van Donk 2002), E. lacustris disappeared from many lakes, as it is a species which is very sensitive to environmental deterioration (Arbačiauskas and Kalytytė 2010). European countries have made great efforts to improve the ecological quality of lakes by reducing external nutrient loading (Sas 1989) or additional restoration measures such as lake biomanipulation, or both (e.g. Benndorf 1990, 2005; Kornijów et al. 1990; Hansson et al. 1998; Mehner et al. 2002). As a consequence, re-oligotrophication is a well-documented phenomenon in many lakes (Jeppesen et al. 2005), and E. lacustris is more common nowadays in deep lakes in central Europe. The high habitat requirements make E. lacustris an excellent indicator of the effectiveness of deep lake restoration. This species is also a very good indicator of the low trophic level and good ecological status of lakes (Karabin 1985; Ejsmont-Karabin and Karabin 2013; Ochocka and Pasztaleniec 2016).
Eurytemora lacustris requires cold and well-oxygenated waters; thus, it was found in freshwater lakes, which were mostly oligotrophic, deep (max depth > 30 m), and oxygenated (>1 mg O2 L−1), with cold hypolimnion (usually <10 °C, with a threshold at 17 °C) (Rylov 1935; Patalas and Patalas 1966; Kasprzak et al. 2005). Based on the literature data, the combined effects of temperature and oxygen concentration in deep waters seem to be fundamental factors influencing the occurrence of E. lacustris. Some studies indicated that E. lacustris exhibits a distinct diurnal vertical migration (Ruttner 1905; Adlerówna 1929), but the migration amplitude clearly decreased during the summer and was closely related to the light intensity (Kasprzak et al. 2005). Other studies reported small differences between day and night in the vertical distribution of this species, e.g. the copepodites and adults were slightly higher at night than during the day (Weiler et al. 2003). The vertical distributions of E. lacustris vary seasonally. In the summer, this species is mainly restricted to the cold, lower water layers, while in the winter and during periods of mixis, it prefers the upper water layers or is uniformly distributed throughout the water column (Weiler et al. 2003). However, the factors which affect the vertical distribution of this species during the summer stagnation are unclear.
The aim of this study was to determine abiotic (temperature, oxygen) and biotic (phytoplankton) factors affecting the vertical distribution of E. lacustris in three mesotrophic lakes (Wigry, Hańcza, Szurpiły) in north-eastern Poland. During the study, it was found that a large part of the E. lacustris population had epibiont ciliates in contrast to other species of zooplankton that did not have any epibionts. Therefore, an additional goal of this study was to assess the infection parameters of E. lacustris populations, such as the prevalence and average intensity of the epibiont suctorian ciliates.
Material and methods
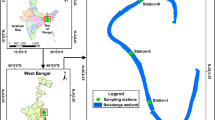
The study was conducted in three mesotrophic lakes in NE Poland: Lake Hańcza (the deepest lake in central and Eastern Europe, with a maximum depth of 108.5 m), Lake Szurpiły (with a maximum depth of 40.0 m) and Lake Wigry (with a maximum depth of 74.0 m). In Lake Wigry, samples were collected from two basins (the North Basin with a maximum depth exceeding 65.0 m and the Central Basin with a maximum depth of 73.0 m). Morphometric and trophic parameters of the studied lakes are shown in Table 1. The trophic state index (TSI) of lakes was calculated from Secchi disc visibility (ZSD) and chlorophyll a concentrations according to Carlson (1977). The study was conducted during the peak of the summer stagnation in July 2015 (Lakes Hańcza and Szurpiły) and July 2016 (Lake Wigry) between 11:00 and 13:00. The zooplankton samples (20 L) were taken every meter from the surface to the upper hypolimnion (0–11 m) by the 5 L Limnos sampler. Additionally, one sample was taken from the centre of the hypolimnion. Temperature, oxygen concentration, and phytoplankton communities were measured every metre from the surface to a depth of 30 m. The temperature and dissolved oxygen concentrations were determined using an HQ40D Multi Meter (Hach-Lange GmbH). The phytoplankton (total chlorophyll a and algal classes) was measured in situ by the submersible spectrofluorometer (FluoroProbe, bbe-Moldaenke), using differences between fluorescence excitation spectra. Changes in chlorophyll a emission allow for the fluorometric estimation of algal classes based on differences in species and class-dependent peripheral antenna pigments (Beutler et al. 2002). The FluoroProbe identifies the four phytoplankton classes: green algae (Chlorophyta and Euglenophyta), cyanobacteria (phycocyanin-rich cyanobacteria), diatoms (Heterokontophyta, Haptophyta, and Dinophyta) and cryptophytes (Cryptophyta and the phycoerythrin-rich cyanobacteria).
The calanoid copepod E. lacustris can be easily distinguished by the long furcal rami (Fig. 1a) and the shape of the fifth leg of males (Fig. 1b) and females (Fig. 1c). Other characteristic features are the 24-segmented antennule (A1) and the lack of “wings” on the 5th thoracic segment (Einsle 1993). Biomass was calculated based on the mean length using the following length-weight relationship (Błędzki and Rybak 2016):
- W:
-
individual body dry weight [μg]
- L:
-
body size [μm]
Species identification of epibiont ciliates was based on Foissner et al. (1995). The biomass of epibionts was calculated from measurements of cell dimensions and simple geometric shapes and then multiplied by a factor of 1.4 (Müller and Geller 1993). Quantifying epibiont numbers in E. lacustris populations includes the measurement of host sample size (N), prevalence (%), and mean epibiont intensity (Rózsa et al. 2000). Only adult specimens were used for analysis.
The analysis of variance was used to determine which explanatory variables (temperature, oxygen, and phytoplankton) provide significant information on the vertical distribution of E. lacustris. Then, we used type I sum of squares analysis to find out if the three variables and their interaction provide the same amount of information to the model. Finally, the relationships between the abundance of E. lacustris and environmental variables were visualized by principal component analysis (PCA). Statistical analyses were performed with XLSTAT-Ecology (Addinsoft).
Results
Water temperature was very similar in the studied lakes (about 20, 12 and 6–7 °C in the epi-, meta- and hypolimnion, respectively) (Fig. 2a). Thermocline developed at a depth of 8–10 m in lakes Hańcza and Wigry and at a depth of 6–9 m in Lake Szurpiły. During the study period, oxygen concentrations were almost the same in the epilimnion of all of the studied lakes (about 10 mg L−1). There were differences in the oxygen concentration in the thermocline between Lake Wigry (metalimnetic oxygen minimum) and Lake Hańcza (metalimnetic oxygen maximum). In lakes Hańcza and Wigry, hypolimnetic waters were quite well oxygenated, whereas the oxygen concentration was relatively low in Lake Szurpiły (Fig. 2b). In lakes Hańcza and Szurpiły, the highest concentration of algae was observed in the metalimnion, while the highest concentration in Lake Wigry was in the epilimnion (Fig. 3). The dominant groups of phytoplankton in the thermocline were cryptophytes in Lake Wigry (Fig. 3a, b), and diatoms with cryptophytes in lakes Hańcza and Szurpiły (Fig. 3c, d).
The lakes were characterised by a large number of zooplankton species, among which Daphnia cucullata (Sars, 1862) dominated. The genus Daphnia was also represented by Daphnia cristata G.O. Sars, 1862, Daphnia longispina O.F. Müller, 1776 and Daphnia longiremis G.O. Sars, 1861. Other microcrustaceans were represented by Bosmina (Eubosmina) crassicornis Lilljeborg, 1887, Bosmina longirostris (O.F. Müller, 1785), Bosmina berolinensis Imhof, 1888, Chydorus sphaericus (O.F. Müller, 1785), Diaphanosoma brachyurum (Lievin, 1848), Leptodora kindtii (Focke, 1844), Eudiaptomus gracilis (G.O. Sars, 1863), Eudiaptomus graciloides (Lilljeborg, 1888), Heterocope appendiculata Sars G.O., 1863, Mesocyclops leuckarti (Claus, 1857), Thermocyclops oithonoides (G.O. Sars, 1863), Cyclops scutifer Sars, 1863, and Cyclops vicinus (Sars, 1863). For more details, see Karpowicz and Ejsmont-Karabin (2017). Eurytemora lacustris was found in the cold water of the hypolimnion and metalimnion of all of the studied lakes (Fig. 2). It should be emphasised that before now, Lake Szurpiły was not a habitat for this species. The highest density (up to 8 ind. L−1) and biomass (up to 0.96 mg L−1) of E. lacustris was noted in Lake Wigry, in which it was a dominant species in the hypolimnion and metalimnion. The abundance and biomass of E. lacustris in lakes Hańcza and Szurpiły did not exceed 2 ind. L−1 and 0.15 mg L−1, respectively (Fig. 2c).
The vertical distribution of the E. lacustris population exhibited a clear heterogeneity with the maximal density in the thermocline of all of the studied lakes (Fig. 2c). In lakes Wigry and Hańcza, the abundance of E. lacustris was about 2–3 times higher in the metalimnion than in the hypolimnion. In Lake Szurpiły, E. lacustris was recorded exclusively in the thermocline. Statistical analysis showed that temperature of the water, oxygen concentration, and phytoplankton were important factors affecting the vertical distribution of E. lacustris (F = 6.83; p = 0.001). The contribution of each effect was evaluated by the Type I SS, and revealed that the most important factors were water temperature (F = 10.78; p = 0.002) and oxygen concentration (F = 8.56; p = 0.005). The results of the PCA analysis clearly divided environmental conditions into vertical profiles. The most epilimnetic stations were positively correlated with the first axis, while metalimnetic and hypolimnetic stations were negatively correlated with the first axis (Fig. 4). The horizontal axis (F1) was linked with temperature, oxygen concentration, and abundance of E. lacustris. The vertical axis (F2) was strongly linked with chlorophyll a concentrations (Table 2). The results of the PCA analysis indicated that E. lacustris preferred a low water temperature and could tolerate low oxygen concentration because of the higher phytoplankton concentrations (Fig. 4). The preferred temperature range of E. lacustris was between 6 and 16 °C. However, maximal densities of this species were observed at 9–11 °C (Fig. 5).
A large part of the E. lacustris populations had epibiont ciliates, while all other species of zooplankton did not have epibionts. The prevalence of epibionts ranged from 33.3% in Lake Szurpiły to 50.0% in Lake Wigry (Table 3). The density of these epibionts varied in a very wide range from 5 to 112 individuals per specimen of E. lacustris (most often it was approximately 40–50 ind.). Up to 95% of all epibionts were attached to the abdomen and furca. These epibionts were difficult to identify but resembled the suctorian Acineta tuberosa (Pallas, 1766) Ehrenberg, 1833 presented in Foissner et al. (1995) by other authors. It should be noted that only one ciliate species was present on a single specimen of E. lacustris, but in different stages of the life cycle (Fig. 6).
Epibionts on the furcal branches of Eurytemora lacustris (a), a single specimen of Acineta tuberosa (b) - Body (25–70 μm in size without stalk and tentacles) with two distinct protrusions, each bearing tentacles up to 55 μm in length. The stalk is two times shorter than the body (usually 12–30 μm long and 2.5–4 μm wide). Lorica, used for the characterisation of species and genera, is not visible, probably due to formalin fixation
Discussion
We found the presence of E. lacustris in three mesotrophic, deep (>40 m) and well oxygenated (>4.5 mg O2 L−1) lakes. This may indicate the good ecological status of the lakes. In Lake Szurpiły, E. lacustris has never been recorded before. In the second half of the twentieth century, E. lacustris disappeared from Lake Wigry due to increasing eutrophication and anthropogenic pressure (Karabin and Ejsmont-Karabin 1999). After a significant reduction in phosphorus load from the catchment and biomanipulation at the end of the twentieth century, water quality radically improved in Lake Wigry (Kamiński 1999). As a result, an increase of the E. lacustris population in Lake Wigry was registered in 2007–2012 (Karpowicz and Górniak 2013). Similar results were found in other lakes, where re-oligotrophication is a well-documented phenomenon (Jeppesen et al. 2005), and E. lacustris has become more common in deep lakes of central Europe (Kasprzak et al. 2005; Maier et al. 2011). This makes E. lacustris an excellent indicator of the effective restoration of deep lakes in Europe. Our study showed that the abundance of E. lacustris in the deep water of Lake Wigry was around 8 ind. L−1, a value which is close to the winter maximum of this species (Błędzki and Rybak 2016). Literature data show that in the summer, this species is generally restricted to the cold hypolimnion, and the majority of its biomass is restricted to layers below the 10 °C isotherm (Weiler et al. 2003; Kasprzak et al. 2005). This study revealed the highest abundance of E. lacustris in thermocline zones. The preferred temperature range was between 6 and 16 °C, with an optimum from 9 to 11 °C. We have also found single individuals of E. lacustris in warm surface water.
The literature data indicated the large amplitude of diurnal vertical migrations of E. lacustris, but this amplitude clearly decreased from May to September. During the summer stagnation, the mean day depth and mean night depth were very similar (Kasprzak et al. 2005). However, the environmental factors which caused the relatively stable vertical distribution of E. lacustris in the summer are unclear. Besides the temperature and oxygen conditions, the light intensity is a factor responsible for their distribution (Kasprzak et al. 2005). Our results suggest that food resources may be an important factor in addition to water temperature and oxygen. We found that E. lacustris could temporarily tolerate warmer water and lower oxygen concentrations probably due to the higher availability of food resources. The food resources in the thermocline zone of lakes Hańcza and Wigry were much higher and better quality than in the hypolimnion. The hypolimnetic zones were strongly dominated by cyanobacteria, which are an inappropriate and poor quality food for zooplankton (e.g. Kosiba et al. 2017). Freshwater calanoids possess numerous chemoreceptors on their antennae and mouthparts that are sensitive to the toxins produced by cyanobacteria and feed selectively by choosing particles of higher quality (DeMott 1986). Kasprzak et al. (2005) suggested that insufficient food supply in combination with low food quality may restrict the occurrence of E. lacustris in some cases. The food resources in the thermocline zone of lakes Hańcza and Wigry were much higher and better quality than in the hypolimnion. Studies by Vezhnovets et al. (2012) on the stomach content of calanoid copepods, examined by scanning electron microscopy, showed that the food pellet of E. lacustris mostly contained small (<40 μm in length) pennate diatoms, that were damaged and difficult to identify. The authors underlined that the dominant algal group/species in lake is the main and easily available food resource for E. lacustris. In turn, the lack of E. lacustris in the hypolimnion of Lake Szurpiły could be the result of the worse aerobic conditions.
Our study revealed that a large part of the E. lacustris population had epibiont ciliates, in contrast to other species of zooplankton. Several studies have reported the presence of epibionts on various species of calanoid copepods (Turner et al. 1979; Nagasawa 1986; Valbonesi and Guglielmo 1988; Chiavelli et al. 1993; Green and Shiel 2000; Utz and Coats 2005, 2008; Visse 2007). Peritrich ciliates, such as Vorticella spp. and Zoothamnium spp. have been found on copepods in the Gulf of Gdańsk (Wiktor and Krajewska-Sołtys 1994). Utz and Coats (2005) found that the common epibiont of estuarine Eurytemora affinis Poppe, 1880 (Chesapeake Bay, USA) was the sessile peritrich ciliate Zoothamnium intermedium Precht, 1935. We have found that relic E. lacustris in lakes of north-eastern Poland had suctorian epibiont ciliates, namely Acineta tuberosa Ehrenberg, 1833. This phenomenon may have not only a local character. It seems that the same suctorian species were illustrated, but not identified and described, on E. lacustris from Ratzeburger Lake complex in Germany (Maier et al. 2011 - Fig. 1b). The epibiont prevalence has generally been observed at the time when the host species are very abundant and dominate plankton communities (Hirche 1974; Chiavelli et al. 1993). The results of this study indicated a strong preference of epibiont ciliates for E. lacustris despite their low share in zooplankton communities.
Epibiosis is essentially a commensal relationship, although new data suggest that epibionts can have negative effects on some life-cycle traits of the Calanoida host (Visse 2007; Souissi et al. 2013). A laboratory experiment with epibionts attached to the calanoid copepod Acartia bifilosa (Giesbrecht, 1881) (Gulf of Riga, Estonia) suggested that animals infested with epibionts were less viable than the non-infested animals. Another laboratory experiment, using 2D infrared video techniques to observe the behavior of heavily infested E. affinis, revealed that epibionts could negatively affect the behavior of the host, in terms of swimming activity, e.g. break, cruise, sink, and jump (Souissi et al. 2013). The high proportion of infestation also dramatically affected the mating success of E. lacustris in laboratory conditions (Souissi et al. 2013). The improvement in water quality has led to an increase in the population of E. lacustris in deep lakes; however, epibiont ciliates could be a threat for E. lacustris populations and may play a substantial role in determining community production and dynamics. Our results showed that E. lacustris, with the mean biomass of 75 μg, had on its body “epibiotic luggage” with a biomass of about 1 μg, accounting for 1.3% of its biomass. This luggage appears to be small but may slow down the movement of E. lacustris and its vertical distribution. However, this assumption requires further studies. It is also necessary to address another question - why do suctorian ciliates colonize only one (in this case less numerous E. lacustris) among many other (more numerous) zooplankton species?
Conclusions
The results of this study revealed the highest abundance of E. lacustris in thermocline zones and indicated that this species could temporarily tolerate warmer water and lower oxygen concentrations probably because of the better food resources. A large part of the E. lacustris population had the epibiont ciliate Acineta tuberosa, while other species of zooplankton did not have suctorian epibiont ciliates. This phenomenon may not be only a local character. Few laboratory experiments on E. species revealed that epibionts could negatively affect the behavior of the host and dramatically affected the mating success. Nowadays, the improvement in water quality has led to an increase in the population of E. lacustris in deep lakes. However, epibiont ciliates could be a threat for E. lacustris populations and may play a substantial role in the production, distribution, and community dynamics.
References
Adlerówna G (1929) Przyczynek do znajomości ustosunkowania ilościowego skorupiaków planktonowych Wigier. Arch Hydrobiol i Ryb 4:169–276
Arbačiauskas K, Kalytytė D (2010) Occurrence and interannual abundance variation of glacial relict calanoids Limnocalanus macrurus and Eurytemora lacustris in Lithuanian Lakes. Acta Zool Litu 20:61–67
Benndorf J (1990) Conditions for effective biomanipulation – conclusions derived from whole-lake experiments in Europe. Hydrobiologia 200:187–203. https://doi.org/10.1007/BF02530339
Benndorf J (2005) Ecotechnology: basis of a new immission concept in water pollution control. Water Sci Technol 52:17–24
Beutler M, Wiltshire KH, Meyer B, Moldaenke C, Lüring C, Meyerhöfer M, Hansen U-P, Dau H (2002) A fluorometric method for the differentiation of algal populations in vivo and in situ. Photosynth Res 72:39–53. https://doi.org/10.1023/A:1016026607048
Błędzki LA, Rybak JI (2016) Freshwater crustacean zooplankton of Europe. Springer, Berlin. https://doi.org/10.1007/978-3-319-29871-9
Carlson RE (1977) A trophic state index for lakes. Limnol Oceanogr 22:361–369. https://doi.org/10.4319/lo.1977.22.2.0361
Chiavelli DA, Mills E, Threkeld ST (1993) Host preference, seasonality, and community interactions of zooplankton epibionts. Limnol Oceanogr 38:574–583. https://doi.org/10.4319/lo.1993.38.3.0574
DeMott WR (1986) The role of taste in food selection by freshwater zooplankton. Oecologia 69:334–340. https://doi.org/10.1007/BF00377053
Einsle U (1993) Crustacea, Copepoda - Calanoida und Cyclopoida. In: Süßwasserfauna von Mitteleuropa. Gustav Fischer Verlag, Stuttgart
Ejsmont-Karabin J, Karabin A (2013) The suitability of zooplankton as lake ecosystem indicator; crustacean trophic state index. Pol J Ecol 61:561–573
Foissner W, Berger H, Blatterer H, Kohmann F (1995) Taxonomische und ökologische Revision der Ciliaten des Saprobiensystems – Band IV: Gymnostomatea, Loxodes, Suctoria. Informationsberichte des Bayer. Landesamtes für Wasserwirtschaft, Heft 1/95, München
Green JD, Shiel RJ (2000) Mobiline peritrich riders on Australian calanoid copepods. Hydrobiologia 437:203–212. https://doi.org/10.1023/A:1026567210125
Gulati RD, Van Donk E (2002) Lakes in the Netherlands, their origin, eutrophication and restoration: review of the state-of-the-art. Hydrobiologia 478:73–106
Hansson L-A, Annadotter H, Bergman E, Hamrin SF, Jeppesen E, Kairesalo T, Luokkanen E, Nilsson P-A, Søndergaard M, Strand J (1998) Biomanipulation as an application of food chain theory: constraints, synthesis and recommendations for temperate lakes. Ecosystems 1:558–574
Hirche HJ (1974) Die Copepoden Eurytemora affinis Poppe und Acartia tonsa Dana und ihre Besiedlung durch Myoschiston centropagidarum Precht (Peritricha) in der Shiel. Kiel Meeresf 30:43–64
Illies J (1978) Limnofauna Europaea. Eine Zusammenstellung aller die europäischen Binnengewässer bewohnenden mehrzelligen Tierarten mit Angaben über ihre Verbreitung und Ökologie. Gustav Fischer Verlag, Stuttgart
Jeppesen E, Søndergaard M, Jensen JP, Havens K, Anneville O, Carvalho L, Coveney MF, Deneke R, Dokulil MT, Foy B, Gerdeaux D, Hampton SE, Kangur K, Köhler J, Körner S, Lammens E, Lauridsen TL, Manea M, Miracle R, Moss B, Nöges P, Persson G, Phillips G, Portielje R, Romo S, Schelske CL, Straile D, Tatrai I, Willén E, Winder M (2005) Lake responses to reduced nutrient loading – an analysis of contemporary long term data from 35 case studies. Freshw Biol 50:1747–1771. https://doi.org/10.1111/j.1365-2427.2005.01415.x
Kamiński M (1999) Lake Wigry, the lake "adopted" by international association of theoretical and applied limnology (SIL "Lake adoption" project). Pol J Ecol 47:215–224
Karabin A (1985) Pelagic zooplankton (Rotatoria + Crustacea) variation in the process of lake eutrophication. I. Structural and quantitative features. Ekologia Polska 33:567–616
Karabin A, Ejsmont-Karabin J (1999) Jezioro Wigry – wieloletnia sukcesja zespołów Rotifera i Crustacea. In: Zdanowski B, Kamiński M (eds) Funkcjonowanie i ochrona ekosystemów wodnych na obszarach chronionych. Wydawnictwo IRS, Olsztyn, pp 371–388
Karpowicz M, Ejsmont-Karabin J (2017) Effect of metalimnetic gradient on phytoplankton and zooplankton (Rotifera, Crustacea) communities in different trophic conditions. Environ Monit Assess 189:367. https://doi.org/10.1007/s10661-017-6055-7
Karpowicz M, Górniak A (2013) Zooplankton skorupiakowy jezior harmonijnych Wigierskiego Parku Narodowego a trofia wód. Monitoring Środowiska Przyrodniczego 14:97–101
Kasprzak P, Reese C, Koschel R, Schulz M, Hambaryan I, Mathes J (2005) Habitat characteristics of Eurytemora lacustris (Poppe, 1887) (Copepoda, Calanoida): the role of lake depth, temperature, oxygen concentration and light intensity. Int Rev Hydrobiol 90:292–309. https://doi.org/10.1002/iroh.200410769
Kinsten B (2012) De glacialrelikta kräftdjurens utbredning I Sverige. Havs- och vattenmyndighetens rapport 2012:1
Kornijów R, Gulati RD, van Donk E (1990) Hydrophyte macroinvertebrate interactions in Zwemlust, a lake undergoing biomanipulation. Hydrobiologia 200:467–474. https://doi.org/10.1007/BF02530364
Kosiba J, Krztoń W, Wilk-Woźniak E (2017) Effect of microcystins on proto- and metazooplankton is more evident in artificial than in natural waterbodies. Microb Ecol 75:293. https://doi.org/10.1007/s00248-017-1058-z
Maier G, Speth B, Arp W, Bahnwart M, Kasprzak P (2011) New records of the rare glacial relict Eurytemora lacustris (Poppe 1887) (Copepoda; Calanoida) in atypical lake habitats of northern Germany. J Limnol 70(1):145–148. https://doi.org/10.3274/JL11-70-1-17
Mehner T, Benndorf J, Kasprzak P, Koschel R (2002) Biomanipulation of lake ecosystems: successful applications and expanding complexity in the underlying science. Freshw Biol 47:2453–2465. https://doi.org/10.1046/j.1365-2427.2002.01003.x
Müller H, Geller W (1993) Maximum growth rates of aquatic ciliated protozoa: the dependence on body size and temperature reconsidered. Arch Hydrobiol 126:315–327
Nagasawa S (1986) The peritrich ciliate Zoothamnium attached to the copepod Centropages abdominalis in Tokyo Bay waters. Bull Mar Sci 38:533–558
Ochocka A, Pasztaleniec A (2016) Sensitivity of plankton indices to lake trophic conditions. Environ Monit Assess 188(622). https://doi.org/10.1007/s10661-016-5634-3
Patalas J, Patalas K (1966) The crustacean plankton communities in polish lakes. Verh Internat Verein Limnol 16:204–215
Rózsa L, Reiczigel J, Majoros G (2000) Quantifying parasites in samples of hosts. J Parasitol 86:228–232. https://doi.org/10.1645/0022-3395(2000)086[0228:QPISOH]2.0.CO;2
Ruttner F (1905) Über das Verhalten des Oberflächenplanktons zu verschiedenen Tageszeiten im Grossen Plöner Sees und in zwei nordböhmischen Teichen. Forschungsber Biol Stat Plön 12:34–62
Rylov WM (1935) Das Zooplankton der Binnengewässer. Einführung in die Systematik und Ökologie des tierischen Limnoplanktons mit besonderer Berücksichtigung der Gewässer Mitteleuropas. In: Thienemann A (ed) Die Binnengewässer, Bd. 15. Schweizerbart, Stuttgart
Sas H (1989) Lake restoration by reduction of nutrient loading. Expectation, experiences, extrapolation. Academia Verlag Richardz, St. Augustin
Souissi A, Souissi S, Hwang J-S (2013) The effect of epibiont ciliates on the behavior and mating success of the copepod Eurytemora affinis. J Exp Mar Bio Ecol 445:38–43
Spikkeland I, Kinsten B, Kjellberg G, Nilssen JP, Väinölä R (2016) The aquatic glacial relict fauna of Norway – an update of distribution and conservation status. Fauna Norv 36:51–65. https://doi.org/10.5324/fn.v36i0.1994
Thienemann A (1950) Verbreitungsgeschichte der Süßwassertierwelt Europas. Versuch einer historischen Tiergeographie der europäischen Binnengewässer. In: Thienemann A (ed) Die Binnegewaesser. E. Schweizerbart’sche Verlagsbuchhandlung, Stuttgart
Turner JT, Postek MT, Collard SB (1979) Infestation of estuarine copepod Acartia tonsa with the ciliate Epistylis. Trans Am Microsc Soc 98:136–139
Utz LRP, Coats DW (2005) Spatial and temporal patterns in the occurrence of peritrich ciliates as epibionts on calanoid copepods in Chesapeake Bay, USA. J Eukaryot Microbiol 52:236–244
Utz LRP, Coats DW (2008) Telotroch formation, survival, and attachment in the epibiotic peritrich Zoothamnium intermedium (Ciliophora, Oligohymenophorea). Invertebr Biol 127:237–248. https://doi.org/10.1111/j.1744-7410.2008.00140.x
Valbonesi A, Guglielmo L (1988) Infestation of a lagoon zooplanktonic community with the epizoic peritrich Zoothamnium intermedium Precht (Peritrichia, Zoothamniidae). Boll Zool 3:179–183
Vezhnovets VV, Zaidykov IY, Naumova EY, Sysova EA (2012) Biological peculiarities of two copepod species (Crustacea, Copepoda, Calanoida) as possible causes of changes in their geographical ranges. Russian Journal of Biological Invasions 3:243–250. https://doi.org/10.1134/S2075111712040054
Visse M (2007) Detrimental effect of peritrich ciliates (Epistylis sp.) as epibionts on the survival of the copepod Acartia bifilosa. Proc Estonian Acad Sci Biol Ecol 56:173–178
Weiler W, Kasprzak P, Schulz M, Flössner D (2003) Habitat requirements of Eurytemora lacustris (Copepoda, Calanoida) and implications for its distribution. Adv Limnol 58:203–214
Wiktor K, Krajewska-Sołtys A (1994) Occurrence of epizoic and parasitic protozoans on Calanoida in the southern Baltic. Bull Sea Fish Inst Gdynia 132:13–25
Acknowledgments
The authors are thankful to employees of the Wigry National Park for their help in sample collection. We thank anonymous reviewers for their valuable comments, which helped us to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Karpowicz, M., Kalinowska, K. Vertical distribution of the relic species Eurytemora lacustris (Copepoda, Calanoida) in stratified mesotrophic lakes. Biologia 73, 1197–1204 (2018). https://doi.org/10.2478/s11756-018-0138-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-018-0138-y