Abstract
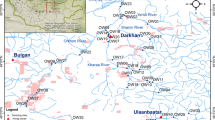
Mining is a relatively highly monitored industry. While chemical pollutants (toxic ions, radionuclides, etc.) have mostly been eliminated from mining waters, other types of environmental pollution (temperature regime alterations, high concentrations of various anions, etc.) can affect benthic invertebrates. In this study, we focused on the effect of mining water effluent on the diversity and density of aquatic Clitellata. Four sampling sites were selected. Three sites in a natural stream (the Nedvědička River, Czech Republic), one upstream and two downstream from the mining effluent, and one site on the mining waters were sampled monthly during 2008–2009. Environmental variables were recorded in and samples were collected from two types of habitats — riffles and pools. The response of clitellate assemblages was evaluated using principal component analysis and generalised estimating equations. The results indicated that the mining effluent caused partial species exchange and had negative effects on clitellate taxa richness and abundance. These responses were specific to both the habitat (riffle/pool) and species sampled. In each of the different taxa studied, we observed one of four typical clitellate responses: (a) elimination of stenotherm species; (b) reduction of clitellate species followed by quick recovery; (c) neutral response; or (d) positive influence. We found that aquatic clitellates, which are considered to be eurytopic with broad ecological valences, are also sensitive to even slight environmental pollution.
Similar content being viewed by others
References
Ames B., McCann J. & Yamasaki E. 1975. Methods for detecting carcinogens and mutagens with the Salmonella/mammalianmicrosome mutagenicity test. Mutation Res. 31(6): 347–364. DOI: 10.1016/0165-1161(75)90046-1
Batty L.C., Atkin L. & Manning D.A.C 2005. Assessment of the ecological potential of mine-water treatment wetland using a baseline survey of macroinvertebrate communities. Environ. Pollut. 138(3): 412–419. DOI: 10.1016/j.envpol.2005.04.022
Bojková J., Schenková J., Horsák M. & Hájek M. 2011. Species richness and composition patterns of clitellate (Annelida) assemblages in the treeless spring fens: the effect of water chemistry and substrate. Hydrobiologia 667(1): 159–171. DOI: 10.1007/s10750-011-0634-3
Brinkhurst R.O. & Cook D.G. 1974. Aquatic earthworms (Annelida: Oligochaeta), pp. 143–156. In: Hart C.W. Jr & Fuller S.L.H. (eds), Pollution Ecology of Freshwater Invertebrates, Academic Press, New York, 389 pp. ISBN-13: 978-0123284501, ISBN-10: 0123284503
Cellot B. & Juget J. 1998. Oligochaete drift in a large river (French Upper Rhôe): the effect of life cycle and discharge. Hydrobiologia 389(1–3): 183–191. DOI: 10.1023/A:1003511916699
Directive 2008/105/EC of European Parliament and of the Council on environmental quality standards in the field of water policy, amending and subsequently repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and amending Directive 2000/60/EC”, OJ L348, pp. 84–97, 24.12.2008
Dumnicka E. 2000. Studies on Oligochaeta taxocens in streams, interstitial and cave waters of southern Poland with remarks on Aphanoneura and Polychaeta distribution. Acta Zool. Cracov. 43(3–4): 339–392.
Dumnicka E. & Galas J. 2006. Distribution of benthic fauna in relation to environmental conditions in an inundated opencast sulphur mine (Piaseczno reservoir, Southern Poland). Aquat. Ecol. 40(2): 203–210. DOI: 10.1007/s10452-005-6040-z
Dumnicka E. & Pasternak K. 1978. The influence of physicochemical properties of water and bottom sediments in the River Nida on the distribution and numbers of Oligochaeta. Acta. Hydrobiol. (Cracow) 20(3): 215–232.
Generlich O. & Giere O. 1996. Osmoregulation in two aquatic oligochaetes from habitats with different salinity and comparison to other annelids. Hydrobiologia 334(1–3): 251–261. DOI: 10.1007/BF00017375
Haidekker A. & Hering D. 2008. Relationship between insects (Ephemeroptera, Plecoptera, Coleoptera, Trichoptera) and temperature in small and medium-sized streams in Germany: A multivariate study. Aquat. Ecol. 42(3): 463–481. DOI: 10.1007/s10452-007-9097-z
Hogg I.D. & Williams D.D. 1996. Response of stream invertebrates to a global-warming thermal regime: an ecosystemlevel manipulation. Ecology 77(2): 395–407. DOI: http://dx.doi.org/10.2307/2265617
Hrabě S. 1981. Vodní máloštětinatci (Oligochaeta) Československa. Acta Univ. Carol. Biol. 1–2, 1979, 168 pp.
Hojsgaard S., Halekoh U. & Yan J. 2006. The R Package geepack for generalized estimating equations. J. Stat. Soft. 15(2): 1–11.
Hudcová H., Badurová J., Rozkošný M., Funková R., Svobodová J. & Sova J. 2012. Ovlivnění jakosti vod a sedimentů v povodí řeky Nedvědičky těžbou a zpracováním uranových rud. VTEI Vodohospodářské Technicko-ekonomické Informace 54(3): 5–10.
Hynes H.B.N. 1970. The Ecology of Running Waters. Liverpool University Press, Liverpool, 555 pp. ISBN: 0802016898, 9780802016898
Korn H. 1963. Studien zur Ökologie der Oligochaeten in der oberen Donau unter Berücksichtigung der Abwassereinwirkungen. Arch. Hydrobiol. 27(2): 131–182. DOI: 10.1127/agdonauforschung/1/1963/131
Krodkiewska M. 2005. The Oligochaeta communities in the benthos of artificially heated Rybnik dam reservoir (Poland). J. Freshwater Ecol. 20(1): 117–122. DOI: 10.1080/02705060.2005.9664944
Krodkiewska M. & Michalik-Kucharz A. 2009. The bottom Oligochaeta communities in sand pits of different trophic status in Upper Silesia. Aquat. Ecol. 43(2): 437–444. DOI: 10.1007/s10452-008-9199-2
Lakly M.B. & McArthur J.V. 2000. Macroinvertebrate recovery of a post-thermal stream: habitat structure and biotic function. Ecol. Engineer. 15(Suppl. 1): S87–S100. DOI: 10.1016/S0925-8574(99)00075-0
Lehmkuhl D.M. 1972. Change in thermal regime as a cause of reduction of benthic fauna downstream of a reservoir. J. Fish. Res. Board Canada, 29(9): 1329–1332. DOI: 10.1139/f72-201
Maret T.R., Cain D.J., MacCoy D.E. & Short T.M. 2003. Response of benthic invertebrate assemblages to metal exposure and bioaccumulation associated with hard-rock mining in northwesterm streams, USA. J. N. Am. Benthol. Soc. 22(4): 598–620.
Martínez-Ansemil E. & Collado R. 1996. Distribution patterns of aquatic oligochaetes inhabiting watercourses in the Northwestern Iberian Peninsula. Hydrobiologia 334(1): 73–83. DOI: 10.1007/BF00017355
Miliša M., Živković V. & Habdija I. 2010. Destructive effect of quarry effluent on life in a mountain stream. Biologia 65(3): 520–526. DOI: 10.2478/s11756-010-0044-4
Nedeau E.J., Merritt R.W. & Kaufman M.G. 2003. The effect of an industrial effluent on an urban stream benthic community: water quality vs. habitat quality. Environ. Pollut. 123(1): 1–13. DOI: 10.1016/S0269-7491(02)00363-9
Nijboer R.C., Wetzel M.J. & Verdonschot P.F.M. 2004. Diversity and distribution of Tubificidae, Naididae, and Lumbriculidae (Annelida: Oligochaeta) in the Netherlands: an evaluation of twenty years of monitoring data. Hydrobiologia 520(1–3): 127–141. DOI: 10.1023/B:HYDR.0000027732.88238.61
Preston R.L. 2009. Osmoregulation in Annelids, pp. 135–160. In: Evans D.H. (ed.), Osmotic and Ionic Regulation: Cells and Animals, CRC Press, Boca Raton, 606 pp. ISBN: 9780849380303
Oksanen J., Blanchet F.G., Kindt R., Legendre P., Minchin P.R., O’Hara R.B., Simpson G.L., Solymos P., Stevens M.H.H. & Wagner H. 2011. vegan: Community Ecology Package. R package version 2.0-2. http://CRAN.R-project.org/package=vegan
Quinn J.M. & Hickey C.W. 1990. Characterisation and classification of benthic invertebrate communities in 88 New Zealand rivers in relation to environmental factors. N. Z. J. Mar. Freshwater Res. 24(3): 387–409. DOI: 10.1080/00288330.1990.9516432
Quinn J.M., Steele G.L., Hickey C.W. & Vickers M.L. 1994. Upper thermal tolerances of twelve New Zealand stream invertebrate species. N. Z. J. Mar. Freshwater Res. 28(4): 391–397. DOI: 10.1080/00288330.1994.9516629
R Development Core Team 2010. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna.
Saltveit S.J., Bremnes T. & Brittain J.E. 1994. Effect of a changed temperature regime on the benthos of a Norwegian regulated river. Regul. Rivers: Res. & Manage. 9(2): 93–102. DOI: 10.1002/rrr.3450090203
Schenková J., Helešic J. & Jarkovsky J. 2006. Seasonal dynamics of Bythonomus lemani and Bothrioneurum vejdovskyanum (Oligochaeta, Annelida) in relation to environmental variables. Biologia 61(5): 517–523. DOI: 10.2478/s11756-006-0085-x
Schenková J., Komárek O. & Zahrádková S. 2001. Oligochaeta of the Morava and Odra River basins (Czech Republic): species distribution and community composition. Hydrobiologia 463: 235–240. DOI: 10.1007/978-94-010-0597-524
Schwank P. 1981. Turbellarien, Oligochaeten und Archianneliden des Breitenbachs und anderer oberhessischer Mittelgebirgsbäche. II. Die Systematik und Autökologie der einzelnen Arten. Schlitzer Produktionsbiologische Studien (43-2). Arch. Hydrobiol. Suppl. 62(1): 86–147.
Straka M., Syrovátka V. & Helešic J. 2012. Temporal and spatial macroinvertebrate variance compared: crutial role of CPOM in a headwater stream. Hydrobiologia 686(1): 119–134. DOI: 10.1007/s10750-012-1003-6
Syrovátka V., Schenková J. & Brabec K. 2009. The distribution of chironomid larvae and oligochaetes within a stony-bottomed river stretch: the role of substrate and hydraulic characteristics. Fund. Appl. Limnol. / Arch. Hydrobiol. 174(1): 43–62. DOI: 10.1127/1863-9135/2009/0174-0043
Taylor B.R. & Dykstra A.N. 2005. Effect of hot ground water on a small swamp-stream in Nova Scotia, Canada. Hydrobiologia 545(1): 129–144. DOI: 10.1007/s10750-005-2745-1
Thomas P. & Liber K. 2001. An estimation of radiation doses to benthic invertebrates from sediments collected near a Canadian uranium mine. Environ. Int. 27(4): 341–353. DOI: 10.1016/S0160-4120(01)00085-X
Uzunov V., Košel V. & Sládeček V. 1988. Indicator value of freshwater Oligochaeta. Acta Hydrochim. Hydrobiol. 16(2): 173–186. DOI: 10.1002/aheh.19880160207
Verdonschot P.F.M. 2006. Beyond masses and blooms: the indicative value of oligochaetes. Hydrobiologia 564: 127–142. DOI: 10.1007/1-4020-5368-1_13
Voelz N.J., Poff N.L. & Ward J.V. 1994. Differential effects of a brief thermal disturbance on caddisflies (Trichoptera) in a regulated river. Am. Midl. Nat. 132(1): 173–182.
Wellborn G.A. & Robinson J.V. 1996. Effects of a thermal effluent on macroinvertebrates in a Central Texas Reservoir. Am. Midl. Nat. 136(1): 110–120.
Živić I., Marković Z. & Brajković M. 2006. Influence of the temperature regime on the composition of the macrozoobenthos community in a thermal brook in Serbia. Biologia 61(2): 179–191. DOI: 10.2478/s11756-006-0029-5
Živić I., Živić M., Milošević D., Bjelanović K., Stanojlović S., Daljević R. & Marković Z. 2013. The effects of geothermal water inflow on longitudinal changes in benthic macroinvertebrate community composition of a temperate stream. J. Therm. Biol. 38(5): 255–263. DOI: 10.1016/j.jtherbio.2013.03.005
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Růžičková, S., Schenková, J., Weissová, V. et al. Environmental impact of heated mining waters on clitellate (Annelida: Clitellata) assemblages. Biologia 69, 1179–1189 (2014). https://doi.org/10.2478/s11756-014-0424-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-014-0424-2