Abstract
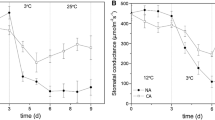
Photosynthesis, chlorophyll (Chl) fluorescence, and antioxidant enzymes were measured in the mulberry (Morus spp.) cultivars Da 10, Hongguo 2, Anza 1, and Taiwan 72C002, which were subjected to salinity and high-temperature stress (STS; 0.1%, 0.3%, and 0.5% NaCl concentrations, 34.5°C–40.5°C/27.8°C–29.2°C day/night temperatures). Control plants were watered with 1 L of full-strength Hoagland’s nutrient solution with no added NaCl. Net photosynthetic rate (P N), stomatal conductance (g s), and effective quantum yield of photosystem II photochemistry (ΦPSII) increased in Anza 1 and Taiwan 72C002 under 0.1% STS but decreased in Da 10 and Hongguo 2 compared with the control. However, all the above parameters, including Chl content, maximum quantum yield of photosystem II photochemistry (Fv/Fm), nonphotochemical quenching (NPQ), and maximum carboxylation velocity of Rubisco (V cmax, decreased in Taiwan 72C002, Honggua 2, and Da 10 under 0.3% and 0.5% STS, suggesting that photoinhibition occurred under severe STS. Under STS, there were no significant changes in P N, Fv/Fm, ΦPSII, ascorbate peroxidase (APX) activity, superoxide dismutase (SOD) activity, catalase activity, superoxide anion radical (O −2 ) content, malondialdehyde (MDA) content, soluble sugar content (SSC), and leaf biomass in Anza 1 even at 0.5% STS, showing that Anza 1 displays high resistance to STS. In addition, peroxidase activity was significantly higher in Anza 1 than in the other mulberry cultivars. Significant adverse effects of severe salinity on photosynthesis and Chl fluorescence parameters were observed in Da 10. Additionally, SOD, peroxidase, and APX activities were lower in Da 10, whereas O −2 and MDA contents were higher in comparison with the other mulberry cultivars under 0.3% and 0.5% STS, suggesting that Da 10 had low resistance to STS. These results show that 0.1% STS had a positive effect on photosynthesis and Chl fluorescence parameters in Anza 1 and Taiwan 72C002, and higher peroxidase activity can to a certain extent explain the higher STS tolerance in Anza 1. Damages to DSM photosystems might be related to lower SOD, POD, and APX activities, which resulted in the accumulation of reactive oxygen species.
Similar content being viewed by others
Abbreviations
- APX:
-
ascorbate peroxidase
- AQY:
-
apparent quantum yield
- C a :
-
atmospheric CO2 concentration
- CE:
-
carboxylation efficiency
- C i :
-
intercellular CO2 concentration
- Chl:
-
chlorophyll
- E :
-
transpiration rate
- F0 :
-
minimum fluorescence level
- Fm :
-
maximum fluorescence level
- Fv/Fm :
-
maximum quantum yield of photosystem II photochemistry
- FS :
-
steady-state yield of PSII fluorescence in the light
- g s :
-
stomatal conductance
- Ls :
-
stomatal limitation value
- MDA:
-
malondialdehyde
- NPQ:
-
non-photochemical quenching
- O −2 :
-
superoxide anion radical
- PAR:
-
photosynthetically active radiation
- P N :
-
net photosynthetic rate
- PPBS:
-
potassium phosphate buffer solution
- PSII:
-
photosystem II
- ΦPSII :
-
effective quantum yield of photosystem II photochemistry
- qp :
-
photochemical quenching coefficient
- ROS:
-
reactive oxygen species
- SOD:
-
superoxide dismutase
- SSC:
-
soluble sugar content
- STS:
-
salinity and high-temperature stress
- V cmax :
-
maximum carboxylation velocity of Rubisco
References
Aebi H. 1974. Catalase, pp. 673–677. In: Bergmeyer H.U. (ed.), Methods of Enzymatic Analysis, Academic Press, New York.
Almeselmani M., Deshmukh P.S., Sairam R.K., Kushwaha S.R. & Singh T.P. 2006. Protective role of antioxidant enzymes under high temperature stress. Plant Sci. 171: 382–388.
Arnon D.I. 1949. Copper enzymes in isolated chloroplasts: Polyphenoloxidase in Beta vulgaris. Plant Physiol. 24: 1–15.
Bates L.S., Waldren R.P. & Teare I.D. 1973. Rapid determination of free proline for water-stress studies. Plant Soil 39: 205–207.
Cardini C.E., Leloir L.F. & Chiriboga J. 1955. The biosynthesis of sucrose. J. Biol. Chem. 214: 149–155.
Chance B. & Maehly A.C. 1955. Assay of catalases and peroxidases. Method. Enzymol. 2: 746–755.
Chen S., Li J., Wang T., Wang S., Polle A. & Hüttermann A. 2002. Osmotic stress and ion-specific effects on xylem abscisic acid and the relevance to salinity tolerance in poplar. J. Plant Growth Regul. 21: 224–233.
Dat J.F., Lopez-Delgado H., Foyer C.H. & Scott I.M. 1998. Parallel changes in H2O2 and catalase during thermotolerance induced by salicylic acid or heat acclimation in mustard seedlings. Plant Physiol. 116: 1351–1357.
Elboutahiri N., Thami Alami I., Ibriz M. & Alfaiz C. 2008. The effect of salinity and high temperature on biomass production of some alfalfa landraces. Options Méditerranéennes Série A 79: 293–297.
Elstner E.F. & Heupel A. 1976. Inhibition of nitrite formation from hydroxylammoniumchloride: A simple assay for superoxide dismutase. Anal. Biochem. 70: 616–620.
Epstein E., Norlyn J.D., Rush D.W., Kingsbury R.W., Kelley D.B., Cunningham G.A. & Wrona A.F. 1980. Saline culture of crops: A genetic approach. Science 210: 399–404.
Esfandiari E., Shekari F., Shekari F. & Esfandiari M. 2007. The effect of salt stress on antioxidant enzymes’ activity and lipid peroxidation on the wheat seedling. Not. Bot. Hort. Agrobot. Cluj. 35: 48–56.
Giannopolitis C.N. & Ries S.K. 1977. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 59: 309–314.
Harinasut P., Poonsopa D., Roengmongkol K. & Charoensataporn R. 2003. Salinity effects on antioxidant enzymes in mulberry cultivar. Science Asia 29: 109–113.
Harinasut P., Srisunak S., Pitukchaisopol S. & Charoensataporn R. 2000. Mechanisms of adaptation to increasing salinity of mulberry: Proline content and ascorbate peroxidase activity in leaves of multiple shoots. Science Asia 26: 207–211.
Henley W.J., Major K.M. & Hironaka J.L. 2002. Response to salinity and heat stress in two halotolerant chlorophyte algae. J. Phycol. 38: 757–766.
Hichem H., El Naceur A. & Mounir D. 2009. Effects of salt stress on photosynthesis, PSII photochemistry and thermal energy dissipation in leaves of two corn (Zea mays L.) varieties. Photosynthetica 47: 517–526.
Hodges D.M., DeLong J.M., Forney C.F. & Prange R.K. 1999. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207: 604–611.
Jia Z.S., Tang M.C., Wu J.M. 1999. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 64: 555–559
Knight H. & Knight M.R. 2001. Abiotic stress signalling pathways: Specificity and cross-talk. Trends Plant Sci. 6: 262–267.
Koca H., Bor M., Özdemir F. & Türkan I. 2007. The effect of salt stress on lipid peroxidation, antioxidative enzymes and proline content of sesame cultivars. Environ. Exp. Bot. 60: 344–351.
Li Z.G. & Gong M. 2005. Improvement of measurement method for superoxide anion radical in plant. Acta Bot. Yunnan. 27: 211–216.
Lu C., Qiu N., Wang B. & Zhang J. 2003. Salinity treatment shows no effects on photosystem II photochemistry, but increases the resistance of photosystem II to heat stress in halophyte Suaeda salsa. J. Exp. Bot. 54: 851–860.
Lu C.M., Qiu N.W., Lu Q.T., Wang B.S. & Kuang T.Y. 2002. Does salt stress lead to increased susceptibility of photosystem II to photoinhibition and changes in photosynthetic pigment composition in halophyte Suaeda salsa grown outdoors? Plant Sci. 163: 1063–1068.
Maricle B.R., Lee R.W., Hellquist C.E., Kiirats O. & Edwards G.E. 2007. Effects of salinity on chlorophyll fluorescence and CO2 fixation in C4 estuarine grasses. Photosynthetica 45: 433–440.
Masojídek J., Torzillo G., Kopecky J., Koblížek M., Nidiaci L., Komenda J., Lukavská A. & Sacchi A. 2000. Changes in chlorophyll fluorescence quenching and pigment composition in the green alga Chlorococcum sp. grown under nitrogen deficiency and salinity stress. J. Appl. Phycol. 12: 417–426.
McMurtrie R.E. & Wang Y.P. 1993. Mathematical models of the photosynthetic responses of tree stands to rising CO2 concentrations and temperatures. Plant Cell Environ. 16: 1–13.
Mishra R.K. & Singhal G.S. 1993. Photosynthetic activity and peroxidation of thylakoid lipids during photoinhibition and high temperature treatment of isolated wheat chloroplasts. J. Plant Physiol. 141: 286–292.
Nagesh Babu R. & Devaraj V.R. 2008. High temperature and salt stress response in French bean (Phaseolus vulgaris). Aust. J. Crop Sci. 2: 40–48
Nakano Y. & Asada K. 1981. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22: 867–880.
Norlyn J.D. & Epstein E. 1984. Variability in salt tolerance of four triticale lines at germination and emergence. Crop Sci. 24: 1090–1092.
Pospíšil P. 2009. Production of reactive oxygen species by photosystem II. BBA-Bioenergetics 1787: 1151–1160.
Qin J., Dong W.Y., He K.N., Chen J., Liu J. & Wang Z.L. 2010. Physiological responses to salinity in Silver buffaloberry (Shepherdia argentea) introduced to Qinghai high-cold and saline area, China. Photosynthetica 48: 51–58.
Qin L.Q., Li L., Bi C., Zhang Y.L., Wan S.B., Meng J.J., Meng Q.W. & Li X.G. 2011. Damaging mechanisms of chilling- and salt stress to Arachis hypogaea L. leaves. Photosynthetica 49: 37–42.
Qiu N. & Lu C. 2003. Enhanced tolerance of photosynthesis against high temperature damage in salt-adapted halophyte Atriplex centralasiatica plants. Plant Cell Environ. 26: 1137–1145.
Rizhsky L., Liang H.J., Shuman J., Shulaev V., Davletova S. & Mittler R. 2004. When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 134: 1683–1696.
Roháček K. 2002. Chlorophyll fluorescence parameters: The definitions, photosynthetic meaning, and mutual relationships. Photosynthetica 40: 13–29.
Stoeva N. & Kaymakanova M. 2008. Effect of salt stress on the growth and photosynthesis rate of bean plants (Phaseolus vulgaris L.). J. Centr. Eur. Agr. 9: 385–391.
Sui N., Li M., Li K., Song J. & Wang B.S. 2010. Increase in unsaturated fatty acids in membrane lipids of Suaeda salsa L. enhances protection of photosystem II under high salinity. Photosynthetica 48: 623–629.
Tiwari A. & Pospíšil P. 2009. Superoxide oxidase and reductase activity of cytochrome b559 in photosystem II. BBA-Bioenergetics 1787: 985–994.
Tsugane K., Koboyashi K., Niwa Y., Ohba Y., Wada K. & Kobayashi H. 1999. A recessive Arabidopsis mutant that grows photoautotrophically under salt stress shows enhanced active oxygen detoxification. Plant Cell 11: 1195–1206.
Wang R.G., Chen S.L., Deng L., Fritz E., Hüttermann A. & Polle A. 2007. Leaf photosynthesis, fluorescence response to salinity and the relevance to chloroplast salt compartmentation and anti-oxidative stress in two poplars. Trees-Struct. Funct. 21: 581–591.
Xu H.L., Wang R., Gauthier L. & Gosselin A. 1999. Tomato leaf photosynthetic responses to humidity and temperature under salinity and water deficit. Pedosphere 9: 105–112.
Xu Z.Z. & Zhou G.S. 2006. Combined effects of water stress and high temperature on photosynthesis, nitrogen metabolism and lipid peroxidation of a perennial grass Leymus chinensis. Planta 224: 1080–1090.
Yin C.Y., Berninger F. & Li C.Y. 2006. Photosynthetic responses of Populus przewalski subjected to drought stress. Photosynthetica 44: 62–68.
Zheng Y.H., Xu X.B., Wang M.Y., Zheng X.H., Li Z.J. & Jiang G.M. 2009. Responses of salt-tolerant and intolerant wheat genotypes to sodium chloride: Photosynthesis, antioxidants activities, and yield. Photosynthetica 47: 87–94.
Zhu J.K. 2001a. Plant salt tolerance. Trends Plant Sci. 6: 66–71.
Zhu J.K. 2001b. Cell signaling under salt, water and cold stresses. Curr. Opin. Plant Biol. 4: 401–406.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, C., Huang, S., Hu, X. et al. Changes in photosynthesis, chlorophyll fluorescence, and antioxidant enzymes of mulberry (Morus ssp.) in response to salinity and high-temperature stress. Biologia 68, 404–413 (2013). https://doi.org/10.2478/s11756-013-0167-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-013-0167-5