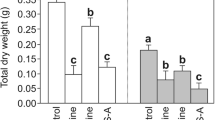
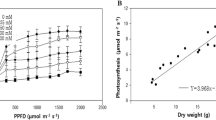
Abstract
In a 16-day study, the effect of increasing soil NaCl on leaf photosynthesis, chlorophyll a fluorescence, chloroplast ion compartmentation, variations of SOD (superoxide dismutase) and POD (peroxidase) isoenzymes and the relevance to salt resistance were investigated in seedlings of Populus euphratica Oliv. (P. euphratica) (salt-resistant) and rooted cuttings of P. “popularis 35–44” (P. popularis) (salt-sensitive). Initial salinity caused a rapid decline of net photosynthetic rate (Pn) and unit transpiration rate (TRN) in P. euphratica, resulting from the NaCl-induced stomatal closure. In a longer-term of salinity, CO2 assimilation in P. popularis was severely reduced whereas stressed P. euphratica maintained a relatively higher and constant level of Pn. Pn–Ci curves showed that salt stress (12 days) reduced CO2 saturation point (CSP), CO2 saturated Pn (CSP n ), and carboxylation efficiency (CE), but increased CO2 compensation point (CCP) in the two genotypes. Similarly, salinity lowered light saturation point (LSP), light saturated Pn (LSP n ), and apparent quantum yield (AQY) in both genotypes but the inhibitory effect of NaCl on light reaction was more pronounced in P. popularis, as compared to P. euphratica. Chlorophyll a fluorescence data indicated that a longer-term of salt stress (12 days) exhibited a marked influence on fluorescence parameters of P. popularis in both dark- and light-adapted states: (a) NaCl inhibited the maximal efficiency of PSII photochemistry (Fv/Fm) due to the salt-induced increase of Fo (the minimal fluorescence) and the marked decline of Fm (the maximal fluorescence); (b) salinity decreased coefficient of photochemical quenching (qP) but markedly elevated coefficient of nonphotochemical quenching (qN) in the light-adapted state. In contrast, there were no corresponding changes of chlorophyll a fluorescence in salinised P. euphratica. X-ray microanalysis results showed that salinity caused salt accumulation in the chloroplasts of P. popularis in which Na+ and Cl− increased up to 42 and 221 mmol dm−3, respectively. Great buildup of Na+ and Cl− in chloroplasts of P. popularis may exhibit direct and indirect restrictions on dark and light reactions. The activity of SOD isoenzymes (CuZn-SOD I and CuZn-SOD II) and POD isoenzymes in P. popularis decreased with increasing exposure period, and leaf malondialdehyde (MDA) content and membrane permeability (MP) increased correspondingly. In contrast to P. popularis, stressed P. euphratica maintained activity of SOD and POD isoenzymes and there was no significant increase of MDA and MP during the period of salt stress. In conclusion, P. euphratica plants exhibited a higher capacity to maintain the activity of anti-oxidant enzymes and restrict salt accumulation in the chloroplasts, the photosynthesis processes were less restricted consequently.





Similar content being viewed by others
References
Almansa MS, del Río LA, Alcaraz C, Sevilla F (1989) Isoenzyme pattern of superoxide dismutase in different varieties of Citrus plants. Physiol Plant 76:563–568
Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutases in controlling oxidative stress in plants. J Exp Bot 53(372):1331–1341
Asada K (1994) Production and action of active oxygen in photosynthetic tissues. In: Foyer CH, Mullineaux PM (eds) Causes of photooxidative stress and amelioration of defense system in plants. CRC, Boca Raton pp 77–104
Asada K, Takahashi M (1987) Production and scavenging of active oxygen in photosynthesis. In: Kyle DJ, Osmond CB, Arntzen CJ (eds) Photoinhibition. Elsevier, Amsterdam, pp 227–287
Badawi GH, Yamauchi Y, Shimada E, Sasaki R, Kawano N, Tanaka K (2004) Enhanced tolerance to salt stress and water deficit by overexpressing superoxide dismutase in tobacco (Nicotiana tabacum) chloroplasts. Plant Sci 166:919–928
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Bowler C, Van Montagu M, Inze D (1992) Superoxide dismutase and stress tolerance. Ann Rev Plant Physiol Plant Mol Biol 43:83–116
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Bridges SM, Salin ML (1981) Distribution of iron-containing superoxide dismutase in vascular plants. Plant Physiol 68:275–278
Chang Y, Chen S, Yin W, Wang R, Liu Y, Shi Y, Shen Y, Li Y, Jiang J, Liu Y (2006) Growth, gas exchange, ABA and CaM response to salt stress in three poplars. J Integr Plant Biol 48:286–293
Chen S (1991) Injury of membrane lipid peroxidation to plant cell. Plant Physiol Commun 27(2):84–90 (in Chinese)
Chen S, Li J, Wang S, Hüttermann A, Altman A (2001) Salt, nutrient uptake and transport, and ABA of Populus euphratica; a hybrid in response to increasing soil NaCl. Trees 15:186–194
Chen S, Li J, Wang T, Wang S, Polle A, Hüttermann A (2002a) Osmotic stress and ion-specific effects on xylem abscisic acid and the relevance to salinity tolerance in poplar. J Plant Growth Regul 21:224–233
Chen S, Li J, Fritz E, Wang S, Hüttermann A (2002b) Sodium and chloride distribution in roots and transport in three poplar genotypes under increasing NaCl stress. For Ecol Manage 168:217–230
Chen S, Li J, Wang S, Fritz E, Hüttermann A, Altman A (2003) Effects of NaCl on shoot growth, transpiration, ion compartmentation and transport in regenerated plants of Populus euphratica and Populus tomentosa. Can J For Res 33:967–975
Dhindsa RS, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalse. J Exp Bot 32:93–101
Elstner EF (1987) Metabolism of activated oxygen species. In: Davies DD (ed) The biochemistry of plants. Biochemistry of metabolism, vol 11. Academic, San Diego, pp 253–315
Flexas J, Bota1 J, Loreto F, Cornic G, Sharkey TD (2004) Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol 6:269–279
Flowers TJ, Yeo AR (1986) Ion relations of plants under drought and salinity. Aust J Plant Physiol 13:75–91
Fridovich I (1986) Superoxide dismutase. Adv Enzymol Relat Areas Mol Biol 58:61–97
Fritz E (1989) X-ray microanalysis of diffusible elements in plant cells after freeze-drying, pressure-infiltration with ether and embedding in plastic. Scan Microsc 3(2):517–526
Fritz E, Jentschke G (1994) Agar standard for quantitative X-ray microanalysis of resin-embedded plant tissues. J Microsc 174:47–50
Fung L, Wang S, Altman A, Hüttermann A (1998) Effect of NaCl on growth, photosynthesis, ion and water relations of four poplar genotypes. For Ecol Manage 107:135–146
Gómez JM, Hernández JA, Jiménez A, del Río LA, Sevilla F (1999) Differential response of antioxidative enzymes of chloroplasts and mitochondria to long-term NaCl stress of pea plants. Free Rad Res 31:S11–S18
Gosset DR, Banks SW, Millhollon EP, Lucas MC (1996) Antioxidant response to NaCl stress in a control and a NaCl-tolerant cotton cell line grown in the presence of paraquat, buthionine sulfoximine, and exogenous glutathione. Plant Physiol 112:803–809
Grassi G, Magnani F (2005) Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant Cell Environ 28:834–849
Greenway H, Munns R (1980) Mechanisms of salt tolerance in nonhalophytes. Ann Rev Plant Physiol 31:149–190
Hassan HM, Scandalios JM (1990) Superoxide dismutases in aerobic organisms. In: Alscher RG, Cumming JR (eds) Stress responses in plants: adaptation and acclimatation mechanisms. Wiley-Liss, New York, pp 175–199
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetigs and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hernández JA, Corpas FJ, Gómez M, del Río LA, Sevilla F (1993) Salt-induced oxidative stress mediated by activated oxygen species in pea leaf mitochondria. Physiol Plant 89:103–110
Hernández JA, Olmos E, Corpas FJ, Sevilla F, del Río LA (1995) Salt-induced oxidative stress in chloroplast of pea plants. Plant Sci 105:151–167
Hernández JA, Campillo A, Jiménez A, Alarcón JJ, Sevilla F (1999) Response of antioxidant systems and leaf water relations to NaCl stress in pea plants. New Phytol 141:241–251
Hernández JA, Jiménez A, Mullineaux P, Sevilla F (2000) Tolerance of pea (Pisum sativum L.) to long-term salt stress is associated with induction of antioxidant defenses. Plant Cell Environ 23:853–862
Ma H, Fung L, Wang S, Altman A, Hüttermann A (1997) Photosynthesis response of Populus euphratica to salt stress. For Ecol Manage 93:55–61
Oertli JJ (1968) Extracellular salt accumulation, a possible mechanism of salt injury in plants. Agrochim 12:461–469
Ottow EA, Brinker M, Teichmann T, Fritz E, Kaiser W, Brosché M, Kangasjärvi J, Jiang X, Polle A (2005a) Populus euphratica displays apoplastic sodium accumulation, osmotic adjustment by decreases in calcium and soluble carbohydrates, and develops leaf succulence under salt stress. Plant Physiol 139:1762–1772
Ottow EA, Polle A, Brosché M, Kangasjärvi J, Dibrov P, Zörb C, Teichmann T (2005b) Molecular characterization of PeNhaD1: the first member of the NhaD Na+/H+ antiporter family of plant origin. Plant Mol Biol 58:73–86
Ros Barceló A, Muñoz R, Sabater F (1987) Lupin peroxidases I. Isolation and characterization of cell wall-bound isoperoxidase activity. Physiol Plant 71:448–454
Savouré A, Thorin D, Davey M, Hua XJ, Mauro S, Van Montagu M, Inzé D, Verbruggen N (1999) NaCl and CuSO4 treatments trigger distinct oxidative defense mechanism in Nicotiana plumbaginifolia L. Plant Cell Environ 22:387–396
Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y, Yoshimura K (2002) Regulation and function of ascorbate peroxidase isoenzymes. J Exp Bot 53:1305–1319
Takahashi MA, Asada K (1983) Superoxide anion permeability of phospholipid membrane and chloroplast thylakoids. Arch Biochem Biophys 226:558–566
Tsugane K, Koboyashi K, Niwa Y, Ohba Y, Wada K, Kobayashi H (1999) A recessive Arabidopsis mutant that grows photoautotrophically under salt stress shows enhanced active oxygen detoxification. Plant Cell 11:1195–1206
Wang Y, Ying Y, Chen J, Wang X (2004) Transgenic Arabidopsis overexpressing Mn-SOD enhanced salt-tolerance. Plant Sci 167:671–677
Wang R, Chen S, Ma H, Liu L, Li H, Weng H, Hao Z, Yang S (2006) Genotypic differences in antioxidative stress and salt tolerance of three poplars under salt stress. Front For Chin 1:82–88
Acknowledgments
The research was supported jointly by Alexander von Humboldt-Stiftung/Foundation (Germany), German Science Foundation through Poplar Research Group Germany (PRG), the key project of National Natural Science Foundation of China (30430430), National Program for High Technology Research and Development (863) of China (No.2006AA10Z131), a Foundation for the Author of National Excellent Doctoral Dissertation of PR China (200152), and the Teaching and Research Award Program for Outstanding Young Teachers in Higher Education Institution of MOE, PRC (2002-323). We thank Dr. Andrea Olbrich for valuable help with operating the microscope, Haiyuan Ma, Liyuan Liu, He Li, Haijiao Weng, Zhiyong Hao, Shuang Yang, Shan Duan and Jie Jiang for assistance in greenhouse and laboratory.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Rennenberg.
Rights and permissions
About this article
Cite this article
Wang, R., Chen, S., Deng, L. et al. Leaf photosynthesis, fluorescence response to salinity and the relevance to chloroplast salt compartmentation and anti-oxidative stress in two poplars. Trees 21, 581–591 (2007). https://doi.org/10.1007/s00468-007-0154-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-007-0154-y