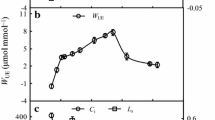
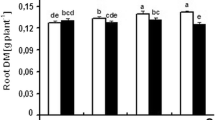
Abstract
Drought and high-temperature stresses have been extensively studied; however, little is known about their combined impact on plants. In the present study, we determined the photosynthetic gas exchange, chlorophyll fluorescence, nitrogen level, and lipid peroxidation of the leaves of a perennial grass (Leymus chinensis (Trin.) Tzvel.) subjected to three constant temperatures (23, 29 and 32°C), and five soil-moisture levels (75–80%, 60–65%, 50–55%, 35–40% and 25–30% of field capacity, respectively). High temperature significantly decreased plant biomass, leaf green area, leaf water potential, photosynthetic rate (A), maximal efficiency of PSII photochemistry (F v/F m), actual PSII efficiency (ΦPSII), the activities of nitrate reductase (NR; EC 1.6.6.1) and glutamine synthetase (GS; EC 6.3.1.2), but markedly increased the ratio of leaf area to leaf weight (SLA), endopeptidase (EP; EC 3.4.24.11) activity, and malondialdehyde (MDA) content, especially under severe water stress conditions. The A and F v/F m were significantly and positively correlated with leaf-soluble protein content, and the activities of NR and GS. However, both photosynthesis parameters were significantly and negatively correlated with EP activity and MDA content (P < 0.05). It is suggested that high temperature, combined with severe soil drought, might reduce the function of PSII, weaken nitrogen anabolism, strengthen protein catabolism, and provoke lipid peroxidation. The results also indicate that severe water stress might exacerbate the adverse effects of high temperature, and their combination might reduce the plant productivity and distribution range of L. chinensis in the future.





Similar content being viewed by others
Abbreviations
- EP:
-
Endopeptidase
- FAA:
-
Free amino acid
- FC:
-
Soil water content at field capacity
- Fv/Fm:
-
Maximal efficiency of PSII photochemistry
- GS:
-
Glutamine synthetase
- g s :
-
Stomatal conductance
- MDA:
-
Malondialdehyde
- NR:
-
Nitrate reductase
- ΦPSII :
-
Actual PSII efficiency
- PSII:
-
Photosystem II
- SRWC:
-
Soil relative water content
- SLA:
-
Specific leaf area
- ψleaf :
-
Leaf water potential
References
Alvim FA, Carolina SMB, Cascardo JCM, Nunnes CC, Martinez CA, Otoni WC, Fontes PB (2001) Enhanced accumulation of BiP in transgenic plant confers tolerance to water stress. Plant Physiol 126:1042–1054
Bai Y, Han X, Wu J, Chen Z, Li L (2004) Ecosystem stability and compensatory effects in the Inner Mongolia grassland. Nature 431:181–184
Baki GKA-E, Siefritz F, Man H-M, Weiner H, Kaldenhoff R, Kaiser WM (2000) Nitrate reductase in Zea mays L. under salinity. Plant Cell Environ 23:515–521
Behera SK, Nayak L, Biswal B (2003) Senescing leaves possess potential for stress adaptation: the developing leaves acclimated to high light exhibit increased to tolerance to osmotic stress during senescence. J Plant Physiol 160:125–131
Bradford MM (1976) A rapid sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cakmak I, Horst WJ (1991) Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase and peroxidase activities in root tips of soybean (Glycine max). Plant Physiol 83:463–468
Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought-from genes to the whole plant. Funct Plant Biol 30:239–264
Cruz LJ, Cagampang GB, Juliano BO (1970) Biochemical factors affecting protein accumulation in the rice grain. Plant Physiol 46:743–747
Faria T, Silvério D, Breia E, Cabral R, Abadía A, Abadía J, Pereira JS, Chaves MM (1998) Differences in the response of carbon assimilation to summer stress (water deficit, high light and temperature) in four Mediterranean tree species. Physiol Plant 102:419–428
Hadži-Tašković Šukalivić V (1986) Activity and distribution of nitrogen-metabolism enzymes in the developing maze kernel. Physiol Plant 67:247–252
Hamerlynck EP, Huxman TE, Loik ME, Smith SD (2000) Effects of extreme high temperature, drought and elevated CO2 on photosynthesis of Mojave Desert evergreen shrub, Larrea dridentata. Plant Ecol 148:183–193
Havaux M (1992) Stress tolerance of photosystem II in vivo: antagonistic effects of water, heat and photoinhibition stress. Plant Physiol 100:424–432
Hernández JA, Almansa MS (2002) Short-term effects of salt stress on antioxidant systems and leaf water relations of leaves. Physiol Plant 115:251–257
Jiang CZ, Ishihara K, Satoh K, Katoh S (1999) Loss of the photosynthetic capacity and protein in senescence leaves at positions of two cultivars of rice in relation to source capacity of the leaves for carbon and nitrogen. Plant Cell Physiol 40:496–503
Jiang Y, Huang B (2001) Drought and heat stress injury to cool season turfgrasses in relation to antioxidant metabolism and lipid peroxidaion. Crop Sci 41:436–442
Keles Y, Öncel I (2002) Response of antioxidative defence system to temperature and water stress combinations in wheat seedlings. Plant Sci 163:783–790
Lam H-M, Coschigano KT, Oliveira IC, Melo-Oliveira R, Coruzzi GM (1996) The molecular-genetics of nitrogen assimilation into amino acids in higher plants. Annu Rev Plant Physiol Plant Mol Biol 47:569–593
Llorens L, Peñuelas J, Estiarte M (2003) Ecophysiological responses of two Mediterranean shrubs, Erica multiflora and Globularia alypum, to experimentally drier and warmer conditions. Physiol Plant 119:231–243
Lu C, Zhang J (1999) Effects of water stress on photosystem II photochemistry and its thermostability in wheat plants. J Exp Bot 50:1139–1206
Mae T (1997) Physiological nitrogen efficiency in rice: nitrogen utilization, photosynthesis, and yield potential. Plant Soil 196:201–210
Maki H, Morohashi Y (2002) Inhibitory effect of polyamines on the activity of endopeptidase in mung bean cotyledons. J Plant Physiol 159:1341–1347
McDonald GK, Paulsen GM (1997) High temperature effects on photosynthesis and water relations of grain legumes. Plant Soil 196:47–58
Monterio de Paula F, Pham Thi AT, Vieira de Silva J, Justin AM, Demandre C (1990) Effects of water stress on the molecular species composition of polar lipid from Vigna unguiculata L. leaves. Plant Sci 66:185–193
Moore S (1970) Amino acid analysis: aqueous dimethylsulfoxide as solvent for the ninhydrin reaction. J Biol Chem 243:6281–6283
Morgan JM (1984) Osmoregulation and water stress in higher plant. Annu Rev Plant Physiol 35:299–319
Munné-Bosch S, Jubany-Mari T, Alegre L (2003) Enhanced photo- and antioxidative protection, and hydrogen peroxide accumulation in drought-stressed Cistus clusii and Cistus albidus plants. Tree Physiol 23:1–12
Niinemets Ü, Kull K (2003) Leaf structure vs. nutrient relationships vary with soil conditions in temperate shrubs and trees. Acta Oecologica 24:209–219
Onoda Y, Hikosaka K, Hirose T (2004) Allocation of nitrogen to cell walls decreases photosynthetic nitrogen-use efficiency. Funct Ecol 18:419–425
Pacifici RE, Davies KJA (1990) Protein degradation as an index of oxidative stress. Method Enzym 186:485–502
Rizhsky L, Liang HJ, Shuman J, Shulaev V, Davletova S, Mittler R (2004) When Defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol 134:1683–1696
Saccardy K, Pineau B, Roche O, Cornic G (1998) Photochemical efficiency of Photosystem II and xanthophyll cycle components in Zea mays leaves exposed to water stress and high light. Photosynth Res 56:57–66
Sack L, Grubb PJ (2002) The combined impacts of deep shade and drought on the growth and biomass allocation of shade-tolerant woody seedlings. Oecologia 131:175–185
Shah NH, Paulsen GM (2003) Interaction of drought and high temperature on photosynthesis and grain-filling of wheat. Plant Soil 257:219–226
Sharkey TD (2005) Effects of moderate heat stress on photosynthesis: importance of thylakoid reactions, rubisco deactivation, reactive oxygen species, and thermotolerance provided by isoprene. Plant Cell Environ 28:269–277
Sibout R, Guerrier G (1998) Solute incompatibility with glutamine synthetase in water-stressed Populus nigra. Environ Exp Bot 40:173–178
Sicher RC, Bunce JA (1997) Relationship of photosynthetic acclimation to changes of Rubisco activity in field-grown winter wheat and barley during growth in elevated carbon dioxide. Photosynth Res 52:27–38
Sinclair TR, Pinter PJ, Kimball BA, Adamsen FJ, LaMorte RL, Wall GW, Hunsaker DJ, Adam N, Brook TJ, Garcia RL, Thompson T, Leavitt S, Mattias A (2000) Leaf nitrogen concentration of wheat subjected to elevated [CO2] and either water or N deficits. Agr Ecosyst Environ 79:53–60
Srivali B, Renu KC (1998) Drought-induced enhancement of protease activity during monocarpic senescence in wheat. Curr Sci 75:1174–1176
Tambussi EA, Nogués S (2005) Ear of durum wheat under water stress: water relations and photosynthetic metabolism. Planta 221:446–458
van Kooten O, Snel JFH (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res 25:147–150
von Caemmerer S, Lawson T, Oxborough K, Baker NR, Andrews TJ, Raines CA (2004) Stomatal conductance does not correlate with photosynthetic capacity in transgenic tobacco with reduced amounts of Rubisco. J Exp Bot 55:1157–1166
Wang W, Vinocur B, Altman A (2003) Plant response to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218:1–14
Wen X, Qiu N, Lu Q, Lu C. (2005) Enhanced thermotolerance of photosystem II in salted-adapted plants of the halophyte Artemisia anethifolia. Planta 220:486–497
Wigley TML, Raper SCB (2001) Interpretation of high projections for global-mean warming. Science 293:451–454
Wittenbach VA (1979) Ribulose bisphosphate carboxylase and proteolytic activity in wheat leaves from anthesis through senescence. Plant Physiol 64:884–887
Wollenweber B, Porter JR, Schellberg J (2003) Lack of interaction between extreme high-temperature events at vegetative and reproductive stages in wheat. J Agr Crop Sci 189:142–150
Xu ZZ, Zhou GS (2005a) Effects of water stress and high nocturnal temperature on photosynthesis and nitrogen level of a perennial grass Leymus chinensis. Plant Soil 269:131–139
Xu ZZ, Zhou GS (2005b) Effects of water stress and nocturnal temperature on carbon allocation in the perennial grass, Leymus chinensis. Physiol Plant 123:272–280
Acknowledgments
The study was supported by the Key Project of Chinese Academy of Sciences (Grant No. KSCX2-SW-133) and the National Natural Science Foundation of China (Grant No. 40231018; 30470338). We thank Bai Li-Ping, Chi Hong-Kang, Jia Bing-Rui, Jiang Yan-Ling, Song Jian, Wang Feng-Yu, Wang Yu-Hui, and Yuan Wen-Ping for their useful help during the experiment. The authors also thank the reviewers for their constructive comments and detailed corrections.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, Z.Z., Zhou, G.S. Combined effects of water stress and high temperature on photosynthesis, nitrogen metabolism and lipid peroxidation of a perennial grass Leymus chinensis . Planta 224, 1080–1090 (2006). https://doi.org/10.1007/s00425-006-0281-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-006-0281-5