Abstract
Objectives
Bevacizumab has been approved by the US Food and Drug Administration as a first-line therapy for metastatic non-small-cell lung cancer (NSCLC), in combination with carboplatin and paclitaxel. A single Latin American center experience was reviewed to determine the safety and efficacy of adding bevacizumab to first-line chemotherapy in a local population.
Methods
We retrospectively identified patients with non-squamous NSCLC treated with bevacizumab plus chemotherapy combinations as first-line chemotherapy between July 1, 2006, and January 30, 2011, at Sirio Libanes Hospital in Sao Paulo, Brazil. We collected data on patient characteristics, treatment combinations, toxicities, response to treatment, and survival. Overall survival (OS) and progression-free survival (PFS) were calculated by Kaplan-Meier analysis, and prognostic factors were identified by the Cox regression model.
Results
A total of 56 patients were included in the final analysis (median age 62.4 years; 70% male). In 35 patients (62.5%), bevacizumab was combined with carboplatin and paclitaxel, and in 16 patients (28.6%), it was combined with pemetrexed and carboplatin. The response rate evaluated by the reference clinical team reached 74.5%, the median PFS was 5.3 months, and the median OS was 14.8 months. In multivariate analysis, use of maintenance therapy was the only predictive factor for OS (hazard ratio 6.85, 95% confidence interval 2.94–15.22). No treatment-related deaths were identified, and the overall incidence of grade 3–4 non-hematologic toxicities was 16%.
Conclusion
Our results confirm the efficacy and safety data of bevacizumab first-line combinations for NSCLC in a Brazilian population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the leading cause of cancer mortality worldwide, being responsible for almost 1 million deaths per year.[1] Lung cancer death rates are decreasing in most developed countries, where tobacco consumption is losing its importance. In contrast, lung cancer rates and mortality are increasing in developing countries, including many examples in Latin America.[2] In Brazil, 27 320 new cases of lung cancer are estimated for the year 2012, most of which will be diagnosed at advanced stages.[3] Non-small-cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancers and, despite recent advances in its treatment, this subtype is still a significant contributor to the burden of lung cancer in the world.
Management of metastatic lung cancer involves palliation of symptoms and prolongation of survival with systemic treatment. Platinum-based doublet chemotherapies are still the standard first-line treatment for patients not harboring an activating mutation, who may benefit from first-line target therapy such as erlotinib, geftinib, and crizotinib. Addition of bevacizumab to the platinum-based backbone has demonstrated efficacy in two randomized phase III trials,[4,5] leading to US Food and Drug Administration approval of this agent as a first-line therapy for non-squamous NSCLC.[6]
In the Eastern Cooperative Oncology Group (ECOG) 4599 trial,[7] bevacizumab added to carboplatin and paclitaxel improved overall survival (OS) and progression-free survival (PFS) compared with the platinum doublet alone in 878 patients with advanced non-squamous NSCLC. The hazard ratios (HRs) for PFS and OS were 0.66 (95% confidence interval [CI] 0.57–0.77, p < 0.001) and 0.79 (95% CI 0.67–0.92, p = 0.003) respectively, in favor of treatment with bevacizumab. The median OS improved from 10.3 months to 12.3 months and response rates increased from 15% to 36% with the addition of bevacizumab. Furthermore, in a subset analysis of patients with adenocarcinoma histology, bevacizumab-based therapy improved the median OS from 10.3 months to 14.2 months.
The AVAiL (Avastin in Lung) trial[5] evaluated the efficacy of two doses of bevacizumab (7.5 mg/kg and 10 mg/kg) or placebo added to a 3-week schedule of cisplatin and gemcitabine. PFS (the primary endpoint of this study) was significantly improved with bevacizumab-based therapy versus the placebo combination (bevacizumab 7.5 mg/kg: HR 0.75, p = 0.003; bevacizumab 15 mg/kg: HR 0.82, p = 0.03). Although the median OS in the AVAiL trial exceeded 13 months in both bevacizumab treatment arms, the PFS benefit seen with bevacizumab therapy did not translate into a statistically significant OS benefit. Both phase III trials[4,5] reported safety profiles for the addition of bevacizumab to chemotherapy, with a mild increase in some toxicities related to bevacizumab, such as hypertension, proteinuria, and bleeding events.
Additionally, a phase IV single-arm study, the SAiL (Safety of Avastin in Lung Cancer) trial,[8] evaluated the safety of first-line bevacizumab together with chemotherapy in a broad patient population. Efficacy data from this study showed a median OS of 14.6 months (95% CI 13.8–15.3) and a median time to tumor progression of 7.8 months (95% CI 7.5–8.1). The disease control rate in patients with post-baseline evaluation was 89%. The incidence of clinically significant (grade ≥3) adverse events of special interest (AESIs) was relatively low, and no new safety signals were reported. Phase IV studies offer the opportunity to mirror usual clinical practice, outside the limited populations and restrictions of phase III trials.
In these pivotal trials of bevacizumab as first-line therapy in metastatic NSCLC, most of the patients included in the analysis were of White (Caucasian) background. In the AVAiL trial,[5] only 5% of patients included in each arm were from Central/South America. Although the SAiL trial[8] intended to describe a broad population, subjects from Hispanic and African American backgrounds represented only 4% of the total population. Despite the approval of bevacizumab for treatment of NSCLC in most countries in Latin America, local experiences of efficacy and safety are diluted in the multitude of data from these studies. The recent publication of the Brazilian experience with breast cancer showed that outcomes in cancer treatment might differ from those reported in developed countries, and heterogeneity in chemotherapy use is among the reasons that could explain this fact.[9]
Considering that lung cancer incidence and mortality continue to increase in Brazil, and that outcomes from use of an agent may differ between populations because of pharmacogenomics and particular clinical practice, analysis of regional experience might be essential for improvement of local oncologic practice. Therefore, we undertook this retrospective review to determine the efficacy and safety of adding bevacizumab to first-line chemotherapy for non-squamous NSCLC in a Brazilian population.
Methods
Patients and Data Collection
We identified all patients from the Sirio Libanes pharmacy registry (Sao Paulo, Brazil) who were treated for NSCLC with bevacizumab between July 2006 and January 2011. In total, 110 patients were identified, and 56 patients who met the following criteria were included in this report: patients were required to have non-squamous NSCLC tumor histology; to have received at least one cycle of first-line chemotherapy with addition of bevacizumab; and to have good quality follow-up data in their medical records, defined as the presence of a clinical description of toxicities that allowed for grading of adverse events according to the US National Cancer Institute’s Common Terminology Criteria for Adverse Events v3.0 (CTCAE),[10] and adequate registration of laboratory and image data. Patients with more than one primary tumor were excluded from the report. Few patients with locally advanced disease undergoing neoadjuvant treatment with bevacizumab were allowed; these patients were only included in the primary response data if their initial clinical stage was IIIB, since this stage was included in the primary response data from large trials. All patients who received bevacizumab prior to a local procedure were excluded from the analysis of PFS and OS. One patient with early-stage NSCLC also received bevacizumab and was included only in the safety analysis.
Patient medical records were reviewed for information regarding demographic data, tumor characteristics, treatment types, treatment responses, and survival. Because of the long period covered by the study and because not all radiologic images were available for our review—some images being from other health institutions—the response evaluation was based on the treating physician’s response assessment and not on the Response Evaluation Criteria in Solid Tumors (RECIST). The tumor stage was determined according to the Seventh Edition of the American Joint Committee on Cancer staging system.[11] All toxicity events were classified according to the CTCAE.[10] All data on adverse events were obtained for up to 28 days after the last bevacizumab infusion, and AESIs were reviewed throughout the entire available follow-up.
Statistical Analysis
We created descriptive summaries for each demographic and clinical variable. The following variables were examined in univariate and multivariate analyses of OS and PFS: age, sex, performance status according to the ECOG scale, smoking status, number of metastatic sites, type of platinum-based chemotherapy backbone, and use of maintenance chemotherapy. Any systemic treatment beyond the planned chemotherapy with platinum was considered to be maintenance therapy, including bevacizumab alone. The Fisher exact test was used to assess the independence between two categoric variables. Survival curves were calculated from the start of chemotherapy, using the Kaplan–Meier method. The two-sided log-rank test was used to test the association between variables and OS and PFS. In the multivariate analysis, a Cox proportional hazard model was used to assess the simultaneous effect of ≥2 variables on OS and PFS. To obtain the best subset of variables in the final model, we performed stepwise model selection. p-Values were derived from two-sided tests, and statistical analyses were carried out using SPSS version 17.0 software (IBM Corporation, Somers, NY, USA).
Results
Patient Characteristics
A total of 110 patients were initially identified from our pharmacy registry as receiving bevacizumab for treatment of lung cancer (figure 1). Thirty-four patients were excluded at the outset because they did not receive bevacizumab as first-line treatment (n = 30) or did not actually initiate the drug (n = 4). Subsequently, a total of 76 patients were selected for careful medical record review. After exclusion of patients with insufficient follow-up data (n = 14) and histologies not classified as non-squamous NSCLC (n = 6), 56 patients were included in our analysis.
Clinical characteristics of the 56 patients who met the inclusion criteria of our study are shown in table I. The median age of the patients was 62.4 years, and the majority were male (69.6%) and former smokers (66.1%). Adenocarcinoma was the most frequent histology among the patients (71.4%). The epidermal growth factor receptor (EGFR) mutation status was unknown for the majority of the patients (91%). In the 51 patients (91.1%) with stage IV disease, the most common metastatic sites were bones (37.5%), pleura (23.2%), the central nervous system (CNS), and lymph nodes (21.4% each).
Treatment Data
Treatment characteristics are summarized in table II. The median number of bevacizumab plus chemotherapy cycles received by the patients was six. Carboplatin and paclitaxel were associated with bevacizumab in 62.5% of patients, while the second choice was carboplatin and pemetrexed in 28.6% of patients. All patients selected for this study received bevacizumab at a dose of 15 mg/kg every 3 weeks. Most patients (57.1%) were started on a maintenance protocol, and the median number of treatment cycles during that phase was 7.5. Among these patients, 25% received bevacizumab and chemotherapy as maintenance therapy (in all cases, pemetrexed was the chemotherapy of choice) and the remainder received bevacizumab as a single agent.
Efficacy Analysis
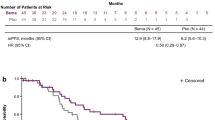
The median follow-up period for the entire cohort was 14.3 months. For the 52 patients who were included in the survival analysis, the median OS was 14.7 months (95% CI 11.5–18) and the median PFS was 5.4 months (95% CI 3.9–6.8). Kaplan–Meier curves for OS and PFS are presented in figure 2.
The overall response rate for the 56 patients was 74.5%, with 37 partial responses (67.2%) and four complete responses (7.2%). One of the complete responses occurred in a patient with locally advanced disease who was referred for surgical resection after the end of treatment, and a pathologically complete response was documented.
Patients who were able to reach the maintenance phase received the greatest survival benefit in our analysis. In this group, the median OS was 22.8 months (95% CI 12.4–33.1). In patients progressing before the opportunity to initiate the maintenance phase, the median OS was remarkably shorter (8.1 months, 95% CI 6.8–9.4).
There was a notable trend toward longer OS in female patients (22.76 months) than in male patients (13.42 months), but the difference did not reach statistical significance (p = 0.22). We also observed a trend toward a longer median OS in patients younger than 63 years (18.5 months) than in older patients (12.4 months), with a p-value of 0.15. Patients with three or more metastatic sites had significantly shorter PFS (3.1 months, 95% CI 1.9–4.4) than patients with fewer than three metastatic sites (6.4 months, 95% CI 4.5–8.7 months), with a p-value of 0.031.
Regarding the chemotherapy backbone associated with bevacizumab, there was no statistical difference in survival outcomes. Patients who received carboplatin and paclitaxel had a median OS of 14.5 months (95% CI 11.4–17.6) and a median PFS of 5.5 months (95% CI 4.1–6.9), while patients receiving carboplatin and pemetrexed had a median OS of 15.4 months (95% CI 8.6–22.11) and a median PFS of 5.4 months (95% CI 4.0–10.35), respectively. Performance status and smoking history also did not influence survival outcomes in our analysis.
Table III shows the results of the multivariate analyses of potential predictors of OS. Initiation of maintenance therapy and female sex were both predictors of longer OS. Although younger age (≤63 years) also tended to predict a better OS outcome, the results were not statistically significant.
Safety Analysis
The most common clinical adverse event observed was fatigue, reported in 31% of patients and classified as grade 3 or higher in seven patients (12.5%). Neutropenia grade 3 or higher was observed in 13 patients (23.2%), but only five patients (9%) developed an episode of neutropenic fever (table IV). A total of 24 patients had an AESI of any grade related to bevacizumab treatment (e.g. hypertension, bleeding, proteinuria, thrombotic events). The most common AESI was hypertension, exhibited by nine patients (16.1%), while the most common AESI of grade 3 or higher was thrombosis. We observed venous thrombosis in three patients (5.4%) and arterial thrombosis in two patients (3.6%). Of those two cases of arterial thrombosis, only one was definitely related to bevacizumab, and it occurred in a cardiac vessel, leading to discontinuation of the treatment by the responsible physician. Although 21% of patients in our series had CNS metastases, none of them had intracranial bleeding during treatment with bevacizumab. The majority of these patients (83%) had their brain lesions treated before initiation of chemotherapy. The only patient who developed CNS bleeding did not have CNS metastases, and this adverse event could be attributed to bevacizumab. No treatment-related deaths were documented in this analysis.
Adverse events observed according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events v3.0 (CTCAE)[10]
Discussion
In this study, we found that the addition of bevacizumab to standard platinum-based chemotherapy for first-line treatment of metastatic non-squamous NSCLC in a Brazilian subset of patients had efficacy similar to that reported in pivotal trials of bevacizumab combinations. In those trials, few patients from Latin America were recruited and, to our knowledge, this is the largest report of first-line bevacizumab in a lung cancer population from this continent.
We observed a median PFS of 5.4 months (compared with 6.2 and 6.5 months in the E4599[4] and AVAiL[5] trials, respectively) and a median OS of 14.7 months (compared with 12.3 and >13 months in E4599 and AVAiL, respectively). In fact, the better comparator for our reported outcomes is the results of the SAiL phase IV trial,[8] since they reflect the experience of community practice similar to that presented here, outside the rigidity of a phase III trial protocol. In the SAiL trial, the median OS was 14.6 months, which is very similar to our finding, and the time to tumor progression, which is usually longer than the PFS, was 7.8 months.
Of note, the response rate observed in our study was higher than those in the phase III trials[4,5] discussed above. Of the patients in our series, 74.5% had some response, compared with 35% and 30.4% in E4599[4] and AVAiL,[5] respectively. One of the hypotheses for this difference is that responses were measured by the RECIST criteria in the phase III trials, whereas in our study, tumor assessments were carried out according to the treating physician’s clinical practice. No specific requirements for assessment or confirmation of responses were implemented, which might have yielded higher responses rates in our study. The fact that the PFS in our study was much closer to those in the pivotal trials suggests that some responses captured in this study were temporary and did not impact final outcomes. In the SAiL trial,[8] responses were likewise not measured using the RECIST guidelines, and response rates were also higher than in previous reports (the response rate in the SAiL trial was 51%). One particularly interesting finding in our study was that one patient who was presented as a complete response had a large lesion not initially considered for surgical resection, which developed into a cavitary lesion after four cycles of platinum chemotherapy plus bevacizumab. Complete surgical resection was possible, and no residual tumor was detected in the pathology report, suggesting that bevacizumab can be considered as a neoadjuvant treatment in some situations.[12]
Regarding identification of the best platinum doublet to use in association with bevacizumab, our study did not favor any specific regimen. The most frequently used backbones were carboplatin plus paclitaxel or carboplatin plus pemetrexed. Interesting, the frequency of the association of carboplatin and paclitaxel reported in our study (62.5%) is very similar to the phase IV experience reported in the ARIES (Avastin Regimens: Investigation of Treatment Effects and Safety) study,[13] in which 64% of patients received carboplatin plus paclitaxel as the regimen of choice. A phase III trial[14] showed that pemetrexed added to cisplatin provided better outcomes in non-squamous NSCLC than gemcitabine/cisplatin. Although bevacizumab was approved for use in combination with carboplatin and paclitaxel,[6] this antibody is frequently added to other chemotherapy combinations. A promising combination is that of bevacizumab with pemetrexed and carboplatin,[15] and new data are expected in the near future for this combination. This retrospective review does not suggest a preferred regimen with which to combine bevacizumab, and future results of new phase III trials are needed to address this question.
The factors associated with improved OS on multivariate analysis were use of maintenance therapy and female sex. Subgroup analysis of the AVAiL trial also showed a better prognosis for female patients exposed to bevacizumab, but the E4599 study suggested the opposite, i.e. better outcomes for male patients than for female patients (HR for OS 0.70 [95% CI 0.57–0.87] vs 0.98 [95% CI 0.77–1.25], respectively). In the analysis reported herein, the median age of the sample used for OS estimation was adopted as a marker of age division (specifically, 62.9 years). This does not mean that we classified patients above that age as elderly; rather, we explored differences in outcomes comparing both percentiles of age distribution. In this analysis, we were not able to detect any influence of age on survival outcomes.
With respect to the maintenance therapy advantage, patients who were able to initiate this phase were nonprogressors, and it was expected that they would have better survival than patients not receiving maintenance therapy, considering that the majority of patients did not initiate maintenance therapy because of tumor progression. Although our analysis did not compare use of maintenance therapy between nonprogressor patients to better analyze the value of this treatment, the median OS of these patients reported here (22.7 months) suggests that this strategy can offer an extended period of disease control for these patients, as has previously been demonstrated by phase III trials.[16,17] Given the limitations of our analysis, we cannot conclude that the use of maintenance therapy was responsible for greater OS in our series of patients entering the maintenance phase. In addition, because of the limitations of our sample size, we combined patients receiving bevacizumab as a single agent and those receiving it in combination with pemetrexed as maintenance therapy, which precludes any suggestion regarding specific regimens.
Our safety results did not reveal any new safety signal, and the outcomes were consistent with those reported previously. The frequency of hypertension, which was the most frequent AESI, can be considered lower than those reported in the literature, considering both all-grade and high-grade events.[18] Arterial and venous thromboembolic events were the most frequent high-grade AESIs. According to meta-analyses, the overall incidence of arterial events during bevacizumab treatment is 2.6%[19] and that of high-grade venous thromboembolic episodes is 6.3%;[20] both are similar to our findings. Although the incidence of high-grade neutropenia in our study was higher than that in the SAiL trial[8] (23.2% vs 6%), a meta-analysis of hematologic toxicities related to bevacizumab supports our results.[21] No patients included in our analysis received primary prophylaxis with myeloid growth factors, and some of them had complete blood counts performed during the second week of treatment even if they were asymptomatic. Both facts may explain the frequency of neutropenia we observed. There were few episodes of neutropenic fever in our series, suggesting that use of primary prophylaxis with granulocyte colony-stimulating factor might not be indicated.
Of note, no patient with CNS metastases in our series presented with CNS bleeding during treatment with bevacizumab. This was also shown by the phase IV ARIES study,[13] in which 101 patients with brain lesions were treated with bevacizumab. Our results reinforce the observation of the European Medicines Agency that patients with brain metastasis can receive bevacizumab safely.[22]
Although they present inherent limitations, analyses of different ethnicities are important, since they can suggest particular responses depending on the genetic background. For example, a subgroup analysis of Asian patients from the AVAiL trial showed an OS benefit that was not present in the main population,[23] reinforcing the assumption that Asian ethnicity can be a positive predictor of increased OS in NSCLC.[24] Since our population was constituted of mixed ethnicity, including individuals of Caucasian, Asian, and Black origin, we cannot assume that there was any influence of genetic constitution on our results.
Despite being the biggest reported clinical experience with bevacizumab in Brazil, this study has several limitations. First, our results are based on a retrospective chart review in which the possibility of underreporting adverse events was real, although most of the clinically significant toxicities were expected to be captured. Second, 14 patients had insufficient follow-up data and were excluded from the analysis, which could have led to a selection bias favoring better results and less toxicity. Third, as previously discussed, response rates were not evaluated by objective criteria and a radiologic review of the images was not performed, so higher response rates than expected could have been reported. Finally, our sample size was not sufficient to permit conclusions about subgroup analysis.
The data reported herein may not provide great novelty for the management of lung cancer worldwide, although patients from South America have not been adequately represented in phase III trials[4,5] and the phase IV trial[8] of bevacizumab. However, our study does provide important data for the oncology community in South America, since it describes an effort by a cancer center in a developing country to share its clinical experience and the encouraging results obtained when advances in oncology are incorporated into routine clinical practice. We attempted to incorporate the experience from other centers in Brazil into our study in order to create a multi-institutional experience, but unfortunately this was not possible. We believe that publications such as this one may encourage other centers to continue monitoring their outcomes and to begin sharing their clinical information with the whole community as well.
Conclusion
Our results confirm efficacy and safety data regarding the addition of bevacizumab to first-line chemotherapy for non-squamous NSCLC reported in major trials, and emphasize that this may be a valid option for such patients in Latin America.
References
Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010; 60: 277–300
Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90
Instituto Nacional de Câncer (INCA). Estimativa 2012: incidência de câncer no Brasil [online]. Available from URL: http://www.inca.gov.br/estimativa/2012/tabelaestados.asp?UF=BR [Accessed 2011 Nov 20]
Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006; 355: 2542–50
Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol 2009; 27: 1227–34
Cohen MH, Gootenberg J, Keegan P, et al. FDA drug approval summary: bevacizumab (Avastin) plus carboplatin and paclitaxel as first-line treatment of advanced/metastatic recurrent nonsquamous non-small cell lung cancer. Oncologist 2007; 12: 713–8
Sandler A, Yi J, Dahlberg S, et al. Treatment outcomes by tumor histology in Eastern Cooperative Group Study E4599 of bevacizumab with paclitaxel/carboplatin for advanced non-small cell lung cancer. J Thorac Oncol 2010; 5: 1416–23
Crino L, Dansin E, Garrido P, et al. Safety and efficacy of first-line bevacizumab-based therapy in advanced non-squamous non-small-cell lung cancer (SAiL, MO19390): a phase 4 study. Lancet Oncol 2010; 11: 733–40
Lee BL, Liedke PE, Barrios CH, et al. Breast cancer in Brazil: present status and future goals. Lancet Oncol 2012; 13: e95–102
Cancer Therapy Evaluation Program [CTEP], National Cancer Institute. Common terminology criteria for adverse events v3.0 (CTCAE). Bethesda (MD): CTEP, 2006 Aug 9 [online]. Available from URL: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf [Accessed 2012 Nov 14]
American Joint Committee On Cancer (AJCO). Lung cancer. In: Edge SB, Byrd DR, Compton CC, et al. AJCC cancer staging manual. 7th ed. New York: Springer, 2010: 479
Bertino E, Villalona-Calero MA, Ross P, et al. Preoperative bevacizumab in combination with paclitaxel and carboplatin in surgically resectable non-small cell lung cancer. Ann Thorac Surg 2011; 91: 640
Fischbach NA, Spigel D, Brahmer J, et al. Preliminary safety and effectiveness of bevacizumab (BV) based treatment in subpopulations of patients (pts) with non-small cell lung cancer (NSCLC) from the ARIES study: a bevacizumab (BV) treatment observational cohort study (OCS) [abstract]. J Clin Oncol 2009; 27(15s): abstract no. 8040 [online]. Available from URL: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=65&abstractID=30542 [Accessed 2012 Nov 14]
Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008; 26: 3543–51
Patel JD, Hensing TA, Rademaker A, et al. Phase II study of pemetrexed and carboplatin plus bevacizumab with maintenance pemetrexed and bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer. J Clin Oncol 2009; 27: 3284–9
Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet 2009; 374: 1432–40
Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010; 11: 521–9
Ranpura V, Pulipati B, Chu D, et al. Increased risk of high-grade hypertension with bevacizumab in cancer patients: a meta-analysis. Am J Hypertens 2010; 23: 460–8
Schutz FA, Je Y, Azzi GR, et al. Bevacizumab increases the risk of arterial ischemia: a large study in cancer patients with a focus on different subgroup outcomes. Ann Oncol 2011; 22: 1404–12
Nalluri SR, Chu D, Keresztes R, et al. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA 2008; 300: 2277–85
Schutz FA, Jardim DL, Je Y, et al. Haematologic toxicities associated with the addition of bevacizumab in cancer patients. Eur J Cancer 2011; 47: 1161–74
European Medicines Agency Committee for Medicinal Products for Human Use. Post-authorisation summary of positive opinion for Avastin [online]. Available from URL: http://www.emea.europa.eu/docs/en_GB/document_library/Summary_of_opinion/human/000582/WC500059419.pdf [Accessed 2012 Nov 20]
Mok TS, Hsia TC, Tsai CM, et al. Efficacy of bevacizumab with cisplatin and gemcitabine in Asian patients with advanced or recurrent non-squamous non-small cell lung cancer who have not received prior chemotherapy: a substudy of the Avastin in Lung trial. Asia Pac J Clin Oncol 2011; 7 Suppl. 2: 4–12
Ou SH, Ziogas A, Zell JA. Asian ethnicity is a favorable prognostic factor for overall survival in non-small cell lung cancer (NSCLC) and is independent of smoking status. J Thorac Oncol 2009; 4: 1083–93
Acknowledgments
No sources of funding were used to conduct this study or to prepare this manuscript. The authors have no conflicts of interest that are directly relevant to the content of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Jardim, D.L.F., de Melo Gagliato, D., Ribeiro, K.B. et al. Bevacizumab as First-Line Therapy in Advanced Non-Small-Cell Lung Cancer. Drugs R D 12, 207–216 (2012). https://doi.org/10.2165/11636760-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11636760-000000000-00000