Abstract
Background
In the FIGHT study (NCT03694522) bemarituzumab, a humanized monoclonal antibody selective for fibroblast growth factor receptor 2b (FGFR2b), plus mFOLFOX6 showed clinically meaningful efficacy in patients with FGFR2b-positive (2+/3+ membranous staining by immunohistochemistry) locally advanced unresectable/metastatic gastric/gastroesophageal cancer (G/GEJC). A meaningful proportion of patients in FIGHT were enrolled in East Asia, reflecting global epidemiology of G/GEJC.
Methods
This subgroup analysis of the global, phase 2, double-blind FIGHT study included all patients enrolled in East Asian sites. Patients were randomized 1:1 to bemarituzumab-mFOLFOX6 (15 mg/kg and one 7.5 mg/kg dose on cycle 1, day 8) or matching placebo-mFOLFOX6. The primary endpoint was investigator-assessed progression-free survival (PFS). Secondary endpoints included overall survival (OS), objective response rate, and safety. Efficacy was evaluated after a minimum follow-up of 24 months.
Results
The East Asian subgroup comprised 89 patients (57% of overall study population); 45 were randomized to bemarituzumab-mFOLFOX6 and 44 to placebo-mFOLFOX6. Median PFS (95% confidence interval [CI]) was 12.9 months (8.8–17.9) with bemarituzumab-mFOLFOX6 and 8.2 months (5.6–10.3) with placebo-mFOLFOX6 (HR 0.50, 95% CI 0.29–0.87); median OS (95% CI) was 24.7 months (13.8–33.1) vs 12.9 months (9.3–21.4), respectively (HR 0.56, 95% CI 0.32–0.96). Treatment benefit was more pronounced in patients with FGFR2b-positive G/GEJC in ≥ 10% of tumor cells. No new safety signals were reported.
Conclusion
In East Asian patients with FGFR2b-positive advanced/metastatic G/GEJC enrolled in the global FIGHT study, bemarituzumab-mFOLFOX6 showed clinically meaningful outcomes over placebo-mFOLFOX6.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric/gastroesophageal junction cancer (G/GEJC) has a higher prevalence in East Asia compared with the rest of the world. Approximately 60% of G/GEJC cases and 58% of G/GEJC deaths in 2018 occurred in East Asia [1, 2]. Moreover, the age-standardized rate per 100,000 people was 22.36 cases and 15.94 deaths in East Asia, compared with 5.80 and 3.40, respectively, in Western Europe and 4.12 and 1.80, respectively, in North America [1, 2]. The incidence and mortality rates of G/GEJC have been successfully reduced in the past few decades across multiple East Asian countries/regions owing to screening accompanied by early treatment, H. pylori eradication, and economic development [3]. Despite the decline in incidence and the reduction in mortality rates for G/GEJC in Asia, 5-year survival rates remain quite poor [3]. Targeted therapies and immune checkpoint inhibitors have shown promising results in advanced G/GEJC, including in analyses/trials focusing on Asian patients [4]. Targeted and immune checkpoint inhibitors are increasingly available in Asia and have been incorporated into regional and national treatment guidelines [5,6,7]. As novel biomarkers emerge, they may reveal more opportunities to improve outcomes in locally advanced unresectable or metastatic G/GEJC via targeted therapeutics.
The IIb splice isoform of the fibroblast growth factor receptor 2 (FGFR2b) has emerged as a target for novel therapeutics in advanced G/GEJC [8]. The incidence of FGFR2b overexpression in G/GEJC has been estimated at 20% to 30% of cases, depending on the cutoff point for immunohistochemistry (IHC) staining [9,10,11,12,13]. The prognostic relevance of FGFR2b overexpression is not fully established, but some studies suggest it has been associated with poor prognosis [9,10,11,12,13]. Targeting FGFR2b in G/GEJC, therefore, represents a promising approach to improve survival outcomes in this patient population.
Bemarituzumab is a first-in-class, humanized, IgG1 monoclonal antibody that selectively inhibits FGFR2b signaling and enhances antibody-dependent cellular cytotoxicity against tumor cells expressing FGFR2b [14]. The phase 2, randomized, double-blind, global FIGHT trial (NCT03694522) evaluated bemarituzumab plus modified 5-fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) vs placebo plus mFOLFOX6 in patients with FGFR2b-overexpressing advanced G/GEJC [13, 15]. In the final analysis of the FIGHT study, bemarituzumab plus mFOLFOX6 extended median progression-free survival (PFS) and median OS with an acceptable safety profile [15]. Median PFS (median follow-up time, 6.8 months) was 9.5 months (95% CI 7.3–13.7) with bemarituzumab plus mFOLFOX6 and 7.4 months (95% CI 5.7–8.4) with placebo plus mFOLFOX6 (HR 0.72; 95% CI 0.49–1.08). Median OS was 19.2 months (95% CI 13.6–24.2) and 13.5 months (95% CI 9.3–15.9), respectively (HR, 0.77; 95% CI, 0.52–1.14) [19]. Most patients enrolled in FIGHT (63%) had FGFR2b overexpression in ≥ 10% of tumor cells as assessed by immunohistochemistry (IHC), defined as moderate (2+) to strong (3+) tumor staining intensity (FGFR2b ≥ 10% subgroup). In the FGFR2b ≥ 10% subgroup, median PFS (HR 0.43 in final analysis) and median OS (HR 0.52) were more pronounced than in the overall population [15].
FIGHT was a global randomized phase 2 trial with the majority of patients (57%) enrolled in East Asian sites. Here, we present the FIGHT final analysis results from all patients enrolled in East Asian sites.
Methods
The trial design, protocol and primary results of the FIGHT study have been reported previously [13, 15]. Briefly, the phase 2 portion of FIGHT was a randomized, double-blind, placebo plus controlled study conducted at 164 sites across 18 countries. Of these, 31 sites were in East Asia (South Korea, China, Japan, and Taiwan).
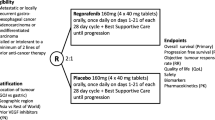
The FIGHT global study evaluated the efficacy and safety of bemarituzumab plus mFOLFOX6 in patients with advanced G/GEJC not known to be HER-2 positive, with FGFR2b overexpression as assessed by IHC and/or FGFR2 gene amplification as assessed by circulating tumor DNA (ctDNA) assay. Patients were randomized 1:1 to bemarituzumab plus mFOLFOX6 or placebo plus mFOLFOX6, and treatment continued through disease progression (Response Evaluation Criteria in Solid Tumors version 1.1 [RECIST v1.1]) or unacceptable toxicity. During the long-term follow-up period, patients were contacted every 3 (± 1) months for 24 months after the last patient was enrolled, or until death, loss to follow-up, consent withdrawal, or study termination, whichever occurred first. Data cutoff for this final analysis was May 13, 2022.
All patients provided written informed consent, and study protocols were approved by institutional ethics committees/institutional review boards at all sites.
Population
Eligible patients were ≥ 18 years of age, had received no prior therapy for histologically confirmed advanced or metastatic G/GEJC, not known to be HER2-positive, had evaluable disease (RECIST version 1.1), had Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1, and FGFR2b overexpression by IHC and/or FGFR2 gene amplification by ctDNA assay. Patients who had received maximum of one dose of mFOLFOX6 during screening were eligible. FGFR2b overexpression by IHC was defined as any tumor cells with moderate (2+) to strong (3+) staining intensity, and cutoff for the ctDNA assay was 1.5X.
Exclusion criteria included untreated/symptomatic central nervous system metastasis, grade ≥ 2 common terminology criteria for adverse events (CTCAE) peripheral sensory neuropathy, acute or unstable corneal abnormalities (including but not limited to those that may increase the risk of developing a corneal ulcer as well as use of contact lenses).
Treatment and randomization
Bemarituzumab dosing was 15 mg/kg every 2 weeks and one additional 7.5 mg/kg dose on cycle 1 day 8. The corresponding placebo treatment was administered with the same schedule as for bemarituzumab. The standard mFOLFOX6 regimen (oxaliplatin 85 mg/m2, leucovorin 400 mg/m2, and 5-fluorouracil 400 mg/m2 bolus followed by 2400 mg/m2 over approximately 48 h) was administered intravenously every 2 weeks in both arms. Randomization was stratified by geographic region (non-Asia [United States, European Union, United Kingdom, and Türkiye] vs China vs rest of Asia), prior treatment status (de novo vs adjuvant/neoadjuvant), and administration of a single dose of mFOLFOX6 during screening (yes vs no). Randomization was done using a permutated block scheme with a block size of four.
Endpoints and assessments
The primary endpoint was PFS, and secondary endpoints included OS, objective response rate (ORR) (complete response [CR] or partial response [PR] according to investigator assessment of tumor lesions per RECIST v1.1), and incidence of adverse events. Duration of response (DOR) was an exploratory endpoint among patients who experienced a CR or PR according to RECIST v1.1. Adverse events were coded using the Medical Dictionary for Regulatory Activities version 25.0 and were graded using CTCAE version 5.0.
Statistical analysis
Efficacy was analyzed in an intention-to-treat (ITT) population that included all randomized patients. Safety was analyzed in the safety analysis set that included patients who had received at least one dose of assigned treatment. The Kaplan–Meier method was used to estimate the median PFS and median OS and the associated 95% CIs in each treatment arm. HRs and 95% CIs were calculated using a stratified Cox regression model. Analysis of outcomes from the FGFR2b ≥ 10% overexpression subgroup (≥ 10% of tumor cells with moderate to strong staining as assessed by IHC) was prespecified in the overall population. Efficacy results in the East Asian subgroup are presented for the ITT population and among patients with FGFR2b ≥ 10% overexpression using the same statistical methodology. Data on adverse events are presented descriptively by number of patients and frequency in the safety population of the East Asian subgroup.
Results
Patient disposition and characteristics
Of the 473 patients prescreened at East Asian sites for FGFR2b overexpression, 45 were randomized to bemarituzumab plus mFOLFOX6 and 44 to placebo plus mFOLFOX6 (ITT; N = 89). The safety population included 44 patients in each treatment arm (ie, those who received at least one dose of the study treatment).
All patients had discontinued the study by the data cutoff date for the final analysis. Radiographic disease progression (n = 11 [24%] bemarituzumab plus mFOLFOX6, n = 25 [57%] placebo plus mFOLFOX6) and treatment-emergent adverse event (TEAE) (n = 21 [47%], n = 3 [7%], respectively) were the primary reasons for bemarituzumab/placebo treatment discontinuation. Death (n = 25 [57%] bemarituzumab plus mFOLFOX6, n = 32 [73%] placebo plus mFOLFOX6) was the primary reason for study discontinuation. Disease progression (n = 17 [38%] bemarituzumab plus mFOLFOX6, n = 27 [61%] placebo plus mFOLFOX6) was most common reason for death.
Baseline demographic and clinical characteristics were balanced between the treatment arms (Table 1). Across both treatment arms, the median age was 57 years and 63 (71%) were male. A total of 54 (61%) were enrolled in South Korea, 27 (30%) in China, 7 (8%) in Japan, and 1 (1%) in Taiwan. Gastric adenocarcinoma was the primary clinical diagnosis of cancer in 85 (96%) of the patients; ECOG status was 0 in 24 (27%) and 1 in 65 (73%); and 92% (n = 82) had disease stage IV at screening. One-third of patients received a single dose of mFOLFOX6 during screening (33% and 30% in the bemarituzumab plus mFOLFOX6 and placebo plus mFOLFOX6 arms, respectively). The median duration of exposure to study drug was 25.9 weeks (range, 2.0–94.6) in the bemarituzumab plus mFOLFOX6 arm and 27.4 weeks (range, 3.0–130.7) in the placebo plus mFOLFOX6 arm. The FGFR2b ≥ 10% subgroup comprised 29 (64%) patients from the bemarituzumab plus mFOLFOX6 arm and 31 (70%) patients from the placebo plus mFOLFOX6 arm.
Progression-free survival
At the final analysis data cutoff, median PFS follow-up time was 8.8 months (range, 0–35.9 months). PFS events occurred in 24 (53.3%) patients in the bemarituzumab plus mFOLFOX6 arm and in 32 (72.7%) patients in the placebo plus mFOLFOX6 arm. Median PFS was 12.9 months (95% CI 8.8–17.9 months) with bemarituzumab plus mFOLFOX6 and 8.2 months (95% CI 5.6–10.3 months) with placebo plus mFOLFOX6 (HR 0.50; 95% CI 0.29–0.87), with a 12 month estimated PFS rate of 55.5% (95% CI 37.5–70.2%) and 28.9% (95% CI 14.8–44.7%), respectively (Fig. 1a).
Progression-free survival (a) and overall survival (b) in the FIGHT East Asian subgroup. Bema bemarituzumab plus mFOLFOX6, CI confidence interval, HR hazard ratio, mFOLFOX6 modified FOLFOX (infusional 5-fluorouracil, leucovorin, and oxaliplatin), mOS median overall survival, mPFS median progression-free survival, OS overall survival, Pbo placebo plus mFOLFOX6, PFS progression-free survival. HRs and 95% CIs were calculated using the unstratified Cox proportional hazards model. Vertical bars indicate censoring
Overall survival
At the final analysis data cutoff, median OS follow-up time was 14.6 months (range, 0–40.5 months). A total of 25 (55.6%) patients in the bemarituzumab plus mFOLFOX6 arm and 32 (72.7%) patients in the placebo plus mFOLFOX6 arm died. Median OS was 24.7 months (95% CI 13.8–33.1 months) with bemarituzumab plus mFOLFOX6 and 12.9 months (95% CI 9.3–21.4 months) with placebo plus mFOLFOX6 (HR 0.56; 95% CI 0.32–0.96). The estimated OS rate at 12 months was 70.3% with bemarituzumab plus mFOLFOX6 and 59.5% with placebo plus mFOLFOX6; the rates at 24 months were 52.0% and 31.5%, respectively (Fig. 1b).
Response rate
The ORR was 48.9% (95% CI 33.7–64.2%) with bemarituzumab plus mFOLFOX6 and 34.1% (95% CI 20.5–49.9%) with placebo plus mFOLFOX6 (Table 2). CR and PR, respectively, was experienced by 3 (6.7%) and 19 (42.2%) patients in the bemarituzumab plus mFOLFOX6 arm and by 1 (2.3%) and 14 (31.8%) patients in the placebo plus mFOLFOX6 arm. Figure 2 displays the best percentage change in tumor size from baseline by individual patient. The median DOR was 15.6 months (95% CI 9.2–NR) with bemarituzumab plus mFOLFOX6 and 7.6 months (95% CI 3.7–14.8) with placebo plus mFOLFOX6.
Efficacy for patients in FGFR2b ≥ 10% subgroup
Efficacy was assessed among patients in the FGFR2b ≥ 10% subgroup. Median PFS in this subgroup was 17.9 months (95% CI 9.5–NR) with bemarituzumab plus mFOLFOX6 and 7.6 months (95% CI 5.5–9.4) with placebo plus mFOLFOX6 (HR 0.28; 95% CI 0.13–0.57), with estimated 12 month PFS rates of 69.2% (95% CI 45.4–84.2%) and 24.1% (95% CI 9.9–41.6%), respectively (Supplementary Fig. 1a). Median OS was 30.1 months (95% CI 17.3-NR) with bemarituzumab plus mFOLFOX6 and 12.9 months (9.1–16.8) with placebo plus mFOLFOX6 (HR 0.43; 95% CI 0.22–0.86) (Supplementary Fig. 1b). The estimated OS rate at 12 months was 77.4% with bemarituzumab plus mFOLFOX6 and 58.6% with placebo plus mFOLFOX6; the rates at 24 months were 65.4% and 28.2%, respectively. The ORR was 51.7% (95% CI 32.5–70.6%) with bemarituzumab plus mFOLFOX6 and 35.5% (95% CI 19.2–54.6%) with placebo plus mFOLFOX6. CR and PR was experienced by 2 (6.9%) and 13 (44.8%) patients in the bemarituzumab plus mFOLFOX6 arm and by 0 and 11 (35.5%) patients in the placebo plus mFOLFOX6 arm.
Subsequent therapies
A total of 27 (61%) and 29 (66%) patients in the bemarituzumab plus mFOLFOX6 and placebo plus mFOLFOX6 arms, respectively, received second-line therapy after disease progression (safety analysis set) (Table 3). Taxanes (50%), pyrimidine analogues (27%), ramucirumab (26%), irinotecan (19%), and programmed cell death protein 1/programmed cell death ligand 1 (PD-1/PD-L1) inhibitors (15%) were the most common agents used as subsequent therapy.
Safety
All patients in the bemarituzumab plus mFOLFOX6 arm and 43 (97.7%) in the placebo plus mFOLFOX6 arm reported TEAEs; 38 (86.4%) and 34 (77.3%) patients reported grade ≥ 3 TEAEs, respectively (Table 4). A total of 40 (90.9%) and 32 (72.7%) patients in the bemarituzumab plus mFOLFOX6 and placebo plus mFOLFOX6 arms, respectively, reported TEAEs related to bemarituzumab/placebo. A total of 21 (47.7%) and 4 (9.1%) patients in the bemarituzumab plus mFOLFOX6 and placebo plus mFOLFOX6 arms, respectively, reported TEAEs leading to discontinuation of bemarituzumab/placebo. Most TEAEs leading to discontinuation of bemarituzumab were corneal adverse events (17/21 [81%]); no corneal adverse events led to discontinuation of placebo. A total of 7 (15.9%) and 26 (59.1%) patients, respectively, in the bemarituzumab plus mFOLFOX6 arm and 7 (15.9%) and 22 (50.0%) patients in the placebo plus mFOLFOX6 arm reported TEAEs leading to dose reduction and dose delay of bemarituzumab/placebo.
A total of 14 (31.8%) and 18 (40.9%) patients in the bemarituzumab plus mFOLFOX6 and placebo plus mFOLFOX6 arms, respectively, reported serious TEAEs. A total of 3 (6.8%) and 2 (4.5%) patients in the bemarituzumab plus mFOLFOX6 and placebo plus mFOLFOX6 arms, respectively, reported fatal TEAEs.
A total of 14 (31.8%) patients in the bemarituzumab plus mFOLFOX6 arm and 0 patients in the placebo plus mFOLFOX6 arm reported grade ≥ 3 corneal adverse events; no patients reported serious or grade ≥ 4 corneal adverse events. Corneal adverse events, regardless of grade, were reported by 30 (68.2%) and 6 (13.6%) patients in the bemarituzumab plus mFOLFOX6 and placebo plus mFOLFOX6 arms, respectively; the median time to onset was 17.1 weeks (range, 0.1–38.6) and 12.4 weeks (range, 6.0–29.0), respectively (Table 5). A total of 24 (54.5%) and 1 (2.3%) patient(s) in the bemarituzumab plus mFOLFOX6 and placebo plus mFOLFOX6 arms, respectively, reported grade ≥ 2 corneal adverse events. In the bemarituzumab plus mFOLFOX6 arm, the median time to onset of grade ≥ 2 corneal adverse events was 28.6 weeks (range, 0.1–56.6). A total of 14 patients in the bemarituzumab plus mFOLFOX6 arm experienced resolution or downgrading of grade ≥ 2 corneal adverse events to grade 1, with a median time to resolution/downgrading of 24.5 weeks (range, 2.1–49.7). One patient in the placebo plus mFOLFOX6 arm experienced resolution of a corneal adverse event, with a time to resolution/downgrading of 2 weeks. All corneal adverse events resolved in 15 (34.1%) and 2 (4.5%) patients in the bemarituzumab plus mFOLFOX6 and placebo plus mFOLFOX6 arms, respectively.
Discussion
East Asian patients treated with bemarituzumab plus mFOLFOX6 experienced a clinically meaningful improvement in median PFS compared with those treated with placebo plus mFOLFOX6 (12.9 vs 8.2 months; HR 0.50; 95% CI 0.29–0.87). Similarly, the treatment benefit in median OS was clinically meaningful with bemarituzumab plus mFOLFOX6 vs placebo plus mFOLFOX6 (24.7 vs 12.9 months; HR 0.56; 95% CI 0.32–0.96). The benefit with bemarituzumab plus mFOLFOX6 was more pronounced in the FGFR2b overexpression ≥ 10% subgroup, with an HR for PFS of 0.28 (95% CI 0.13–0.57) and OS of 0.43 (95% CI 0.22–0.86). Most TEAEs were similar between the two treatment arms except for corneal adverse events, which occurred more frequently with bemarituzumab plus mFOLFOX6 and led to most of the adverse event-related discontinuations in that treatment arm.
As in the overall FIGHT population, patients in the East Asian subgroup experienced robust improvement across efficacy outcomes with bemarituzumab plus mFOLFOX6 [15]. The HR for PFS and OS were 0.72 (95% CI 0.49–1.08) and 0.77 (95% CI 0.52–1.14) in the final analysis of the overall population. ORR was 48.1% (95% CI 36.5–59.7%) with bemarituzumab plus mFOLFOX6 and 33.3% (95% CI 23.1–44.9%) with placebo plus mFOLFOX6 in the overall population, with a median DOR of 11.9 months (95% CI 6.9–17.3) and 7.5 months (95% CI 4.3–13.8), respectively. Treatment benefit was more pronounced in patients with FGFR2b overexpression ≥ 10% in the East Asian subgroup, which was also observed in the overall population (HR for PFS, 0.43 [95% CI 0.26–0.73]; HR for OS, 0.52 [95% CI 0.31–0.85]) [15]. Notably, the HRs for PFS and OS were numerically lower in the East Asian subgroup than those observed in the FIGHT overall population. The study was not sufficiently powered to determine whether this is a meaningful difference. Numerical differences indicating prolonged OS or PFS among Asian patients compared with Western or global trial populations have been observed across trials evaluating immunotherapy for gastric or gastroesophageal cancer [4]. A meta-analysis found statistically significant differences in OS between Asian and Western populations in immunotherapy and first-line gastric cancer clinical trials [4]. Prespecified and post-hoc analyses of PD-1/PD-L1 inhibitors and novel targeted agents in first-line gastric cancer have found that patients enrolled in Asia have numerically improved PFS and OS compared with overall, global study populations [16,17,18,19,20,21]. The numerical difference in PFS and OS results by region of enrolment in FIGHT and other similar clinical trials may reflect differing treatment patterns in Asia.
A similar proportion of patients reported grade ≥ 3 TEAEs in the overall population (82.9% with bemarituzumab plus mFOLFOX6 and 75.3% with placebo plus mFOLFOX6) and East Asian subgroup [15]. As in the overall population, discontinuation of bemarituzumab were mostly driven by corneal adverse events. This may be due to a protocol requirement that patients in the bemarituzumab were withdrawn if grade ≥ 2 corneal adverse events did not resolve completely or to grade 1. No new safety signals were reported from the East Asian subgroup analysis of the FIGHT trial.
Treatment efficacy of bemarituzumab plus mFOLFOX6 in the FIGHT East Asian subgroup was in line with or improved relative to results of other combinations in front-line gastric cancer, including PD-1/PD-L1 inhibitors and other novel targeted agents. In a systematic meta-analysis, Zhang and colleagues found a significant treatment benefit in Asian patients across clinical trials of immunotherapy (HR, 0.80; 95% CI, 0.65–0.98) [4]. The FIGHT study enrolled patients with untreated, HER-2 non-positive advanced G/GEJC. Studies of novel agents in Asian patients with HER-2 non-positive G/GEJC—the population of the present analysis from the FIGHT trial—demonstrated meaningful treatment benefit. Asian patients with HER-2 non-positive G/GEJC experienced treatment benefit with nivolumab in CheckMate 649 (Chinese patient subgroup analysis) [16, 18] and ATTRACTION-4 (all patients from East Asia) [22], pembrolizumab in KEYNOTE-859 and KEYNOTE-062 (Asian subgroups) [19,20,21], and zolbetuximab in SPOTLIGHT and GLOW (Asian subgroup) [17]. These results, as well as those of the FIGHT East Asian subgroup, support the use of biomarker-driven agents and immunotherapies in first-line treatment of advanced G/GEJC in East Asian populations.
The results presented here should be considered in the context of strengths and limitations of the analysis. Key strengths of this analysis are that the East Asian subgroup comprised a meaningful proportion of the FIGHT study overall population (reflecting the epidemiology of G/GEJC), and region was a stratification factor in randomization. The key limitations are that this was a post-hoc subgroup analysis, and the sample size was relatively small. Phase 3 global studies of bemarituzumab added to chemotherapy are underway, with enrollment planned across East Asia. Both the FORTITUDE-101 (NCT05052801) [23] and FORTITUDE-102 (NCT05111626) [24] phase 3 trials investigate bemarituzumab plus chemotherapy in previously untreated G/GEJC, and the primary endpoint will be assessed among patients with FGFR2b overexpression in ≥ 10% of tumor cells. The trials will compare bemarituzumab or placebo; all patients will receive mFOLFOX6 in FORTITUDE-101 and nivolumab plus mFOLFOX6/CAPOX in FORTITUDE-102 [23, 24].
Conclusions
In East Asian patients with advanced or metastatic G/GEJC (N = 89), bemarituzumab plus mFOLFOX6 continued to show clinically meaningful outcomes over placebo plus mFOLFOX6 after 24 months of follow-up. As was observed in the global FIGHT final analysis, East Asian patients with FGFR2b overexpression in ≥ 10% of tumor cells by IHC experienced more pronounced treatment benefit. Randomized phase 3 trials are ongoing to confirm the observed clinical benefit of bemarituzumab added to chemotherapy in patients with advanced G/GEJC with FGFR2b overexpression in ≥ 10% of tumor cells by IHC (NCT05111626, NCT05052801) [23, 34]
Data availability
Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: http://www.amgen.com/datasharing.
References
Arnold M, Abnet CC, Neale RE, et al. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology. 2020;159:335-49.e15. https://doi.org/10.1053/j.gastro.2020.02.068.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. https://doi.org/10.3322/caac.21492.
Shin WS, Xie F, Chen B, et al. Updated epidemiology of gastric cancer in Asia: decreased incidence but still a big challenge. Cancers. 2023;15:2639. https://doi.org/10.3390/cancers15092639.
Zhang Z, Liu Z, Chen Z. Comparison of treatment efficacy and survival outcomes between Asian and Western patients with unresectable gastric or gastro-esophageal adenocarcinoma: a systematic review and meta-analysis. Front Oncol. 2022;12:831207. https://doi.org/10.3389/fonc.2022.831207.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2021 (6th edition). Gastric Cancer. 2023;26:1–25. https://doi.org/10.1007/s10120-022-01331-8.
Kim TH, Kim IH, Kang SJ, et al. Korean practice guidelines for gastric cancer 2022: an evidence-based, multidisciplinary approach. J Gastric Cancer. 2023;23:3–106. https://doi.org/10.5230/jgc.2023.23.e11.
Muro K, Cutsem EV, Narita Y, et al. Pan-Asian adapted ESMO clinical practice guidelines for the management of patients with metastatic gastric cancer: a JSMO–ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS Ann Oncol. 2019;30:19–33. https://doi.org/10.1093/annonc/mdy502.
Dieci MV, Arnedos M, Andre F, Soria JC. Fibroblast growth factor receptor inhibitors as a cancer treatment: from a biologic rationale to medical perspectives. Cancer Discov. 2013;3:264–79. https://doi.org/10.1158/2159-8290.cd-12-0362.
Ahn S, Lee J, Hong M, et al. FGFR2 in gastric cancer: protein overexpression predicts gene amplification and high H-index predicts poor survival. Mod Pathol. 2016;29:1095–103. https://doi.org/10.1038/modpathol.2016.96.
Han N, Kim MA, Lee HS, Kim WH. Evaluation of fibroblast growth factor receptor 2 expression, heterogeneity and clinical significance in gastric cancer. Pathobiology. 2015;82:269–79. https://doi.org/10.1159/000441149.
Hyung S, Han B, Jung J, et al. Incidence of FGFR2 amplification and FGFR2 fusion in patients with metastatic cancer using clinical sequencing. J Oncol. 2022;2022:9714570. https://doi.org/10.1155/2022/9714570.
Nagatsuma AK, Aizawa M, Kuwata T, et al. Expression profiles of HER2, EGFR, MET and FGFR2 in a large cohort of patients with gastric adenocarcinoma. Gastric Cancer. 2015;18:227–38. https://doi.org/10.1007/s10120-014-0360-4.
Wainberg ZA, Enzinger PC, Kang Y-K, et al. Bemarituzumab in patients with FGFR2b-selected gastric or gastro-oesophageal junction adenocarcinoma (FIGHT): a randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 2022;23:1430–40. https://doi.org/10.1016/s1470-2045(22)00603-9.
Xiang H, Chan AG, Ahene A, et al. Preclinical characterization of bemarituzumab, an anti-FGFR2b antibody for the treatment of cancer. MAbs. 2021;13:1981202. https://doi.org/10.1080/19420862.2021.1981202.
Wainberg ZA, Kang Y, Lee K, et al. Bemarituzumab as first-line treatment for locally advanced or metastatic gastric/gastroesophageal junction adenocarcinoma: final analysis of the randomized phase 2 FIGHT trial. Gastric Cancer. 2024. https://doi.org/10.1007/s10120-024-01466-w.
Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27–40. https://doi.org/10.1016/s0140-6736(21)00797-2.
Liang Z, Liu L, Li W, et al. Efficacy and safety of zolbetuximab for first-line treatment of advanced Claudin 18.2-positive gastric or gastro-esophageal junction adenocarcinoma: a systematic review and meta-analysis of randomized controlled trials. Front Oncol. 2023;13:1258347. https://doi.org/10.3389/fonc.2023.1258347.
Liu T, Bai Y, Lin X, et al. First-line nivolumab plus chemotherapy vs chemotherapy in patients with advanced gastric, gastroesophageal junction and esophageal adenocarcinoma: CheckMate 649 Chinese subgroup analysis. Int J Cancer. 2023;152:749–60. https://doi.org/10.1002/ijc.34296.
Oh D-Y, Bai Y, Ryu MH, et al. 138MO pembrolizumab (pembro) or placebo (Pbo) plus chemotherapy (Chemo) for advanced HER2-negative gastric/gastroesophageal junction (G/GEJ) adenocarcinoma (KEYNOTE-859): Asia subgroup analysis. Ann Oncol. 2023;34(4):S1526. https://doi.org/10.1016/j.annonc.2023.10.273.
Rha SY, Oh D-Y, Yañez P, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24:1181–95. https://doi.org/10.1016/s1470-2045(23)00515-6.
Satake H, Lee K-W, Chung HC, et al. Pembrolizumab or pembrolizumab plus chemotherapy versus standard of care chemotherapy in patients with advanced gastric or gastroesophageal junction adenocarcinoma: Asian subgroup analysis of KEYNOTE-062. Jpn J Clin Oncol. 2023;53:221–9. https://doi.org/10.1093/jjco/hyac188.
Kang YK, Chen LT, Ryu MH, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23:234–47. https://doi.org/10.1016/s1470-2045(21)00692-6.
Smyth EC, Chao J, Muro K, et al. Trial in progress: phase 3 study of bemarituzumab + mFOLFOX6 versus placebo + mFOLFOX6 in previously untreated advanced gastric or gastroesophageal junction (GEJ) cancer with FGFR2b overexpression (FORTITUDE-101). J Clin Oncol. 2022;40:TPS4164–264. https://doi.org/10.1200/jco.2022.40.16_suppl.tps4164.
Wainberg ZA, Cutsem EV, Moehler MH, et al. Trial in progress: phase 1b/3 study of bemarituzumab + mFOLFOX6 + nivolumab versus mFOLFOX6 + nivolumab in previously untreated advanced gastric and gastroesophageal junction (GEJ) cancer with FGFR2b overexpression (FORTITUDE-102). J Clin Oncol. 2022;40:4165–265. https://doi.org/10.1200/jco.2022.40.16_suppl.tps4165.
Acknowledgements
We thank the patients, investigators, and study staff who contributed to this study. Medical writing support was provided by Tim Peoples, MA, ELS, contractor to Amgen Inc. We thank Erica Sommermann, MEd, PhD (Amgen Inc.) for operational planning assistance. This study was funded by Five Prime Therapeutics, Inc. Five Prime Therapeutics, Inc. is a wholly owned subsidiary of Amgen Inc.
Funding
This study was funded by Five Prime Therapeutics, Inc. Five Prime Therapeutics, Inc. is a wholly owned subsidiary of Amgen Inc.
Author information
Authors and Affiliations
Contributions
Conception and design: AZ-K. Provision of study materials or patients: YKK, SQ, K-WL, SCO, I-HK, JGK, YL, ZY, JL, L-YB, KY. Data analysis and interpretation: AZ-T, KT, CC, AY. Manuscript writing: All authors. Final approval of manuscript: All authors. Accountable for all aspects of the work: All authors.
Corresponding author
Ethics declarations
Conflict of interests
Yoon-Koo Kang: Consulting fees (self): Amgen, Novartis, Roche, Daehwa, Zymeworks, Blueprint, Surface Oncology, ALX Oncology, Macrogenics, BMS, Merck, Liscure. Shukui Qin: None to disclose. Keun-Wook Lee: All support for the present manuscript: Five Prime Therapeutics; Grants or contracts from any entity: All to institution for conducting clinical trials—AstraZeneca, Ono pharmaceutical, Merck Sharp and Dohme, Merck KGaA, Roche, Pfizer, Beigene, Leap therapeutics, ALX Oncology, Zymeworks, Astellas, Macrogenics, Amgen, Seagen, Bolt therapeutics, Trishula therapeutics, Oncologie, Pharmacyclics, MedPacto, Green Cross Corp, ABLBIO, Y-BIOLOGICS, Daiichi Sankyo, Taiho Pharmaceutical, InventisBio, Elevar Therapeutics, Metafines, Idience, Genome & Company, Exelixis; Honoraria for lectures: Ono pharmaceutical, Boryung, Daiichi Sankyo, Astellas, Sanofi-Aventis; Participation on a Data Safety Monitoring Board or Advisory Board: ALX Oncology, Metafines. Sang Cheul Oh: None to disclose. In-Ho Kim: None to disclose. Jong Gwang Kim: None to disclose. Yong Li: None to disclose. Zhuchen Yan: None to disclose. Jin Li: Research Grants: Roche; Speakers Bureau: Eli Lilly, AstraZeneca. Li-Yuan Bai: None to disclose. Catherine Chan: Employee and stockholder, Amgen Inc. Akeem Yusuf: Employee and stockholder, Amgen Inc. Anita Zalten-Kümeli: Employee and stockholder, Amgen Inc. Kate Taylor: Employee and stockholder, Amgen Inc. Kensei Yamaguchi: Research Grants: Taiho Pharm; Speakers Bureau: Daiichi Sankyo Co., Ltd.,Chugai Pharmaceutical Co., Ltd.,Bristol-Myers Squibb K.K., Eli Lilly Japan K.K., Taiho Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Merck Biopharm Co., Ltd.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent to be included in the study, or the equivalent, was obtained from all patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kang, YK., Qin, S., Lee, KW. et al. Bemarituzumab plus mFOLFOX6 as first-line treatment in East Asian patients with FGFR2b-overexpressing locally advanced or metastatic gastric/gastroesophageal junction cancer: subgroup of FIGHT final analysis. Gastric Cancer (2024). https://doi.org/10.1007/s10120-024-01516-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10120-024-01516-3