Abstract
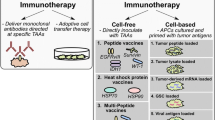
Malignant gliomas are the most common and aggressive form of brain tumour. Current combinations of aggressive surgical resection, radiation therapy and chemotherapy regimens do not significantly improve long-term patient survival for these cancers. Therefore, investigative therapies including tumour vaccines have targeted this devastating condition. This article reviews evidence and data on chemotherapy and immunotherapy for a personalized medicine approach in order to enable physicians to select the appropriate treatment for glioma patients. Dendritic cell- and peptide-based therapy for gliomas seems to be safe and without major adverse effects. Gene-modified vaccines have also shown promise in the treatment of malignant gliomas. The concept of ‘personalized medicine’ is currently important in oncology treatment development. Using a personalized medicine approach, it may be necessary to evaluate the molecular genetic abnormalities in individual patient tumours, and such findings should be the mainstay of immunotherapeutic strategies designed for the individual patient.
Similar content being viewed by others
References
Daumas-Duport C, Scheithauer B, O’Fallon J, et al. Grading of astrocytomas: a simple and reproducible method. Cancer 1988; 62(10): 2152–65
Legler JM, Gloeckler Ries LA, Smith MA, et al. Response: re: brain and other central nervous system cancers: recent trends in incidence and mortality [letter]. J Natl Cancer Inst 1999; 91(23): 2050–1A
Mitchell DA, Sampson JH. Toward effective immunotherapy for the treatment of malignant brain tumors. Neurotherapeutics 2009; 6(3): 527–38
Lutterbach J, Bartelt S, Ostertag C. Long-term survival in patients with brain metastases. J Cancer Res Clin Oncol 2002; 128(8): 417–25
Nussbaum ES, Djalilian HR, Cho KH, et al. Brain metastases: histology, multiplicity, surgery, and survival. Cancer 1996; 78(8): 1781–8
Laws ER, Parney IF, Huang W, et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg 2003; 99(3): 467–73
Stupp R, Dietrich PY, Ostermann Kraljevic S, et al. Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J Clin Oncol 2002; 20(5): 1375–82
Chao ST, Barnett GH, Liu SW, et al. Five-year survivors of brain metastases: a single-institution report of 32 patients. Int J Radiat Oncol Biol Phys 2006; 66(3): 801–9
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100(1): 57–70
Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005; 352(10): 987–96
Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet 2002; 359(9311): 1011–8
Brada M, Hoang-Xuan K, Rampling R, et al. Multicenter phase II trial of temozolomide in patients with glioblastoma multiforme at first relapse. Ann Oncol 2001; 12(2): 259–66
Stupp R, Hegi ME, Gilbert MR, et al. Chemoradiotherapy in malignant glioma: standard of care and future directions. J Clin Oncol 2007; 25(26): 4127–36
Groothuis DR, Vick NA. Brain tumors and the blood-brain barrier. Trends Neurosci 1982; 5: 232–5
Rapoport SI. Osmotic opening of the blood-brain barrier: principles, mechanism, and therapeutic applications. Cell Mol Neurobiol 2000; 20(2): 217–30
Levin VA. Pharmacological principles of brain tumor chemotherapy. Adv Neurol 1976; 15: 315–25
Muldoon LL, Soussain C, Jahnke K, et al. Chemotherapy delivery issues in central nervous system malignancy: a reality check. J Clin Oncol 2007; 25(16): 2295–305
Langer R. Drug delivery and targeting. Nature 1998; 392 (6679 Suppl.): 5–10
Lengyel JS, Milne JL, Subramaniam S. Electron tomography in nanoparticle imaging and analysis. Nanomed 2008; 3(1): 125–31
Veiseh O, Sun C, Fang C, et al. Specific targeting of brain tumors with an optical/magnetic resonance imaging nanoprobe across the blood-brain barrier. Cancer Res 2009; 69(15): 6200–7
Sarin H, Kanevsky AS, Wu H, et al. Effective transvascular delivery of nanoparticles across the blood-brain tumor barrier into malignant glioma cells. J Transl Med 2008; 6: 80
Sarin H, Kanevsky AS, Wu H, et al. Physiologic upper limit of pore size in the blood-tumor barrier of malignant solid tumors. J Transl Med 2009; 7: 51
Frederick L, Wang XY, Eley G, et al. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res 2000; 60(5): 1383–7
Libermann TA, Nusbaum HR, Razon N, et al. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature 1985; 313(5998): 144–7
Plate KH, Breier G, Weich HA, et al. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature 1992; 359(6398): 845–8
Berkman RA, Merrill MJ, Reinhold WC, et al. Expression of the vascular permeability factor/vascular endothelial growth factor gene in central nervous system neoplasms. J Clin Invest 1993; 91(1): 153–9
Gammeltoft S, Ballotti R, Kowalski A, et al. Expression of two types of receptor for insulin-like growth factors in human malignant glioma. Cancer Res 1988; 48(5): 1233–7
Constam DB, Philipp J, Malipiero UV, et al. Differential expression of transforming growth factor-beta 1, -beta 2, and -beta 3 by glioblastoma cells, astrocytes, and microglia. J Immunol 1992; 148(5): 1404–10
Kjellman C, Olofsson SP, Hansson O, et al. Expression of TGF-beta isoforms, TGF-beta receptors, and SMAD molecules at different stages of human glioma. Int J Cancer 2000; 89(3): 251–8
Yancopoulos GD, Davis S, Gale NW, et al. Vascular-specific growth factors and blood vessel formation. Nature 2000; 407(6801): 242–8
Folkman J. Angiogenesis. Annu Rev Med 2006; 57: 1–18
Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature 2005; 438(7070): 967–74
Ferrara N, Hillan KJ, Gerber HP, et al. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 2004; 3(5): 391–400
Iwamoto FM, Fine HA. Bevacizumab for malignant gliomas. Arch Neurol 2010; 67(3): 285–8
Kreisl TN, Kim L, Moore K, et al. Phase II trial of singleagent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 2009; 27(5): 740–5
Bokstein F, Shpigel S, Blumenthal DT. Treatment with bevacizumab and irinotecan for recurrent high-grade glial tumors. Cancer 2008; 112(10): 2267–73
Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology 2008; 70(10): 779–87
De Groot JF, Wen PY, Lamborn K, et al. Phase II single arm trial of aflibercept in patients with recurrent temozo-lomide-resistant glioblastoma: NABTC 0601 [abstract]. J Clin Oncol (Meeting Abstracts) 2008; 26 (15 Suppl.): 2020
Norden AD, Drappatz J, Wen PY. Antiangiogenic therapies for high-grade glioma. Nat Rev Neurol 2009; 5(11): 610–20
De Groot JF, Prados T, Urquhart S, et al. A phase II study of XL184 in patients (pts) with progressive glioblastoma multiforme (GBM) in first or second relapse [abstract]. J Clin Oncol 2009; 27 (15 Suppl.): 2047
Hood JD, Meininger CJ, Ziche M, et al. VEGF upregulates ecNOS message, protein, and NO production in human endothelial cells. Am J Physiol 1998; 274 (3 Pt 2): H1054–8
Fukumura D, Gohongi T, Kadambi A, et al. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci U S A 2001; 98(5): 2604–9
Erber R, Thurnher A, Katsen AD, et al. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J 2004; 18(2): 338–40
Du R, Lu KV, Petritsch C, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell 2008; 13(3): 206–20
Narayana A, Golfinos JG, Fischer I, et al. Feasibility of using bevacizumab with radiation therapy and temozolomide in newly diagnosed high-grade glioma. Int J Radiat Oncol Biol Phys 2008; 72(2): 383–9
Using the immune response to attack tumors. In: Janeway CA, Shlomchik MJ, Travers P, et al., editors. Immunobiology. 6th ed. New York: Garland Science, 2005: 613–64
Noguchi M, Itoh K, Suekane S, et al. Immunological monitoring during combination of patient-oriented peptide vaccination and estramustine phosphate in patients with metastatic hormone refractory prostate cancer. Prostate 2004; 60(1): 32–45
Noguchi M, Itoh K, Yao A, et al. Immunological evaluation of individualized peptide vaccination with a low dose of estramustine for HLA-A24+ HRPC patients. Prostate 2005; 63(1): 1–12
Sampath P, Hanes J, DiMeco F, et al. Paracrine immuno-therapy with interleukin-2 and local chemotherapy is synergistic in the treatment of experimental brain tumors. Cancer Res 1999; 59(9): 2107–14
Wheeler CJ, Das A, Liu G, et al. Clinical responsiveness of glioblastoma multiforme to chemotherapy after vaccination. Clin Cancer Res 2004; 10(16): 5316–26
Fecci PE, Mitchell DA, Archer GE, et al. The history, evolution, and clinical use of dendritic cell-based immunization strategies in the therapy of brain tumors. J Neurooncol 2003; 64(1-2): 161–76
Tang J, Flomenberg P, Harshyne L, et al. Glioblastoma patients exhibit circulating tumor-specific CD8+ T cells. Clin Cancer Res 2005; 11(14): 5292–9
Ueda R, Low KL, Zhu X, et al. Spontaneous immune responses against glioma-associated antigens in a long term survivor with malignant glioma. J Transl Med 2007; 5: 68
Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med 2004; 10(9): 909–15
Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol 2000; 18: 767–811
Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998; 392(6673): 245–52
Cho HI, Kim HJ, Oh ST, et al. In vitro induction of carcino-embryonic antigen (CEA)-specific cytotoxic T lymphocytes by dendritic cells transduced with recombinant adenoviruses. Vaccine 2003; 22(2): 224–36
Lambert LA, Gibson GR, Maloney M, et al. Intranodal immunization with tumor lysate-pulsed dendritic cells enhances protective antitumor immunity. Cancer Res 2001; 61(2): 641–6
Candido KA, Shimizu K, McLaughlin JC, et al. Local administration of dendritic cells inhibits established breast tumor growth: implications for apoptosis-inducing agents. Cancer Res 2001; 61(1): 228–36
Godelaine D, Carrasco J, Lucas S, et al. Polyclonal CTL responses observed in melanoma patients vaccinated with dendritic cells pulsed with a MAGE-3.A1 peptide. J Immunol 2003; 171(9): 4893–7
Boczkowski D, Nair SK, Snyder D, et al. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med 1996; 184(2): 465–72
Condon C, Watkins SC, Celluzzi CM, et al. DNA-based immunization by in vivo transfection of dendritic cells. Nat Med 1996; 2(10): 1122–8
Yu JS, Wheeler CJ, Zeltzer PM, et al. Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res 2001; 61(3): 842–7
Yu JS, Liu G, Ying H, et al. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res 2004; 64(14): 4973–9
Yamanaka R, Homma J, Yajima N, et al. Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: results of a clinical phase I/II trial. Clin Cancer Res 2005; 11(11): 4160–7
Liau LM, Prins RM, Odesa SK, et al. Dendritic cell vaccination in combination with TLR-7 agonist, imiquimod, following radio-chemotherapy for newly diagnosed glioblastoma [abstract]. J Clin Oncol (Meeting Abstracts) 2007; 25 (18Suppl.): 2021
De Vleeschouwer S, Fieuws S, Rutkowski S, et al. Postoperative adjuvant dendritic cell-based immunotherapy in patients with relapsed glioblastoma multiforme. Clin Cancer Res 2008; 14(10): 3098–104
Liu G, Black KL, Yu JS. Sensitization of malignant glioma to chemotherapy through dendritic cell vaccination. Expert Rev Vaccines 2006; 5(2): 233–47
Liu G, Akasaki Y, Khong HT, et al. Cytotoxic T cell targeting of TRP-2 sensitizes human malignant glioma to chemotherapy. Oncogene 2005; 24(33): 5226–34
Tsuboi K, Saijo K, Ishikawa E, et al. Effects of local injection of ex vivo expanded autologous tumor-specific T lymphocytes in cases with recurrent malignant gliomas. Clin Cancer Res 2003; 9(9): 3294–302
Zhang JG, Eguchi J, Kruse CA, et al. Antigenic profiling of glioma cells to generate allogeneic vaccines or dendritic cell-based therapeutics. Clin Cancer Res 2007; 13 (2 Pt 1): 566–75
Prins RM, Cloughesy TF, Liau LM. Cytomegalovirus immunity after vaccination with autologous glioblastoma lysate. N Engl J Med 2008; 359(5): 539–41
Mintz A, Debinski W. Cancer genetics/epigenetics and the X chromosome: possible new links for malignant glioma pathogenesis and immune-based therapies. Crit Rev Oncog 2000; 11(1): 77–95
Skog J. Glioma-specific antigens for immune tumor therapy. Expert Rev Vaccines 2006; 5(6): 793–802
Pelloski CE, Ballman KV, Furth AF, et al. Epidermal growth factor receptor variant III status defines clinically distinct subtypes of glioblastoma. J Clin Oncol 2007; 25(16): 2288–94
Sampson JH, Archer GE, Mitchell DA, et al. Tumor-specific immunotherapy targeting the EGFRvIII mutation in patients with malignant glioma. Semin Immunol 2008; 20(5): 267–75
Sampson JH, Archer GE, Bigner DD, et al. Effect of EGFRvIII-targeted vaccine (CDX-110) on immune response and TTP when given with simultaneous standard and continuous temozolomide in patients with GBM [abstract]. J Clin Oncol (Meeting Abstracts) 2008; 26 (20 Suppl): 2011
Izumoto S, Tsuboi A, Oka Y, et al. Phase II clinical trial of Wilms tumor 1 peptide vaccination for patients with recurrent glioblastoma multiforme. J Neurosurg 2008; 108(5): 963–71
Fakhrai H, Mantil JC, Liu L, et al. Phase I clinical trial of a TGF-beta antisense-modified tumor cell vaccine in patients with advanced glioma. Cancer Gene Ther 2006; 13(12): 1052–60
Okada H, Lieberman FS, Walter KA, et al. Autologous glioma cell vaccine admixed with interleukin-4 gene trans-fected fibroblasts in the treatment of patients with malignant gliomas. J Transl Med 2007; 5: 67
Okada H, Pollack IF, Lotze MT, et al. Gene therapy of malignant gliomas: a phase I study of IL-4-HSV-TK gene-modified autologous tumor to elicit an immune response. Hum Gene Ther 2000; 11(4): 637–53
Bruserud O, Ersvaer E, Olsnes A, et al. Anticancer immunotherapy in combination with proapoptotic therapy. Curr Cancer Drug Targets 2008; 8(8): 666–75
Peres E, Wood GW, Poulik J, et al. High-dose chemotherapy and adoptive immunotherapy in the treatment of recurrent pediatric brain tumors. Neuropediatrics 2008; 39(3): 151–6
Mine T, Sato Y, Noguchi M, et al. Humoral responses to peptides correlate with overall survival in advanced cancer patients vaccinated with peptides based on pre-existing, peptide-specific cellular responses. Clin Cancer Res 2004; 10(3): 929–37
Yajima N, Yamanaka R, Mine T, et al. Immunologic evaluation of personalized peptide vaccination for patients with advanced malignant glioma. Clin Cancer Res 2005; 11(16): 5900–11
ten Bokkel Huinink W, Lane SR, Ross GA. Long-term survival in a phase III, randomised study of topotecan versus paclitaxel in advanced epithelial ovarian carcinoma. Ann Oncol 2004; 15(1): 100–3
Hsu DS, Balakumaran BS, Acharya CR, et al. Pharmaco-genomic strategies provide a rational approach to the treatment of cisplatin-resistant patients with advanced cancer. J Clin Oncol 2007; 25(28): 4350–7
Walker EB, Haley D, Miller W, et al. gp100 (209-2M) peptide immunization of human lymphocyte antigen-A2+ stage I-III melanoma patients induces significant increase in antigen-specific effector and long-term memory CD8+ T cells. Clin Cancer Res 2004; 10(2): 668–80
Romero P, Cerottini JC, Speiser DE. Monitoring tumor antigen specific T-cell responses in cancer patients and phase I clinical trials of peptide-based vaccination. Cancer Immunol Immunother 2004; 53(3): 249–55
Acknowledgements
X.-J. Dai and W.-J. Jiang contributed equally to this work.
This work was supported by grants from China Postdoctoral Science Foundation (no. 20070420766), the natural Science Foundation of Guangdong Province (001122) and the Fund of the 10th 5-Year Medical Research Project of the People’s Liberation Army (01MA038).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dai, Xj., Jiang, Wj., Wang, Wm. et al. Drug or Vaccine?. Drugs 70, 1477–1486 (2010). https://doi.org/10.2165/11538040-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11538040-000000000-00000