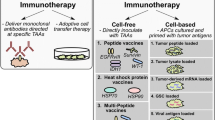
Abstract
Glioblastoma multiforme (GBM), a highly aggressive tumor, poses significant challenges in achieving successful treatment outcomes. Conventional therapeutic modalities including surgery, radiation, and chemotherapy have demonstrated limited efficacy, primarily attributed to the complexities associated with drug delivery to the tumor site and tumor heterogeneity. To address this critical need for innovative therapies, the potential of cancer vaccines utilizing tumor cells and dendritic cells has been explored for GBM treatment. This article provides a comprehensive review of therapeutic vaccinations employing cell-based vaccine strategies for the management of GBM. A meticulous evaluation of 45 clinical trials involving more than 1500 participants revealed that cell-based vaccinations have exhibited favorable safety profiles with minimal toxicity. Moreover, these vaccines have demonstrated modest improvements in overall survival and progression-free survival among patients. However, certain limitations still persist. Notably, there is a need for advancements in the development of potent antigens to evoke immune responses, as well as the optimization of dosage regimens. Consequently, while cell-based vaccinations show promise as a potential therapeutic approach for GBM, further research is imperative to overcome the current limitations. The ultimate objective is to surmount these obstacles and establish cell-based vaccinations as a standard therapeutic modality for GBM.


Similar content being viewed by others
Data availability
Not applicable.
References
Cunha M, Maldaun MVC. Metastasis from glioblastoma multiforme: a meta-analysis. Rev Assoc Med Bras. 2019;65(3):424–33.
Stupp R, Taillibert S, Kanner AA, Kesari S, Steinberg DM, Toms SA, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015;314(23):2535–43.
Shireman JM, Gonugunta N, Zhao L, Pattnaik A, Distler E, Her S, et al. GM-CSF and IL-7 fusion cytokine engineered tumor vaccine generates long-term Th-17 memory cells and increases overall survival in aged syngeneic mouse models of glioblastoma. Aging Cell. 2023. https://doi.org/10.1111/acel.13864.
Ganipineni LP, Danhier F, Préat V. Drug delivery challenges and future of chemotherapeutic nanomedicine for glioblastoma treatment. J Control Release. 2018;281:42–57.
Khatami SH, Karami N, Taheri-Anganeh M, Taghvimi S, Tondro G, Khorsand M, et al. Exosomes: promising delivery tools for overcoming blood-brain barrier and glioblastoma therapy. Mol Neurobiol. 2023;60(8):4659–78.
Zhan C, Lu W. The blood-brain/tumor barriers: challenges and chances for malignant gliomas targeted drug delivery. Curr Pharm Biotechnol. 2012;13(12):2380–7.
Arvanitis CD, Ferraro GB, Jain RK. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat Rev Cancer. 2020;20(1):26–41.
Mo F, Pellerino A, Soffietti R, Rudà R. Blood-brain barrier in brain tumors: biology and clinical relevance. Int J Mol Sci. 2021;22(23):12654.
Dymova MA, Kuligina EV, Richter VA. Molecular mechanisms of drug resistance in glioblastoma. Int J Mol Sci. 2021;22(12):6385.
Fisher R, Pusztai L, Swanton C. Cancer heterogeneity: implications for targeted therapeutics. Br J Cancer. 2013;108(3):479–85.
Arrieta VA, Dmello C, McGrail DJ, Brat DJ, Lee-Chang C, Heimberger AB, et al. Immune checkpoint blockade in glioblastoma: from tumor heterogeneity to personalized treatment. J Clin Invest. 2023. https://doi.org/10.1172/JCI163447.
Hanif F, Muzaffar K, Perveen K, Malhi SM, Simjee ShU. Glioblastoma multiforme: a review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac J Cancer Prev. 2017;18(1):3–9.
Janjua TI, Rewatkar P, Ahmed-Cox A, Saeed I, Mansfeld FM, Kulshreshtha R, et al. Frontiers in the treatment of glioblastoma: past, present and emerging. Adv Drug Deliv Rev. 2021;171:108–38.
Aly A, Singh P, Korytowsky B, Ling Y-L, Kale HP, Dastani HB, et al. Survival, costs, and health care resource use by line of therapy in US Medicare patients with newly diagnosed glioblastoma: a retrospective observational study. Neuro-Oncol Pract. 2019;7(2):164–75.
Weller M, van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021;18(3):170–86.
Wick W, Osswald M, Wick A, Winkler F. Treatment of glioblastoma in adults. Ther Adv Neurol Disord. 2018;11:1756286418790452.
Franceschi E, Minichillo S, Brandes AA. Pharmacotherapy of glioblastoma: established treatments and emerging concepts. CNS Drugs. 2017;31(8):675–84.
Oh HC, Shim JK, Park J, Lee JH, Choi RJ, Kim NH, et al. Combined effects of niclosamide and temozolomide against human glioblastoma tumorspheres. J Cancer Res Clin Oncol. 2020;146(11):2817–28.
Arora A, Somasundaram K. Glioblastoma vs temozolomide: can the red queen race be won? Cancer Biol Ther. 2019;20(8):1083–90.
Sevastre AS, Costachi A, Tataranu LG, Brandusa C, Artene SA, Stovicek O, et al. Glioblastoma pharmacotherapy: a multifaceted perspective of conventional and emerging treatments (Review). Exp Ther Med. 2021;22(6):1408.
Xiao ZZ, Wang ZF, Lan T, Huang WH, Zhao YH, Ma C, et al. Carmustine as a supplementary therapeutic option for glioblastoma: a systematic review and meta-analysis. Front Neurol. 2020;11:1036.
Barrascout E, Lamuraglia M. Glioblastoma and bevacizumab in elderly patients: monocentric study. J Oncol Pharm Pract. 2021;27(4):842–6.
Narita Y. Bevacizumab for glioblastoma. Ther Clin Risk Manag. 2015;11:1759–65.
Rominiyi O, Vanderlinden A, Clenton SJ, Bridgewater C, Al-Tamimi Y, Collis SJ. Tumour treating fields therapy for glioblastoma: current advances and future directions. Br J Cancer. 2021;124(4):697–709.
Bernard-Arnoux F, Lamure M, Ducray F, Aulagner G, Honnorat J, Armoiry X. The cost-effectiveness of tumor-treating fields therapy in patients with newly diagnosed glioblastoma. Neuro Oncol. 2016;18(8):1129–36.
Zhao T, Li C, Ge H, Lin Y, Kang D. Glioblastoma vaccine tumor therapy research progress. Chin Neurosurg J. 2022;8(1):2.
Zhao M, van Straten D, Broekman MLD, Préat V, Schiffelers RM. Nanocarrier-based drug combination therapy for glioblastoma. Theranostics. 2020;10(3):1355–72.
Bruinsmann FA, Richter Vaz G, Soares Alves ADC, Aguirre T, Raffin Pohlmann A, Stanisçuaski Guterres S, et al. Nasal drug delivery of anticancer drugs for the treatment of glioblastoma: preclinical and clinical trials. Molecules. 2019;24(23):4312.
Yang Q, Zhou Y, Chen J, Huang N, Wang Z, Cheng Y. Gene therapy for drug-resistant glioblastoma via lipid-polymer hybrid nanoparticles combined with focused ultrasound. Int J Nanomed. 2021;16:185–99.
Stephens AJ, Burgess-Brown NA, Jiang S. Beyond just peptide antigens: the complex world of peptide-based cancer vaccines. Front Immunol. 2021;12:696791.
Da Ros M, De Gregorio V, Iorio AL, Giunti L, Guidi M, de Martino M, et al. Glioblastoma chemoresistance: the double play by microenvironment and blood-brain barrier. Int J Mol Sci. 2018;19(10):2879.
Kong Z, Wang Y, Ma W. Vaccination in the immunotherapy of glioblastoma. Hum Vaccine Immunother. 2018;14(2):255–68.
Kanaly CW, Ding D, Heimberger AB, Sampson JH. Clinical applications of a peptide-based vaccine for glioblastoma. Neurosurg Clin N Am. 2010;21(1):95–109.
Frederico SC, Hancock JC, Brettschneider EES, Ratnam NM, Gilbert MR, Terabe M. Making a cold tumor hot: the role of vaccines in the treatment of glioblastoma. Front Oncol. 2021;11:672508.
Balan S, Saxena M, Bhardwaj N. Dendritic cell subsets and locations. Int Rev Cell Mol Biol. 2019;348:1–68.
Munson PV, Butterfield LH, Adamik J. Chapter 7 - Novel dendritic cell vaccine strategies. In: Buonaguro L, Van Der Burg S, editors. Cancer vaccines as immunotherapy of cancer. Cambridge: Academic Press; 2022. p. 109–35.
Reardon D, Mitchell D. The development of dendritic cell vaccine-based immunotherapies for glioblastoma. Sem Immunopathol. 2017;39:225–239.
Cintolo JA, Datta J, Mathew SJ, Czerniecki BJ. Dendritic cell-based vaccines: barriers and opportunities. Future Oncol. 2012;8(10):1273–99.
Boudousquié C, Boand V, Lingre E, Dutoit L, Balint K, Danilo M, et al. Development and optimization of a GMP-compliant manufacturing process for a personalized tumor lysate dendritic cell vaccine. Vaccines (Basel). 2020. https://doi.org/10.3390/vaccines8010025.
Strome SE, Voss S, Wilcox R, Wakefield TL, Tamada K, Flies D, et al. Strategies for antigen loading of dendritic cells to enhance the antitumor immune response. Cancer Res. 2002;62(6):1884–9.
Chiang CL, Kandalaft LE, Tanyi J, Hagemann AR, Motz GT, Svoronos N, et al. A dendritic cell vaccine pulsed with autologous hypochlorous acid-oxidized ovarian cancer lysate primes effective broad antitumor immunity: from bench to bedside. Clin Cancer Res. 2013;19(17):4801–15.
Chiang CL, Maier DA, Kandalaft LE, Brennan AL, Lanitis E, Ye Q, et al. Optimizing parameters for clinical-scale production of high IL-12 secreting dendritic cells pulsed with oxidized whole tumor cell lysate. J Transl Med. 2011;9:198.
Tanyi JL, Bobisse S, Ophir E, Tuyaerts S, Roberti A, Genolet R, et al. Personalized cancer vaccine effectively mobilizes antitumor T cell immunity in ovarian cancer. Sci Transl Med. 2018. https://doi.org/10.1126/scitranslmed.aao5931.
Chiang CL, Ledermann JA, Rad AN, Katz DR, Chain BM. Hypochlorous acid enhances immunogenicity and uptake of allogeneic ovarian tumor cells by dendritic cells to cross-prime tumor-specific T cells. Cancer Immunol Immunother. 2006;55(11):1384–95.
Minasian LM, O’Mara A, Mitchell SA. Clinician and patient reporting of symptomatic adverse events in cancer clinical trials: using CTCAE and PRO-CTCAE(®) to provide two distinct and complementary perspectives. Patient Relat Outcome Meas. 2022;13:249–58.
Sloan AE, Dansey R, Zamorano L, Barger G, Hamm C, Diaz F, et al. Adoptive immunotherapy in patients with recurrent malignant glioma: preliminary results of using autologous whole-tumor vaccine plus granulocyte-macrophage colony-stimulating factor and adoptive transfer of anti-CD3-activated lymphocytes. Neurosurg Focus. 2000;9(6): e9.
Plautz GE, Barnett GH, Miller DW, Cohen BH, Prayson RA, Krauss JC, et al. Systemic T cell adoptive immunotherapy of malignant gliomas. J Neurosurg. 1998;89(1):42–51.
Liau LM, Prins RM, Kiertscher SM, Odesa SK, Kremen TJ, Giovannone AJ, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11(15):5515–25.
Vik-Mo EO, Nyakas M, Mikkelsen BV, Moe MC, Due-Tønnesen P, Suso EM, et al. Therapeutic vaccination against autologous cancer stem cells with mRNA-transfected dendritic cells in patients with glioblastoma. Cancer Immunol Immunother. 2013;62(9):1499–509.
Liau LM, Ashkan K, Brem S, Campian JL, Trusheim JE, Iwamoto FM, et al. Association of autologous tumor lysate-loaded dendritic cell vaccination with extension of survival among patients with newly diagnosed and recurrent glioblastoma: a phase 3 prospective externally controlled cohort trial. JAMA Oncol. 2023;9(1):112–21.
Chang CN, Huang YC, Yang DM, Kikuta K, Wei KJ, Kubota T, et al. A phase I/II clinical trial investigating the adverse and therapeutic effects of a postoperative autologous dendritic cell tumor vaccine in patients with malignant glioma. J Clin Neurosci. 2011;18(8):1048–54.
Wen PY, Reardon DA, Armstrong TS, Phuphanich S, Aiken RD, Landolfi JC, et al. A randomized double-blind placebo-controlled phase II trial of dendritic cell vaccine ICT-107 in newly diagnosed patients with glioblastoma. Clin Cancer Res. 2019;25(19):5799–807.
Hu JL, Omofoye OA, Rudnick JD, Kim S, Tighiouart M, Phuphanich S, et al. A phase I study of autologous dendritic cell vaccine pulsed with allogeneic stem-like cell line lysate in patients with newly diagnosed or recurrent glioblastoma. Clin Cancer Res. 2022;28(4):689–96.
Lv L, Huang J, Xi H, Zhou X. Efficacy and safety of dendritic cell vaccines for patients with glioblastoma: a meta-analysis of randomized controlled trials. Int Immunopharmacol. 2020;83: 106336.
Neth BJ, Webb MJ, Parney IF, Sener UT. The current status, challenges, and future potential of therapeutic vaccination in glioblastoma. Pharmaceutics. 2023;15(4):1134.
Thomas AA, Fisher JL, Ernstoff MS, Fadul CE. Vaccine-based immunotherapy for glioblastoma. CNS Oncol. 2013;2(4):331–49.
Sayegh ET, Oh T, Fakurnejad S, Bloch O, Parsa AT. Vaccine therapies for patients with glioblastoma. J Neurooncol. 2014;119(3):531–46.
Jackson C, Ruzevick J, Brem H, Lim M. Vaccine strategies for glioblastoma: progress and future directions. Immunotherapy. 2013;5(2):155–67.
Funding
This research did not receive any funding.
Author information
Authors and Affiliations
Contributions
MEP collected data and wrote the main manuscript text. MEP and SGM prepared figures and tables. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pour, M.E., Moghadam, S.G., Shirkhani, P. et al. Therapeutic cell-based vaccines for glioblastoma multiforme. Med Oncol 40, 354 (2023). https://doi.org/10.1007/s12032-023-02220-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02220-5