Abstract
Background and objective: Cation-containing drugs have the potential to affect the absorption of quinolones. The current study was conducted to assess whether the bioavailability of garenoxacin was affected by administration as crushed tablets with and without concomitant enteral nutrition.
Methods: This was a randomised, open-label, three-period, single-dose, crossover study carried out in healthy male volunteers who received study treatments at a clinical facility. The study included 18 subjects (mean age 30 ± 6 years) who were treatment-naive to garenoxacin and had a body mass index of ≥18 kg/m2 and ≤30 kg/m2. Subjects received garenoxacin 600mg orally in three treatments: (A) intact tablets; (B) crushed tablets suspended in water delivered via a nasogastric (NG) tube; and (C) treatment B plus concomitant enteral feeding (Osmolite®; 600mL at 100 mL/h). Serial plasma samples were collected post-dose for pharmacokinetic analysis. Pharmacokinetic parameters were determined by noncompartmental methods. Geometric mean ratio with 90% CI for area under the concentration-time curve from time 0 extrapolated to infinity (AUC∞) and maximum observed plasma concentration (Cmax) were used to assess potential effects of the different conditions of administration. Absence of an effect was concluded if the 90% CIs for the ratio of geometric means for Cmax and AUC∞ were within 0.7–1.43 and 0.8–1.25, respectively. The pharmacokinetics (AUC∞ and Cmax) and safety of garenoxacin were assessed.
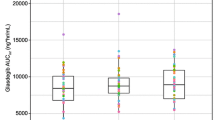
Results: Geometric means for Cmax were 8.3, 8.5 and 8.3 μg/mL and for AUC∞ were 103.3, 97.2 and 93.4 μ·h/mL for treatments A, B and C, respectively. The 90% CIs for geometric mean ratios for AUC8 and Cmax of garenoxacin administered as crushed tablets and crushed tablets with Osmolite? via NG tube relative to intact tablets administered orally were within 0.80–1.25, suggesting that the bioavailability of garenoxacin was not affected by delivery of crushed tablets via NG tube, regardless of concomitant enteral feeding, compared with oral delivery of intact tablets. Half-life (mean range 12–13 hours) was similar for all three groups.
Conclusions: The relative bioavailability of garenoxacin was not affected by administration as crushed tablets, regardless of enteral feeding. Garenoxacin can be administered as crushed tablets in the presence or absence of concomitant Osmolite®.




Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Van Wart S, Phillips L, Ludwig EA, et al. Population pharmacokinetics and pharmacodynamics of garenoxacin in patients with community-acquired respiratory tract infections. Antimicrob Agents Chemother 2004 Dec; 48 (12): 4766–77
Bello A, Stewart C, O’Mara E, et al. Absolute oral bioavailability of garenoxacin tablets and the bioequivalence of the intravenous formulation with respect to oral tablets. 37th Annual American Society of Health-System Pharmacists Midyear Clinical Meeting; 2002 Dec 8–12; Atlanta (GA)
Yuk JH, Williams Jr TW. Drug interaction with quinolone antibiotics in intensive care unit patients. Arch Intern Med 1991 Mar; 151 (3): 619
Lomaestro BM, Bailie GR. Quinolone-cation interactions: a review. DICP, Ann Pharmacol 1991 Nov; 25 (11): 1249–58
Marshall AJ, Piddock LJ. Interaction of divalent cations, quinolones and bacteria. J Antimicrob Chemother 1994 Oct; 34 (4): 465–83
Toussaint B, Chedin M, Bordin G, et al. Determination of (fluoro)quinolone antibiotic residues in pig kidney using liquid chromatography-tandem mass spectrometry: I. Laboratoryvalidated method. J Chromatogr A 2005 Sep 23; 1088 (1–2): 32–9
Toussaint B, Chedin M, Vincent U, et al. Determination of (fluoro)quinolone antibiotic residues in pig kidney using liquid chromatography-tandem mass spectrometry: II. Intercom parison exercise. J Chromatogr A 2005 Sep 23; 1088 (1–2): 40–8
Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 1998 Jan; 26 (1): 1–10
Healy DP, Brodbeck MC, Clendening CE. Ciprofloxacin absorption is impaired in patients given enteral feedings orally and via gastrostomy and jejunostomy tubes. Antimicrob Agents Chemother 1996 Jan; 40 (1): 6–10
Mueller BA, Brierton DG, Abel SR, et al. Effect of enteral feeding with ensure on oral bioavailabilities of ofloxacin and ciprofloxacin. Antimicrob Agents Chemother 1994 Sep; 38 (9): 2101–5
Debon R, Breilh D, Boselli E, et al. Pharmacokinetic parameters of ciprofloxacin (500mg/5mL) oral suspension in critically ill patients with severe bacterial pneumonia: a comparison of two dosages. J Chemother 2002 Apr; 14 (2): 175–80
Vincent J, Teng R, Pelletier SM, et al. The bioavailability of nasogastric versus tablet-form oral trovafloxacin in healthy subjects. Am J Surg 1998 Dec; 176 (6A Suppl.): 23S–6S
Kanji S, McKinnon PS, Barletta JF, et al. Bioavailability of gatifloxacin by gastric tube administration with and without concomitant enteral feeding in critically ill patients. Crit Care Med 2003 May; 31 (5): 1347–52
Osmolite® (Isotonic nutrition). Full prescribing information, Ross Products, division of Abbott Laboratories. Columbus (OH): 2004
Gajjar DA, Bello A, Ge Z, et al. Multiple-dose safety and pharmacokinetics of oral garenoxacin in healthy subjects. Antimicrob Agents Chemother 2003 Jul; 47 (7): 2256–63
Acknowledgements
This study was supported in part by a grant from Bristol-Myers Squibb Company, Princeton, NJ, USA. Schering-Plough Research Institute, Kenilworth, NJ, USA is now responsible for development of garenoxacin. Robert Noveck and Ramon Vargas have no personal conflicts to disclose; however, the clinical centre with which they are affiliated has received grant research support from Bristol-Myers Squibb Company and Schering-Plough Research Institute. Gopal Krishna and Zaiqi Wang are employees of Schering-Plough Research Institute. Dennis Grasela is an employee of Bristol-Myers Squibb Company and owns stock in the company.
Robert Noveck was the principal investigator in this study, and Dennis Grasela was primarily responsible for study protocol development and management, analysis and interpretation of the study data. Gopal Krishna and Zaiqi Wang participated in study data analysis and interpretation. The authors thank Harold Amkraut, Schering-Plough Research Institute, Kenilworth, NJ, for statistical review of this manuscript. The authors also wish to thank Ken Kauffman and Maryann Travaglini, PharmD, who provided medical writing and editorial assistance on behalf of Schering-Plough Research Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krishna, G., Noveck, R., Vargas, R. et al. Nasogastric Administration of Garenoxacin as Crushed Tablets With and Without Concomitant Enteral Feeding in Healthy Subjects. Drugs R D 8, 43–50 (2007). https://doi.org/10.2165/00126839-200708010-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00126839-200708010-00004