Abstract
Purpose
This phase I open-label study investigated the oral bioavailability of two novel maleate salt-based glasdegib (PF-04449913) tablet formulations (small- and large-particle size) relative to the current clinical formulation (diHCl salt-based). In addition, the effect of a gastric pH-altering agent (rabeprazole) and food on the pharmacokinetics of the large-particle size formulation of glasdegib were evaluated. The pharmacokinetics of glasdegib oral solution was also assessed.
Methods
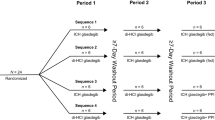
Thirty-four healthy subjects received glasdegib 100 mg as three different formulations in the fasted state (diHCl salt or small- or large-particle size maleate formulation); 13 received the large-particle maleate formulation (fed), and 14 concurrently with rabeprazole (fasted); six subjects received glasdegib 50 mg oral solution (fasted).
Results
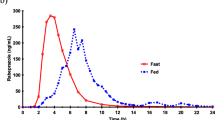
For both new tablet formulations of glasdegib, ratios (Test:Reference) of adjusted geometric means (90% confidence interval) of area under the concentration–time curve from 0 to infinity and maximum plasma concentration were within 80–125% compared with the diHCl formulation (fasted). For the large-particle size formulation (fed), these ratios were 86.3% (81.0–92.0%) and 75.7% (65.3–87.7%), respectively, compared with fasted. When the large-particle maleate formulation was administered concurrently with rabeprazole versus alone (fasted), these ratios were 111.9% (102.8–121.9%) and 87.2% (75.9–100.3%), respectively. The pharmacokinetics of oral solution was similar to the tablet.
Conclusions
The maleate salt-based tablet formulations were bioequivalent to the diHCl tablet formulation. The extent of the observed effect of a high-fat, high-calorie meal or concurrent rabeprazole treatment on glasdegib exposure is not considered clinically meaningful.



Similar content being viewed by others
References
Ingham PW, McMahon AP (2001) Hedgehog signaling in animal development: paradigms and principles. Genes Dev 15:3059–3087. doi:10.1101/gad.938601
Irvine DA, Copland M (2012) Targeting hedgehog in hematologic malignancy. Blood 119:2196–2204. doi:10.1182/blood-2011-10-383752
McMillan R, Matsui W (2012) Molecular pathways: the hedgehog signaling pathway in cancer. Clin Cancer Res 18:4883–4888. doi:10.1158/1078-0432.CCR-11-2509
Dierks C, Grbic J, Zirlik K et al (2007) Essential role of stromally induced hedgehog signaling in B-cell malignancies. Nat Med 13:944–951. doi:10.1038/nm1614
Peacock CD, Wang Q, Gesell GS et al (2007) Hedgehog signaling maintains a tumor stem cell compartment in multiple myeloma. Proc Natl Acad Sci 104:4048–4053. doi:10.1073/pnas.0611682104
Zhao C, Chen A, Jamieson CH et al (2009) Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature 458:776–779. doi:10.1038/nature07737
Munchhof MJ, Li Q, Shavnya A et al (2011) Discovery of PF-04449913, a potent and orally bioavailable inhibitor of Smoothened. ACS Med Chem Lett 3:106–111. doi:10.1021/ml2002423
Martinelli G, Oehler VG, Papayannidis C et al (2015) Treatment with PF-04449913, an oral Smoothened antagonist, in patients with myeloid malignancies: a phase 1 safety and pharmacokinetics study. Lancet Haematol 2:e339-e46. doi:10.1016/S2352-3026(15)00096-4
Berge SM, Bighley LD, Monkhouse DC (1977) Pharmaceutical salts. J Pharm Sci 66:1–19. doi:10.1002/jps.2600660104
Sun CC, Hou H, Gao P, Ma C, Medina C, Alvarez FJ (2009) Development of a high drug load tablet formulation based on assessment of powder manufacturability: moving towards quality by design. J Pharm Sci 98:239–247. doi:10.1002/jps.21422
FDA CDER (2002) Guidance for industry: food-effect bioavailability and fed bioequivalence studies
Shaik MN, LaBadie RR, Rudin D, Levin WJ (2014) Evaluation of the effect of food and ketoconazole on the pharmacokinetics of the Smoothened inhibitor PF-04449913 in healthy volunteers. Cancer Chemother Pharmacol 74:411–418. doi:10.1007/s00280-014-2502-0
Numico G, Fusco V, Franco P, Roila F (2017) Proton pump inhibitors in cancer patients: How useful they are? A review of the most common indications for their use. Crit Rev Oncol/Hematol 111:144–151. doi:10.1016/j.critrevonc.2017.01.014
Smelick GS, Heffron TP, Chu L et al (2013) Prevalence of acid-reducing agents (ARA) in cancer populations and ARA drug-drug interaction potential for molecular targeted agents in clinical development. Mol Pharm 10:4055–4062. doi:10.1021/mp400403s
Budha NR, Frymoyer A, Smelick GS et al (2012) Drug absorption interactions between oral targeted anticancer agents and PPIs: is pH-dependent solubility the Achilles heel of targeted therapy? Clin Pharmacol Ther 92:203–213. doi:10.1038/clpt.2012.73
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was sponsored by Pfizer and was conducted at the Pfizer Clinical Research Unit in New Haven, CT. Medical writing support was provided by David Sunter at Engage Scientific Solutions and was funded by Pfizer.
Conflict of interest
Nagdeep Giri, Robert R. LaBadie, Joseph F. Krzyzaniak, Hong Jiang, Brian Hee, Yali Liang, and M. Naveed Shaik are employees of Pfizer Inc and hold stock and/or stock options in Pfizer Inc; Lisa H. Lam was a paid postdoctoral fellow with Pfizer through the Skaggs School of Pharmacy and Pharmaceutical Sciences at the University of California, San Diego during the conduct of this study and development of this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
This study was sponsored by Pfizer and was conducted at the Pfizer Clinical Research Unit in New Haven, CT, USA.
Rights and permissions
About this article
Cite this article
Giri, N., Lam, L.H., LaBadie, R.R. et al. Evaluation of the effect of new formulation, food, or a proton pump inhibitor on the relative bioavailability of the smoothened inhibitor glasdegib (PF-04449913) in healthy volunteers. Cancer Chemother Pharmacol 80, 1249–1260 (2017). https://doi.org/10.1007/s00280-017-3472-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3472-9