Abstract
Purpose
The purpose of this study was to determine the impact of food on gastric pH and the ability of over the counter betaine hydrochloride (BHCl) acid to reacidify gastric pH after food-induced elevations in gastric pH.
Methods
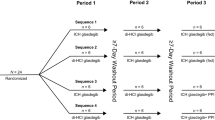
This open-label cross over clinical study (NCT02758015) included 9 subjects who were randomly assigned to one of 16 possible, 4-period cross-over sequences to determine the impact and relationship of food and gastric pH with acid supplementation. Subjects were administered various doses (1500 mg, 3000 mg and 4500 mg) of betaine hydrochloride (BHCl) to determine the ability of acid supplementation to reacidify gastric pH after the elevation of gastric pH caused by the ingestion of food.
Results
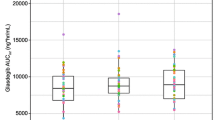
Following the administration of food and the resulting elevation in gastric pH, time to return to baseline gastric pH levels without acid supplementation was 49.7 ± 14.0 min. Administering 4500 mg of BHCl acid in capsules was able to reacidify gastric pH levels back to baseline following the administration of food in approximately 17.3 ± 5.9 min. AUCpH of each treatment were similar and not statistically different. Mean max pH following the administration of food was 3.20 ± 0.55.
Conclusion
The ability of food to elevate and maintain gastric pH levels in the presence of acid supplementation was made evident throughout the study. A 4500 mg dose of BHCl was required to reacidify gastric pH after the administration of food. This study details the difficulty faced by clinicians in dosing a poorly soluble, weakly basic drug to patients receiving acid reducing agents where administration with food is recommended to avoid gastric side effects.
Trial Registration: https://clinicaltrials.gov/ct2/show/NCT02758015



Similar content being viewed by others
Abbreviations
- ARA:
-
Acid reducing agent
- BHCl:
-
Betaine hydrochloride
- GERD:
-
Gastroesophageal reflux disease
- PPI:
-
Proton pump inhibitor
References
Yago MR, Frymoyer AR, Smelick GS, Frassetto LA, Budha NR, Dresser MJ, et al. Gastric reacidification with betaine HCl in healthy volunteers with rabeprazole-induced hypochlorhydria. Mol Pharm. 2013;10(11):4032–7. https://doi.org/10.1021/mp4003738.
Smith DA, van de WH, Walker DK. Pharmacokinetics and metabolism in drug design: Wiley-VCH Verlag GmbH; 2001.
Abuhelwa AY, Williams DB, Upton RN, Foster DJR. Food, gastrointestinal pH, and models of oral drug absorption. Eur J Pharm Biopharm. 2017;112:234–48. https://doi.org/10.1016/j.ejpb.2016.11.034.
Chin TWF, Loeb M, Fong IW. Effects of an acidic beverage (Coca-Cola) on absorption of ketoconazole. Antimicrob Agents Chemother. 1995;39(8):1671–5. https://doi.org/10.1128/AAC.39.8.1671.
Yago MR, Frymoyer A, Benet LZ, Smelick GS, Frassetto LA, Ding X, et al. The use of betaine HCl to enhance dasatinib absorption in healthy volunteers with rabeprazole-induced hypochlorhydria. AAPS J. 2014;16(6):1358–65. https://doi.org/10.1208/s12248-014-9673-9.
Faber KP, Wu H-F, Yago MR, Xu X, Kadiyala P, Frassetto LA, et al. Meal effects confound attempts to counteract rabeprazole-induced hypochlorhydria decreases in atazanavir absorption. Pharm Res. 2017;34(3):619–28. https://doi.org/10.1007/s11095-016-2090-2.
Eley T, Luo FR, Agrawal S, Sanil A, Manning J, Li T, et al. Phase I study of the effect of gastric acid pH modulators on the bioavailability of oral dasatinib in healthy subjects. J Clin Pharmacol. 2009;49:700–9. https://doi.org/10.1177/0091270009333854.
Kletzl H, Giraudon M, Abt M, Hamilton M, Lum BL. Effect of gastric pH on erlotinib pharmacokinetics in healthy individuals: omeprazole and ranitidine. Anti-Cancer Drugs. 2015;26:565–72. https://doi.org/10.1097/CAD.0000000000000212.
Everhart J. Burden of digestive diseases in the United States report. 2008.
Gawron AJ, Pandolfino JE, Miskevics S, Lavela SL. Proton pump inhibitor prescriptions and subsequent use in US veterans diagnosed with gastroesophageal reflux disease. J Gen Intern Med. 2013;28(7):930–7. https://doi.org/10.1007/s11606-013-2345-0.
Smelick GS, Heffron TP, Chu L, Dean B, West DA, DuVall SL, et al. Prevalence of acid-reducing agents (ARA) in cancer populations and ARA drug-drug interaction potential for molecular targeted agents in clinical development. Mol Pharm. 2013;10(11):4055–62. https://doi.org/10.1021/mp400403s.
Dawson RM, et al. Data for biochemical research. 3rd ed. New York: Oxford University Press; 1986.
William RO 3rd, Sykora MA, Mahaguna V. Method to recover a lipophilic drug from hydroxypropyl methylcellulose matrix tablets. AAPS PharmSciTech. 2001;2(2):E8.
Institute of Medicine. Dietary reference intakes for energy, carbohydrate, Fiber, fat, fatty acids, cholesterol, protein, and amino acids; 2005. https://doi.org/10.17226/10490.
Knapp MJ, Berardi RR, Dressman JB, Rider JM, Carver PL. Modification of gastric pH with oral glutamic acid hydrochloride. Clin Pharm. 1991;10(11):866–9.
Falcon RW, Kakuda TN. Drug interactions between HIV protease inhibitors and acid-reducing agents. Clin Pharmacokinet. 2008;47(2):75–89. https://doi.org/10.2165/00003088-200847020-00001.
Budha NR, Frymoyer A, Smelick GS, Jin JY, Yago MR, Dresser MJ, et al. Drug absorption interactions between oral targeted anticancer agents and PPIs: is pH-dependent solubility the Achilles heel of targeted therapy? Clin Pharmacol Ther. 2012;92(2):203–13. https://doi.org/10.1038/clpt.2012.73.
Zhang L, Wu F, Lee SC, Zhao H. pH-dependent drug-drug interactions for weak base drugs: potential implications for new drug development. Clin Pharmacol Ther. 2014;96(2):266–77. https://doi.org/10.1038/clpt.2014.87.
van Leeuwen RWF, Peric R, Hussaarts KG, et al. Influence of the acidic beverage cola on the absorption of erlotinib in patients with non – small-cell lung cancer. J Clin Oncol. 2016;34(12):1309–15. https://doi.org/10.1200/JCO.2015.65.2560.
Prilosec® [Prescribing Information]. AstraZeneca Pharmaceuticals LP. Wilmington, DE. June 2018.
Kis O, J a Z, Ramaswamy M, Bendayan R. pH dependence of organic anion-transporting polypeptide 2B1 in Caco-2 cells : potential role in antiretroviral drug oral bioavailability and drug – drug interactions. J Pharmacol Exp Ther. 2010;334(3):1009–22. https://doi.org/10.1124/jpet.110.166314.
Zhu L, Persson A, Mahnke L, Eley T, Li T, Xu X, et al. Effect of low-dose omeprazole (20 mg daily) on the pharmacokinetics of multiple-dose atazanavir with ritonavir in healthy subjects. J Clin Pharmacol. 2011;51(3):368–77. https://doi.org/10.1177/0091270010367651.
Dressman JB, Berardi RR, Dermentzoglou LC, Russell TL, Schmaltz SP, Barnett JL, et al. Upper gastrointestinal (GI) pH in young, healthy men and women. Pharm Res. 1990;7(7):756–61. https://doi.org/10.1023/A:1015827908309.
Singh B. Effects of food on clinical pharmacokinetics. Clin Pharmacokinet. 1999;37(3):213–55.
Netzer P, Eschenmoser K, Halter F, Inauen W. Impact of food intake on the antisecretory effect of low-dose ranitidine and famotidine. Aliment Pharmacol Ther. 1999;13(3):407–12.
FDA. Guidance for industry: food-effect bioavailability and fed bioequivalence studies. 2002.
Walravens J, Brouwers J, Spriet I, Tack J, Annaert P, Augustijns P. Effect of pH and comedication on gastrointestinal absorption of posaconazole. Clin Pharmacokinet. 2011;50(11):725–34. https://doi.org/10.2165/11592630-000000000-00000.
Agarwala SG, Eley T, Wang Y, Hughes E, Grasela D. Pharmacokinetic interaction between Atazanavir and omeprazole in healthy subjects. In: Presented at the 3rd IAS conference on HIV pathogenesis and treatment. http://www.medadvocates.org/resources/conferences/3rd_ias/05-156b_agarwala_109.pdf. Published; 2005.
Acknowledgments and Disclosures
This study was funded by the Benet Fund for Excellence through contributions and through Dr. Benet’s consultation and expert witness fees, as well as Board of Directors remunerations, all of which are made payable to the Regents of the University of California to support the research studies of the Benet Laboratory. The clinical portion of the study was supported in part by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 TR000004. Conflict of Interests: None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Surofchy, D.D., Frassetto, L.A. & Benet, L.Z. Food, Acid Supplementation and Drug Absorption – a Complicated Gastric Mix: a Randomized Control Trial. Pharm Res 36, 155 (2019). https://doi.org/10.1007/s11095-019-2693-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-019-2693-5