Abstract
Objective: To investigate the effects of mild to moderate hepatic impairment on the pharmacokinetics and pharmacodynamics of landiolol hydrochloride, a new ultra-short-acting β1-adrenergic antagonist.
Methods: Six patients with hepatic impairment and six healthy volunteers were enrolled in the open-label, parallel-group study. Landiolol hydrochloride was given intravenously with a 1-minute loading infusion of 0.06 mg/kg/min, followed by a 60-minute infusion of 0.02 mg/kg/min using an automated infusion pump. Venous blood was drawn just before (predose) and 1, 2, 5, 15, 30 and 61 minutes after beginning the continuous intravenous infusion (during infusion); 2, 5, 10 and 30 minutes and 1, 4 and 8 hours after the end of the infusion (after infusion); and 24 hours after beginning the infusion (next day). Urine samples were collected up to 24 hours after beginning the infusion. Before subjects were discharged, an indocyanine green elimination test, clinical laboratory testing, physical examination and recording of ECGs and vital signs were performed.
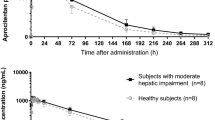
Results: The geometric mean maximum plasma concentration and area under the concentration-time curve values for the patients with hepatic impairment were 42% and 44% higher, respectively, than those observed for the healthy volunteers, indicating that hepatic impairment affected the disposition of landiolol hydrochloride. There were no significant changes in the elimination half-life of the drug. There were no clinically significant differences between the two groups in terms of reductions in heart rate or blood pressure.
Conclusion: The pharmacokinetic and pharmacodynamic characteristics of this ultra-short-acting β1-blocker were maintained even in the patients with hepatic impairment. Although we did not observe any drug-related adverse events in these patients, hypotension or bradycardia should be considered, necessitating continuous monitoring of both heart rate and BP in patients with hepatic impairment who receive landiolol hydrochloride.






Similar content being viewed by others
References
Iguchi S, Iwamura H, Nishizaki M, et al. Development of a highly cardioselective ultra short-acting β-blocker, ONO-1101. Chem Pharm Bull 1992; 40: 1462–9
Nakashima M, Kanamaru M. Phase I study of ONO-1101, a new ultra short acting β1-blocking agent in healthy volunteers (in Japanese). Rinsho Iyaku 2000; 16: 1531–56
Kitamura A, Sakamoto A, Inoue T, et al. Efficacy of an ultrashort-acting β-adrenoceptor blocker (ONO-1101) in attenuating cardiovascular responses to endotracheal intubation. Eur J Clin Pharmacol 1997; 51: 467–71
Atarashi H, Kuruma A, Yashima M, et al. Pharmacokinetics of landiolol hydrochloride, a new ultra-short-acting β-blocker, in patients with cardiac arrhythmias. Clin Pharmacol Ther 2000; 68: 143–50
US Food and Drug Administration: Guidance for industry. Pharmacokinetics in patients with impaired hepatic function: study design, data analysis, and impact on dosing and labeling. Rockville (MD): US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, 2003
Tsunekawa K, Imawaka H, Yamamoto K, et al. Studies on the metabolic fate of ultra short acting β1 blocker ONO-1101 (3): metabolism and protein binding (in Japanese). Drug Metab Pharmacokinet 1997; 12: 31–41
Morgan DJ, McLean AJ. Clinical pharmacokinetic and pharmacodynamic considerations in patients with liver disease: an update. Clin Pharmacokinet 1995; 29: 370–91
Thomson PD, Melmon KL, Richardson JA, et al. Lidocaine pharmacokinetics in advanced heart failure, liver disease and renal failure in humans. Ann Intern Med 1973; 78: 499–508
Crouthamel WG. The effect of congestive heart failure on quinidine pharmacokinetics. Am Heart J 1975; 90: 335–9
Koch-Weser J, Klein SW. Procainamide dosage schedules, plasma concentrations, and clinical effects. JAMA 1971; 215: 1454–60
Rabinowitz JL, Staeffen J, Blanquet P, et al. Sources of serum [14C]-octanoate in cirrhosis of the liver and hepatic encephalopathy. J Lab Clin Med 1978; 91: 223–7
Shrestha R, McKinley C, Showalter R, et al. Quantitative liver function tests define the functional severity of liver disease in early-stage cirrhosis. Liver Transpl Surg 1997; 3: 166–73
Acknowledgements
The authors thank Akihiro Ohnishi, MD, PhD, from the Department of Laboratory Medicine, Daisan Hospital, Jikei University School of Medicine, Tokyo, Japan, for medical advice about adverse events and medical consideration from a third-person persepctive throughout this study. The study was supported by Ono Pharmaceutical Co. Ltd, Osaka, Japan.
The authors have no potential conflicts of interest that are directly relevant to the contents of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takahata, T., Yasui-Furukori, N., Sakamoto, J. et al. Influence of Hepatic Impairment on the Pharmacokinetics and Pharmacodynamics of Landiolol Hydrochloride, an Ultra-Short-Acting β1-Blocker. Drugs in R D 6, 385–394 (2005). https://doi.org/10.2165/00126839-200506060-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00126839-200506060-00006