Summary
Abstract
Interferon-β-1b (Betaseron®, Betaferon®) is a non-glycosylated recombinant human interferon-β approved for high-frequency, subcutaneous (SC) administration in the treatment of multiple sclerosis (MS). Its mechanism of action is unknown, but may involve modulation of the autoimmune pathogenic processes of MS.
In a randomised, double-blind trial in patients with relapsing-remitting MS (RRMS), SC interferon-β-1b 250μg (8 million International Units [MIU]) every other day reduced the annual relapse rate and increased the proportion of relapse-free patients compared with placebo. It also reduced relapse severity, hospitalisations, and disease activity assessed by magnetic resonance imaging (MRI), and increased the time to first relapse. Progression of disability showed a trend towards reduction relative to placebo and baseline, but did not reach statistical significance.
SC interferon-β-1b 250μg every other day was shown in a randomised trial to be superior to intramuscular (IM) interferon-β-1a 30μg (6 MIU) once weekly with respect to reductions in relapse-related parameters, disability progression and MRI-assessed disease activity.
In patients with secondary progressive MS (SPMS), SC interferon-β-1b 250μg every other day slowed progression of the disease relative to placebo in one randomised, double-blind trial, but not in another. In both studies, interferon-β-1b 250μg recipients had fewer relapses and less MRI-assessed disease activity than placebo recipients. The difference in primary outcome may reflect differences in patient entry criteria.
Interferon-β-1b is generally well tolerated and the common adverse events (e.g. injection site reactions, asthenia and an influenza-like symptom complex) are clinically manageable. In a randomised trial, the tolerability of SC interferon-β-1b 250μg every other day was generally similar to that of IM interferon-β-1a 30μg once weekly, except for higher incidences of injection site reactions and neutralising anti-interferon-β antibodies with SC interferon-β-1b.
In conclusion, SC interferon-β-1b 250μg every other day reduces the frequency and severity of relapses and MRI measures of disease activity and may delay the progression of disability in RRMS. The drug appeared to be more effective than, and as well tolerated as, IM interferon-β-1a 30μg once weekly. Interferon-β-1b also has positive effects on relapse rates and disease activity in patients with SPMS, although its effects on disease progression remain uncertain. The drug is generally well tolerated, and the common adverse events are clinically manageable. Thus, interferon-β-1b is a valuable first-line therapy for patients with RRMS and a potentially useful option in those with SPMS.
Pharmacodynamic Properties
The mechanism of action of interferon-β-1b in multiple sclerosis (MS) is not known and could involve any of the known actions of interferon-β, which include antiviral, anti-inflammatory, antiproliferative and immunomodulatory activities. Owing to the autoimmune nature of MS, current hypotheses favour the immunomodulatory actions of interferon-β-1b as those responsible for its therapeutic efficacy in MS. Interferon-β-1b is thought to interfere with T-cell activation, cytokine production and cellular activities responsible for the inflammatory and destructive processes occurring within the CNS. Interferon-β-1b may also be instrumental in maintaining the integrity of the blood-brain barrier and preventing the penetration of activated autoimmune T-cells into the CNS.
Interferon-β-1b is immunogenic and many patients develop binding antibodies to interferon-β. A proportion of these are neutralising antibodies (NAB) that inhibit the biological activity of interferon-β. The reported prevalence of NAB is variable (28–38% in pivotal clinical trials) since it is highly dependent on the assay method used. NAB develop early after initiation of therapy; titres peak at 1.5–2 years, and then decline with continued therapy. Many patients initially positive for NAB to interferon-β-1b revert to an NAB-negative status. A recent consensus statement suggests that NAB-related loss of clinical efficacy of interferon-β may occur, depending on the duration and severity of decreased bioactivity. However, current data on the clinical impact of NAB in trials of interferon-β-1b, as with other interferon-β products, are conflicting. NAB are cross-reactive between interferon-β-1b and interferon-β-1a. In the absence of guidelines on how to manage the NAB-positive patient, it is appropriate to base treatment decisions on clinical, rather than NAB, status.
Pharmacokinetic Properties
The pharmacokinetic properties of interferon-β-1b are not well characterised as a result of technical difficulties in quantifying serum concentrations of the drug. Following single-dose subcutaneous (SC) administration of 500μg (16 million International Units [MIU]) in 12 healthy adult volunteers, peak serum concentrations were attained at 1–8 hours. The bioavailability was 51%. There was no serum accumulation following SC administration of 500μg once daily for 8 days in healthy volunteers. Peak serum concentrations of interferon-β-1b in three patients with MS receiving long-term therapy with SC interferon-β-1b 250μg every other day were 260–290 IU/mL at 8–24 hours; they declined to near pre-injection levels (75–90 IU/mL) by 48 hours. Following intravenous administration in 9 healthy volunteers, the elimination half-life was 4.29 hours, the clearance was 0.76 L/h/kg, and the steady state volume of distribution was 2.88 L/ kg.
Blood levels of biological response markers (BRMs) induced by interferon-β, such as neopterin, β2-microglobulin and human myxovirus protein A (MxA), are more frequently used to assess the kinetics of interferon-β-1b activity. After single-dose administration of SC interferon-β-1b 250μg in 5 healthy volunteers, peak blood levels of the BRMs occurred at 24–48 hours and then declined to baseline levels by 120–168 hours. Blood levels of BRMs increased in a dose-dependent manner with SC interferon-β-1b 62.5–375μg. During multiple-dose SC administration of interferon-β-1b 250μg every other day in healthy volunteers, maximum blood levels of various BRMs were attained after 40–123 hours.
Therapeutic Efficacy
A small dose-finding study in 30 patients with relapsing-remitting MS (RRMS) indicated that SC interferon-β-1b 250μg every other day was an appropriate dosage regimen based on efficacy and tolerability.
The pivotal multicentre, randomised, double-blind trial in 338 patients with RRMS demonstrated that SC interferon-β-1b 250μg every other day significantly reduced the mean annual relapse rate and increased the proportion of relapse-free patients compared with placebo (primary endpoints assessed at 2 years). In addition, interferon-β-1b was more effective than placebo for other endpoints (measured over 2 or 3 years), with a longer median time to first relapse, reductions in relapse severity and MS-related hospitalisations, and improvement in all magnetic resonance imaging (MRI) measures of disease activity. Improvements in relapse rates and annual activity monitored by MRI were maintained throughout the 5-year study period. Interferon-β-1b did not significantly reduce disease progression over 5 years, relative to placebo or baseline, as assessed by changes in Kurtzke’s Expanded Disability Status Scale (EDSS) scores, although the trial was not sufficiently powered to show such an effect.
In the only head-to-head, randomised, active comparator trial of interferon-β-1b, the Independent Comparison of Interferons (INCOMIN) trial in patients with RRMS, SC interferon-β-1b 250μg every other day for 2 years (n = 96) was superior to intramuscular (IM) interferon-β-1a 30μg (6 MIU) once weekly (n =92) for all efficacy parameters, including the primary endpoint of proportion of patients remaining relapse free after 2 years (51% vs 36%, p < 0.05). Mean annual relapse rates, progression of disability (assessed using EDSS) and MRI measures of disease activity were all significantly lower in the interferon-β-1b group.
In the randomised, double-blind European study of SC interferon-β-1b 250μg every other day for 3 years in patients with secondary progressive MS (SPMS), interferon-β-1b significantly delayed the time to confirmed disease progression according to changes in the EDSS scores, and reduced the proportion of patients with confirmed progression by 21.7% and the proportion of patients becoming wheelchair bound by 32% relative to placebo. In addition, interferon-β-1b increased the time to first relapse and reduced the annual relapse rate, the proportion of moderate or severe relapses, MS-related hospital admissions and corticosteroid use, and MRI-assessed newly active lesions and total lesion volume. The relative difference in EDSS scores between placebo and interferon-β-1b recipients observed at 36 months was maintained during open-label treatment in a subset of patients assessed at 96 months.
In contrast, a randomised, double-blind study conducted in North America in patients with SPMS showed that SC interferon-β-1b 250μg or 160 μg/m2 (5 MIU/ m2) every other day for 3 years had no significant effect on disease progression, as measured by changes in EDSS scores, compared with placebo, although at the fixed 250μg dosage the annual relapse rate was significantly reduced, and both dosages significantly reduced MRI-assessed lesions, relative to placebo. Neither dosage affected the proportion of moderate or severe relapses, MS-related hospitalisations or the number of patients requiring corticosteroids (although there were fewer corticosteroid courses with the 250μg dosage).
Patients with SPMS treated with SC interferon-β-1b 250μg every other day for up to 3 years had mean total Sickness Impact Profile scores not significantly different from those in placebo recipients. Between-group differences in favour of interferon-β-1b for the physical dimension components of this health-related quality of life (HR-QOL) assessment scale reached statistical significance at 6 and 12 months and at the last visit, while those for the psychosocial dimension were significant only at 18 months.
HR-QOL in patients with RRMS treated for >2 years (mean duration approximately 5 years) with interferon-β-1b 250μg every other day (n = 80) was assessed using the Short-Form Health Survey (SF-36) questionnaire. Relative to historical control patients, those with an EDSS score <3.0 had significant improvements in 4 of 8 SF-36 domains, those with an EDSS score 3.0–6.0 had no improvement, and those with an EDSS score >6.0 had improvement in the physical function domain.
Pharmacoeconomic Considerations
Using a £30 000/quality-adjusted life year (QALY) threshold and a 20-year time frame, interferon-β-1b approached a 50% probability of being cost effective for both RRMS and SPMS, a level of cost effectiveness less likely to be achieved by three other interferon-β products or glatiramer acetate.
Relative to no treatment, interferon-β-1b appeared cost effective over 10 years (cost/QALY, €7800) and cost saving over 15-25 years when progression frorr RRMS to SPMS, and costs and utilities at all disease/disability levels, were considered. The cost/QALY over 20 years for a patient starting treatment at EDSS level 3.0 had a >80% probability of being below €50 000. Nonetheless, uncertainty over the cost-utility of interferon-β-1b remains and firm conclusions cannot be drawn at this time.
Tolerability
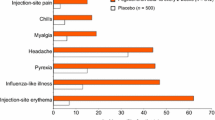
SC interferon-β-1b is generally well tolerated at the recommended dosage of 250μg every other day in patients with MS. The most frequent adverse events, occurring in ≥30% of patients, are injection site reaction, asthenia, influenza-like symptoms, headache, pain, hypertonia, myasthenia, fever and arthralgia. Injection site necrosis occurred in 5% of patients. Lymphopenia is the most common laboratory abnormality, but is generally mild and intermittent. Elevated liver enzymes occur in 3% (AST) and 10% (ALT) of patients. Autoimmune disease and/or autoantibodies may occur in a small proportion of patients and most commonly manifest clinically as thyroid or liver dysfunction. Although depression may increase with therapy with interferon-β, the incidence of depression with interferon-β-1b was similar to that with placebo in clinical trials. Interferon-β-1b at dosages higher than those currently recommended appears to have acceptable tolerability in ongoing trials.
In the 2-year INCOMIN trial in 186 patients with RRMS, the tolerability profile of SC interferon-β-1b 250μg every other day was similar to that of IM interferon-β-1a 30μg once weekly, except for significantly higher incidences of injection site reactions and NAB with interferon-β-1b.
A randomised, rater-blinded, 4-week study in 64 healthy volunteers indicated that SC interferon-β-1b 250μg every other day caused approximately one-third less injection site pain than SC interferon-β-1a 44μg (12MIU) three times weekly (p < 0.0001), as well as almost one-half the number of injection site reactions (p < 0.0001), which were of lower severity (p < 0.01).
Dosage and Administration
Interferon-β-1b is approved in the US for the treatment of relapsing forms of MS to reduce the frequency of clinical relapses, and in Europe for the treatment of active RRMS and SPMS; the difference in indications reflects the disparate results of the two pivotal trials in SPMS conducted in the US and Europe. The recommended dosage in both the US and Europe is 250μg injected SC every other day; in the US, the dosage should be titrated to this level over a 6-week period. The duration of therapy is undefined, but consensus guidelines recommend that therapy be continued indefinitely where possible. Therapy should be restricted to adult patients (aged 18–64 years).
Interferon-β-1b is contraindicated in patients with a history of hypersensitivity to natural or recombinant interferon-β, pregnant women and breast-feeding mothers. Special caution should be exercised in patients with active depression. There are no known interactions between interferon-β-1b and other drugs.






Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
O’Connor P. Key issues in the diagnosis and treatment of multiple sclerosis: an overview. Neurology 2002 Sep 24; 59(6 Suppl 3): S1–33
Compston A, Coles A. Multiple sclerosis. Lancet 2002; 359(9313): 1221–31
National Multiple Sclerosis Society. The National MS Society Information Sourcebook: epidemiology [online]. Available from URL: http://www.nationalmssociety.org/sourcebook.asp [Accessed 2003 Dec 3]
Corboy JR, Goodin DS, Frohman EM. Disease-modifying therapies for multiple sclerosis. Curr Treat Options Neurol 2003 Jan; 5(1): 35–54
Miltenburger C, Kobelt G. Quality of life and cost of multiple sclerosis. Clin Neuro Neurosurg 2002; 104(3): 272–5
Rudick RA. Disease-modifying drugs for relapsing-remitting multiple sclerosis and future directions for multiple sclerosis therapeutics. Arch Neurol 1999 Sep; 56(9): 1079–84
Goodin DS. Therapeutic developments in multiple sclerosis. Expert Opin Investig Drugs 2000 Apr; 9(4): 655–70
Karussis DM, Abramsky O. Immunomodulating therapeutic approaches for multiple sclerosis. J Neurol Sci 1998; 153(2): 239–50
Freedman MS, Blumhardt LD, Brochet B, et al. International consensus statement on the use of disease-modifying agents in multiple sclerosis. Mult Scler 2002 Feb; 8(1): 19–23
Goodin DS, Frohman EM, Garmany GP Jr, et al. Disease modifying therapies in multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the MS Council for Clinical Practice Guidelines [published erratum appears in Neurology 2002 Aug 13; 59 (3):480]. Neurology 2002 Jan 22; 58(2): 169–78
Chiron Corporation, Berlex Laboratories. Betaseron®: interferon beta-1b, US prescribing information [online]. 2003
Biogen Inc. Avonex® (interferon beta-1a): US prescribing information [online]. Available from URL: http://www.avonex.com/forms/Avonex_Lyo_PI.pdf [Accessed 2003 Nov 24]
Serono Inc. Rebif® (interferon beta-1a): US prescribing information [online]. Available from URL: http://www.rebif.com/assets/pdfs/Rebif_PI.pdf [Accessed 2003 Nov 24]
Faulds D, Benfield P. Interferon beta-1b in multiple sclerosis: an initial review of its rationale for use and therapeutic potential. Clin Immunother 1994 Jan; 1: 79–87
Dhib-Jalbut S. Mechanisms of action of interferons and glatiramer acetate in multiple sclerosis. Neurology 2002 Apr 23; 58(8 Suppl 4): S3–9
Horowski R. Multiple sclerosis and interferon β-1b, past, present and future. Clin Neurol Neurosurg 2002 Jul; 104(3): 259–64
Rolak LA. Multiple sclerosis treatment 2001. Neurol Clin 2001 Feb; 19(1): 107–18
Huynh HK, Oger J, Dorovini-Zis K. Interferon-β downregulates interferon-γ-induced class II MHC molecule expression and morphological changes in primary cultures of human brain microvessel endothelial cells. J Neuroimmunol 1995; 60(1-2): 63–73
Genc K, Dona DL, Reder AT. Increased CD80+ B cells in active multiple sclerosis and reversal by interferon β-1b therapy. J Clin Invest 1997 Jun 1; 99(11): 2664–71
Ozenci V, Kouwenhoven M, Huang YM, et al. Multiple sclerosis: levels of interleukin-10-secreting blood mononuclear cells are low in untreated patients but augmented during interferon-β-1b treatment. Scand J Immunol 1999 May; 49(5): 554–61
Nicoletti F, Di Marco R, Patti F, et al. Blood levels of transforming growth factor-beta 1 (TGF-β1) are elevated in both relapsing remitting and chronic progressive multiple sclerosis (MS) patients and are further augmented by treatment with interferon-beta 1b (IFN-β1b). Clin Exp Immunol 1998 Jul; 113(1): 96–9
Brod SA, Marshall GD Jr, Henninger EM, et al. Interferon-β1b treatment decreases tumor necrosis factor-α and increases interleukin-6 production in multiple sclerosis. Neurology 1996 Jun; 46(6): 1633–8
Calabresi PA, Tranquill LR, Dambrosia JM, et al. Increases in soluble VCAM-1 correlate with a decrease in MRI lesions in multiple sclerosis treated with interferon β-1b. Ann Neurol 1997 May; 41(5): 669–74
Prat A, Al-Asmi A, Duquette P, et al. Lymphocyte migration and multiple sclerosis: relation with disease course and therapy. Ann Neurol 1999; 46(2): 253–6
Uhm JH, Dooley NP, Stuve O, et al. Migratory behavior of lymphocytes isolated from multiple sclerosis patients: effects of interferon β-1b therapy. Ann Neurol 1999 Sep; 46(3): 319–24
Trojano M, Avolio C, Liuzzi GM, et al. Changes of serum sICAM-1 and MMP-9 induced by rIFNβ-1b treatment in relapsing-remitting MS. Neurology 1999 Oct 22; 53(7): 1402–8
Pachner AR. Measurement of antibodies to interferon beta in patients with multiple sclerosis. Arch Neurol 2001 Aug; 58(8): 1299–300
Rice G. The significance of neutralizing antibodies in patients with multiple sclerosis treated with interferon beta. Arch Neurol 2001 Aug; 58(8): 1297–8
Brickelmaier M, Hochman PS, Baciu R, et al. ELISA methods for the analysis of antibody responses induced in multiple sclerosis patients treated with recombinant interferon-β. J Immunol Methods 1999 Jul 30; 227(1-2): 121–35
The IFNB Multiple Sclerosis Study Group, University of British Columbia MS/MRI Analysis Group. Neutralizing antibodies during treatment of multiple sclerosis with interferon beta-1b: experience during the first three years. Neurology 1996 Oct; 47(4): 889–94
Polman C, Kappos L, White R, et al. Neutralizing antibodies during treatment of secondary progressive MS with interferon β-1b. Neurology 2003 Jan 14; 60(1): 37–43
US FDA. Medical officer clinical review of the North American study of Betaseron® in the treatment of secondary progressive multiple sclerosis [online]. Available from URL: http://www.fda.gov/cder/biologics/review/ifnbchi031403r2.pdf [Accessed 2003 Oct 15]
Hurwitz B, Polman C. Neutralizing antibodies to interferon beta-1b (Betaferon®/Betaseron®): real-world data [poster P181]. 19th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS); 2003 Sep 17–20; Milan, Italy
Knobler RL, Greenstein JI, Johnson KP, et al. Systemic recombinant human interferon-β treatment of relapsing-remitting multiple sclerosis: pilot study analysis and six-year follow-up. J Interferon Res 1993 Oct; 13(5): 333–40
Kremenchutzky M. Long-term evolution of anti-IFNb antibodies in IFNb-treated MS patients: the London, Canada, MS Clinic experience. Neurology 2003 Nov; 61Suppl. 5: S29–30
Khan OA, Xia Q, Bever Jr CT, et al. Interferon beta-1b serum levels in multiple sclerosis patients following subcutaneous administration. Neurology 1996 Jun; 46(6): 1639–43
Deisenhammer F, Reindl M, Harvey J, et al. Bioavailability of interferon beta 1b in MS patients with and without neutralizing antibodies. Neurology 1999 Apr 12; 52(6): 1239–43
Milano E. IFNβ bioavailability in MS patients treated with three different IFNβ preparations: a 96-hour time-course study [abstract no. P01.140]. Neurology 2002 Apr; 58Suppl. 3: A72
Bertolotto A, Gilli F, Sala A, et al. Persistent neutralizing antibodies abolish the interferon β bioavailability in MS patients. Neurology 2003; 60(4): 634–9
Pachner AR. Anti-IFNβ antibodies in IFNβ-treated MS patients: summary. Neurology 2003 Nov; 61Suppl. 5: Sl–5
Petkau AJ. Statistical approaches to assessing the effects of neutralizing antibodies: IFNβ-1b in the pivotal trial of relapsing-remitting multiple sclerosis. Neurology 2003; 61Suppl. 5: S35–7
Petkau AJ, White RA, Ebers GC, et al. Longitudinal analyses of the effects of neutralizing antibodies on interferon beta-1b in relapsing-remitting multiple sclerosis. Mult Scler 2004; 10: 126–38
Rudick RA. Biological impact of interferon antibodies, and complexities in assessing their clinical significance. Neurology 2003; 61Suppl. 5: S31–4
Bertolotto A, Malucchi S, Sala A, et al. Differential effects of three interferon betas on neutralising antibodies in patients with multiple sclerosis: a follow up study in an independent laboratory. J Neurol Neurosurg Psychiatry 2002 Aug; 73(2): 148–53
Scagnolari C, Bellomi F, Turriziani O, et al. Neutralizing and binding antibodies to IFN-β: relative frequency in relapsing-remitting multiple sclerosis patients treated with different IFN-β preparations. Journal of Interferon & Cytokine Research 2002; 22(2): 207–13
Ross C, Clemmesen KM, Svenson M, et al. Immunogenicity of interferon-β in multiple sclerosis patients: influence of preparation, dosage, dose frequency, and route of administration. Ann Neurol 2000; 48(5): 706–12
Kivisakk P, Alm GV, Fredrikson S, et al. Neutralizing and binding anti-interferon-β (IFN-β) antibodies. A comparison between IFN-β-2a and IFN-β-2b treatment in multiple sclerosis. Eur J Neurol 2000 Jan; 7(1): 27–34
Cook SD, Quinless JR, Jotkowitz A, et al. Serum IFN neutralizing antibodies and neopterin levels in a cross-section of MS patients. Neurology 2001 Sep 25; 57(6): 1080–4
Perini P, Facchinetti A, Bulian P, et al. Interferon-beta (IFN-β) antibodies in interferon-β la- and interferon-β1b-treated multiple sclerosis patients. Prevalence, kinetics, cross-reactivity, and factors enhancing interferon-β immunogenicity in vivo. Eur Cytokine Netw 2001 Mar; 12(1): 56–61
Durelli L, Verdun E, Barbero P, et al. Every-other-day interferon beta-1b versus once-weekly interferon beta-1a for multiple sclerosis: results of a 2-year prospective randomised multicentre study (INCOMIN). Lancet 2002 Apr 27; 359(9316): 1453–60
Panitch H, Goodin DS, Francis G. Randomized, comparative study of interferon b-la treatment regimens in MS: the EVIDENCE trial. Neurology 2002 Nov 26; 59(10): 1496–506
Khan OA, Dhib-Jalbut SS. Neutralizing antibodies to interferon β-la and interferon β-1b in MS patients are cross-reactive. Neurology 1998 Dec; 51(6): 1698–702
Bertolotto A, Malucchi S, Milano E, et al. Interferon β neutralizing antibodies in multiple sclerosis: neutralizing activity and cross-reactivity with three different preparations. Immunopharmacology 2000 Jul 20; 48(2): 95–100
Deisenhammer F, Mayringer I, Harvey J, et al. A comparative study of the relative bioavailability of different interferon beta preparations. Neurology 2000 Jun 13; 54(11): 2055–60
Sturzebecher S, Maibauer R, Heuner A, et al. Pharmacodynamic comparison of single doses of IFN-βla and IFN-β1b in healthy volunteers. J Interferon Cytokine Res 1999; 19(11): 1257–64
Chiang J, Gloff CA, Yoshizawa CN, et al. Pharmacokinetics of recombinant human interferon-β ser in healthy volunteers and its effect on serum neopterin. Pharm Res 1993 Apr; 10(4): 567–72
Bertolotto A, Gilli F, Sala A, et al. Evaluation of bioavailability of three types of IFNβ in multiple sclerosis patients by a new quantitative-competitive-PCR method for MxA quantification. J Immunol Methods 2001; 256(1-2): 141–52
Williams GJ, Witt PL. Comparative study of the pharmacodynamic and pharmacologie effects of Betaseron and AVONEX. J Interferon Cytokine Res 1998 Nov; 18(11): 967–75
The IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebocontrolled trial. Neurology 1993 Apr; 43(4): 655–61
Itoyama Y, Saida T, Tashiro K, et al. Japanese multicenter, randomized, double-blind trial of interferon β-1b in relapsingremitting multiple sclerosis: two-year results [abstract no. 269. Ann Neurol 2000 Sep; 48: 487
European Study Group on Interferon β-1b in Secondary Progressive MS. Placebo-controlled multicentre randomised trial of interferon β-1b in treatment of secondary progressive multiple sclerosis. Lancet 1998 Nov 7; 352: 1491–7
Goodkin DE, CA and the North American Study Group on Interferon beta-1b in Secondary Progressive MS. Interferon beta-1b in secondary progressive MS: clinical and MRI results of a 3-year randomized controlled trial [abstract no. LBN.004]. 52nd Annual Meeting of the American Academy of Neurology Late Breakers; 2000 Apr 29–May 6; San Diego, USA
Kurtzke JF. Rating neurological impairment in multiple sclerosis: and expanded disability status scale (EDSS). Neurology 1983 Nov; 33(11): 1444–52
Arnold DL, Matthews PM. MRI in the diagnosis and management of multiple sclerosis. Neurology 2002; 58(8 Suppl. 4): S23–31
Durelli L, Oggero A, Verdun E, et al. Does high-dose interferon beta-1b improve clinical response in more severely disabled multiple sclerosis patients? J Neurol Sci 2000 Sep; 178: 37–41
Durelli L, Verdun E, Barbero P, et al. The OPTimization of interferon for MS (OPTIMS) trial: a multicenter trial comparing two different doses (250 μg and 375 μg) of IFNB-1b [poster]. 56th American Academy of Neurology (AAN) Meeting; 2004 April 24–May 1 May; San Francisco (CA)
Paty DW, Li DK. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. II. MRI analysis results of a multicenter, randomized, double-blind, placebo-controlled trial. UBC MS/MRI Study Group and the IFNB Multiple Sclerosis Study Group. Neurology 1993 Apr; 43(4): 662–7
The IFNB Multiple Sclerosis Study Group, University of British Columbia MS/MRI Analysis Group. Interferon beta-1b in the treatment of multiple sclerosis: final outcome of the randomized controlled trial. Neurology 1995 Jul; 45: 1277–85
Schering AG. Longest ever follow-up of beta interferon in multiple sclerosis: sustained benefits and tolerability over 12 years for Betaferon (R) therapy [media release]. Available from URL: http://www.schering.de/eng [Accessed 2002 Mar 11]
Karlik S, Kirk S, Nicolle E, et al. Evidence for very long term MRI efficacy of interferon beta 1b in relapsing remitting MS patients [abstract]. Presented at the 17th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS); 2001 Sep 12–15; Dublin, Ireland
Khan OA, Tselis AC, Kamholz JA, et al. A prospective, open-label treatment trial to compare the effect of IFN beta-1a (Avonex), IFNbeta-1b (Betaseron), and glatiramer acetate (Copaxone) on the relapse rate in relapsing-remitting multiple sclerosis. Eur J Neurol 2001 Mar; 8(2): 141–8
Milanese C, La Mantia L, Salmaggi A, et al. Azathioprine and interferon β-1b treatment in relapsing-remitting multiple sclerosis [letter]. J Neurol Neurosurg Psychiatry 2001 Mar; 70(3): 413–4
Pakdaman H. Response to beta-interferon in Iranian multiple sclerosis patients [abstract no. P-146]. Mult Scler 2001 Sep; 7Suppl. 1: S54
Haas J, Firzlaff M, Schmidt M. Comparison of new immunomodulatory treatments in the early stages of MS [abstract no. O-28]. Mult Scler 2001 Sep; 7Suppl. 1: S15
Flechter S, Vardi J, Pollak L, et al. Comparison of glatiramer acetate (Copaxone) and interferon b-1b (Betaferon) in multiple sclerosis patients: an open-label 2-year followup. J Neurol Sci 2002 May 15; 197(1-2): 51–5
Öztekin N, Öztekin MF. An open label trial comparing the effects of IFNβ-1a (Rebif), (Avonex), and IFNβ-1b (Betaferon) on the relapse rate, lesion load on MRI and disease progression in patients with relapsing-remitting multiple sclerosis: results of 24 months of therapy [abstract no. P-312]. Mult Scler 2001 Sep; 7Suppl. 1: S96
Trojano M, Liguori M, Paolicelli D, et al. Interferon beta in relapsing-remitting multiple sclerosis: an independent post-marketing study in southern Italy. Mult Scler 2003; 9: 451–7
Sormani MP, Bruzzi P, Beckmann K, et al. MRI metrics as surrogate endpoints for EDSS progression in SPMS patients treated with IFN β-1b. Neurology 2003 May 13; 60(9): 1462–6
Kuhle J, Hardmeier M, D’Hooghe M. Long-term follow-up of the European Study of Interferon beta-1b (EUSPMS) in secondary progressive MS [abstract no. P329, plus poster]. 19th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS); 2003 Sep 17–20; Milan, Italy
Molyneux PD, Kappos L, Polman C, et al. The effect of interferon beta-1b treatment on MRI measures of cerebral atrophy in secondary progressive multiple sclerosis. European Study Group on Interferon beta-1b in secondary progressive multiple sclerosis. Brain 2000 Nov; 123 (Pt 11): 2256–63
Brex PA, Molyneux PD, Smiddy P, et al. The effect of IFNβ-1b on the evolution of enhancing lesions in secondary progressive MS. European Study Group on Interferon beta-1b in Secondary Progressive MS. Neurology 2001 Dec 26; 57(12): 2185–90
Barkhof F, van Waesberghe JH, Filippi M, et al. T1 hypointense lesions in secondary progressive multiple sclerosis: effect of interferon beta-1b treatment. Brain 2001 Jul; 124 (Pt 7): 1396–402
Freeman JA, Thompson AJ, Fitzpatrick R, et al. Interferon-β1b in the treatment of secondary progressive MS: impact on quality of life. Neurology 2001 Nov 27; 57(10): 1870–5
Rice GP, Oger J, Duquette P, et al. Treatment with interferon beta-1b improves quality of life in multiple sclerosis. Can J Neurol Sci 1999 Nov; 26(4): 276–82
Schwartz CE, Coulthard-Morris L, Cole B, et al. The quality-of-life effects of interferon beta-1b in multiple sclerosis. An extended Q-TWiST analysis. Arch Neurol 1997 Dec; 54(12): 1475–80
Chilcott J, McCabe C, Tappenden P, et al. Modelling the cost effectiveness of interferon beta and glatiramer acetate in the management of multiple sclerosis. BMJ 2003; 326(7388): 522–5
Kobelt G, Jönsson L, Fredrikson S. Cost-utility of interferon β1b in the treatment of patients with active relapsing-remitting or secondary progressive multiple sclerosis. Eur J Health Econom 2003; 4(1): 50–9
TappendenP, Chilcott J, O’HaganT, et al. Cost effectiveness of beta interferons and glatiramer acetate in the management of multiple sclerosis: final report to the National Institute for Clinical Excellence [online]. Available from URL: http://www.nice.org.uk/pdf/msscharrreport.pdf [Accessed 2004 Mar 30]
Parkin D, McNamee P, Jacoby A, et al. A cost-utility analysis of interferon beta for multiple sclerosis. Health Technology Assessment (Winchester, England) 1998; 2(4): 1–67
Orlewska E, Mierzejewski P, Czlonkowska A. Cost-effectiveness and cost-utility analysis of interferon beta-1B in relapsing-remitting multiple sclerosis in Poland (2-years perspective) [abstract no. PNR1]. Value Health 2000 Sep–Oct; 31: 362–3
Forbes RB, Lees A, Waugh N, et al. Population based cost utility study of interferon beta-1b in secondary progressive multiple sclerosis. BMJ 1999 Dec 11; 319(7224): 1529–33
Kobelt G, Jonsson L, Henriksson F, et al. Cost-utility analysis of interferon beta-1b in secondary progressive multiple sclerosis. Int J Technol Assess Health Care 2000 Summer; 16(3): 768–80
Kobelt G, Jonsson L, Miltenburger C, et al. Cost-utility analysis of interferon beta-1B in secondary progressive multiple sclerosis using natural history disease data. Int J Technol Assess Health Care 2002 Winter; 18(1): 127–38
Touchette DR, Durgin TL, Wanke LA, et al. A cost-utility analysis of mitoxantrone hydrochloride and interferon beta-1b in the treatment of patients with secondary progressive or progressive relapsing multiple sclerosis. Clin Ther 2003 Feb; 25(2): 611–34
National Institute for Clinical Excellence. Beta interferon and glatiramer acetate for the treatment of multiple sclerosis [online]. Available from URL: http://www.nice.org.uk [Accessed 2004 Mar 30]
Schering AG. Betaferon®: summary of product characteristics, European prescribing information [online]. Available from URL: http://www.emea.eu.int/humandocs/humans/EPAR/Betaferon/Betaferon.htm [Accessed 2003 Oct 15]
Durelli L, Ferrero B, Oggero A, et al. Autoimmune events during interferon beta-1b treatment for multiple sclerosis. J Neurol Sci 1999 Jan 1; 162(1): 74–83
Ferrero B, Durelli L, Ecari U, et al. The Betaferon Safety Trial: prospective multicentric monitoring of autoimmunity during interferon-beta-1b (IFNB) therapy for multiple sclerosis (MS) [abstract no. P01.071]. Neurology 1998 Apr; 50(4 Suppl.): 33
Kreisler A, de Seze J, Stojkovic T, et al. Multiple sclerosis, interferon beta and clinical thyroid dysfunction. Acta Neurol Scand 2003 Feb; 107(2): 154–7
Rotondi M, Oliviero A, Profice P, et al. Occurrence of thyroid autoimmunity and dysfunction throughout a nine-month follow-up in patients undergoing interferon-β therapy for multiple sclerosis. J Endocrinol Invest 1998; 21(11): 748–52
Mohr DC, Goodkin DE, Likosky W, et al. Treatment of depression improves adherence to interferon beta-1b therapy for multiple sclerosis. Arch Neurol 1997 May; 54(5): 531–3
Blundo C, Ricci M, Galgani S, et al. Depressive disorders in relapsing-remitting multiple sclerosis patients treated with interferon β-1b: preliminary results after 12 months of therapy. Eur J Neurol 1998 Sep; 5Suppl. 3: 125
Borras C, Rio J, Porcel J, et al. Emotional state of patients with relapsing-remitting MS treated with interferon beta-1b. Neurology 1999 May 12; 52(8): 1636–9
Metz LM, Francis G, Grumser Y. Depression in MS: The relationship to interferon therapy [abstract no. P03.025]. Neurology 2002 Apr; 58Suppl. 3: A188
Durelli L, Oggero A, Verdun E, et al. Interferon-β; dose and efficacy: the OPTIMS study. Neurological Sciences 2001; 22(2): 201–3
Verdun E, Ricci A, Clerico M, et al. Evaluation of high-dose interferon beta-1b in relapsing-remitting MS non-responder patients: the OPTIMS trial. Safety data [abstract no. S21.004]. Neurology 2003 Mar 11; 60Suppl. 1: A168
HurwitzB,ArnasonBG,Bigley GK, et al. Betaseron®/Betafer-on® efficacy yielding outcomes of a new dose (BEYOND) —safety and tolerability of 500 mcg vs 250 mcg of interferon beta-1b. Presented at the 19th Congress of the European Committee for Treatment and Research in Multiple Sclerosis.
Schering AG. BEYOND program shows new, higher Betafer-on® dose is safe and well tolerated [media release]. 2003 Sep 18
Goodin DS. A comparison of the biological response and tolerability between the two high-dose, high-frequency beta interferons in healthy volunteers [poster]. Presented at the 13th European Neurological Society Annual Meeting; 2003 Jun 14–18; Istanbul, Turkey
Lublin FD, Whitaker JN, Eidelman BH, et al. Management of patients receiving interferon beta-1b for multiple sclerosis: report of a consensus conference. Neurology 1996 Jan; 46(1): 12–8
PRISMS Study Group. Randomised double-blind placebo-controlled study of interferon β-1a in relapsing/remitting multiple sclerosis. Lancet 1998; 352: 1498–504
Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. Multiple Sclerosis Collaborative Research Group. Ann Neurol 1996; 39: 285–94
Johnson KP, Brooks BR, Cohen JA, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind, placebo-controlled trial. Neurology 1995 Jul; 45: 1268–76
Comi G, Filippi M, Wolinsky JS. European/Canadian multicenter, double-blind, randomized, placebo-controlled study of the effects of glatiramer acetate on magnetic resonance imaging-measured disease activity and burden in patients with relapsing multiple sclerosis. European/Canadian Glatiramer Acetate Study Group. Ann Neurol 2001 Mar; 49(3): 290–7
Durelli L, Barbero P, Verdun E, et al. Reducing interferon beta dose in chronically-treated MS patients may have long-term deleterious effects on disease activity [abstract no. S21.002]. Neurology 2003 Mar 11; 60Suppl. 1: A167
Gupta A. ABOVE: interferon beta-1a, interferon beta-1b,observation of efficacy. Presented at the 19th Congress of the European Committee for Treatment and Research in Multiple Sclerosis, Milan, Italy, 17–20 Sep 2003 [online]. Available from URL: http://www.akm.ch/ectrims2003/ [Accessed 2003 Sep 23]
Comi G. Why treat early multiple sclerosis patients? Curr Opin Neurol 2000 Jun; 13(3): 235–40
Jacobs LD, Beck RW, Simon JH, et al. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group. N Engl J Med 2000 Sep 28; 343(13): 898–904
Comi G, Filippi M, Barkhof F, et al. Effect of early interferon treatment on conversion to definite multiple sclerosis: a randomised study. Early Treatment of Multiple Sclerosis Study Group. Lancet 2001 May 19; 357(9268): 1576–82
Freedman MS, Edan G, Hartung HP, et al. Betaferon®/Betaser-on® (interferon beta-1b) in early treatment of multiple sclerosis: the Benefit Study [abstract no. P06.114]. Neurology 2003 Mar 11; 60Suppl. 1: 483
Inglese M, Van Waesberghe JH, Rovaris M, et al. The effect of interferon β-1b on quantities derived from MT MRI in secondary progressive MS. Neurology 2003 Mar 11; 60(5): 853–60
Monta1ban X, Brieva L, Tintoré M, et al. Single centre, DBPC, randomised trial of interferon-β 1b in primary progressive and transitional progressive multiple sclerosis: an exploratory phase II study. Neurology 2003 Mar 11; 60 (Suppl. 1): 483. Plus poster presented at the 55th Annual Meeting of the American Academy of Neurology; 2003 Mar 29–Apr 5, Honolulu (HA)
Tornatore C, Bartlett D. Use of an automated injection device for the delivery of interferon β-1b [abstract no. 188]. Ann Neurol 2001 Sep; 50(Suppl. 1): 58–9
Tornatore C, Bartlett D. Tolerability of interferon beta-1b (Betaferon®/Betaseron®) can be significantly improved using both interferon-free needle and automated injection techniques [abstract no. P814]. J Neurol 2002; 249 Suppl. 1: 1–205. Plus poster presented at the 12th Meeting of the European Neurological Society; 2002 Jun 22–26; Berlin
Schering A. Comparative MS study examines drug activity, tolerability of Betaferon and Rebif [media release]. 2003 Jun 16
Hadjimichael O, Vollmer TL. Adherence to injection therapy in multiple sclerosis: patients survey [abstract no. S74.003]. Neurology 1999 Apr 12; 52Suppl. 2: 549
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: G. Comi, Department of Neurology, Ospedale San Raffaele, Milan, Italy; F. Deisenhammer, Department of Neurology, University of Innsbruck, Innsbruck, Austria; O. Fernandez, Institute of Neurosciences, Hospital Regional Universitario Carlos Haya, Malaga, Spain; M. Filippi, Scientific Institute and University San Raffaele, Milan, Italy; R.B. Forbes, Department of Neurology, Royal Victoria Hospital, Belfast, Northern Ireland; M.J. Nuijten, MEDTAP International, Amsterdam, The Netherlands; T. Traboulsee, University of British Columbia, Vancouver, British Columbia, Canada.
Data Selection Sources: Medical literature published in any language since 1980 on interferon-beta-1b, identified using Medline and EMBASE, supplemented by AdisBase (a proprietary database of Adis International). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: Medline search terms were ‘interferon-beta-1b’ or ‘beta-1b’. EMBASE search terms were ‘interferon beta serine’ or ‘interferon-beta-1b’ or ‘beta-1b’. AdisBase search terms were ‘interferon-beta-1b’ or ‘beta-1b’. Searches were last updated 22 April 2004.
Selection: Studies in patients with relapsing forms of multiple sclerosis who received interferon-β-1b. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Interferon-beta-1b, multiple sclerosis, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability.
Rights and permissions
About this article
Cite this article
McCormack, P.L., Scott, L.J. Interferon-β-1b. CNS Drugs 18, 521–546 (2004). https://doi.org/10.2165/00023210-200418080-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00023210-200418080-00004