Abstract
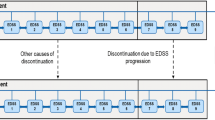
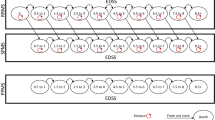
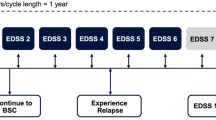
Economic evaluations of treatments for multiple sclerosis have focused either on relapsing-remitting or secondary progressive disease.None has addressed specifically the question of how treatments affect disease progression from diagnoses to severe disability;however, in both clinical and economic terms this is one of the key questions. This study therefore created a disease model that includes both relapsing and progressive multiple sclerosis and covers the full range of disease severity. In addition, the issue of covariates influencing the cost per QALY and the uncertainty in the estimates is addressed.The model is based on our earlier approach using a Markov model,where disease progression is measured as functional disability (EDSS), costs and utilities associated with different disability levels, and outcome expressed as QALY.Treatment is based on a subgroup of patients with active disease in two placebo-controlled clinical trials and open-label extensions with interferon-β1b in relapsing-remitting or secondary progressive multiple sclerosis, and natural history data are used for the extrapolation beyond the trials.Disease progression (transition probabilities) was estimated using a probit model controlling for differences in patient and disease characteristics, and costs and utilities for different disability levels are taken from large population-based observational studies.Results are presented for Sweden and compared to the United Kingdom. When treatment is given for 36 months (adjusted for compliance) and no further effect is included, the cost per QALY is EURO 7800 (direct, indirect, and informal care costs included, discounted at 3%) over 10 years.The total number of QALYs increases from 3.45 in the no-treatment group to 3.64 in the treatment group.When treatment is prolonged to 54 months, the cost per QALY is EURO 38,700. Using the full range of costs and utilities, the probability that the cost per QALY over a 20-year time-frame is below EURO 50,000 for patients starting treatment at EDSS 3.0 is 80%.In view of the progression of MS over many years models are required to estimate the cost-effectiveness of new treatments. The model proposed here allows estimating disease progression, costs, and QALYs for patients with different characteristics and types of MS, and it can be adapted to different countries. It can hence be used to assess the cost-effectiveness of a defined intervention, such as interferon-β1b.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Gisela Kobelt HDI, France, 492 chemin des Laurens, 06530 Spéracèdes, France, e-mail: kobelt.gisele@wanadoo.fr
Rights and permissions
About this article
Cite this article
Kobelt, G., Jönsson, L. & Fredrikson, S. Cost-utility of interferon β1b in the treatment of patients with active relapsing-remitting or secondary progressive multiple sclerosis. Eur J Health Econom 4, 50–59 (2003). https://doi.org/10.1007/s10198-002-0163-0
Issue Date:
DOI: https://doi.org/10.1007/s10198-002-0163-0