Abstract
The risk of ‘hangover’ effects, e.g. residual daytime sleepiness and impairment of psychomotor and cognitive functioning the day after bedtime administration, is one of the main problems associated with the use of hypnotics. However, the severity and duration of these effects varies considerably between hypnotics and is strongly dependent on the dose administered.
This article reviews epidemiological evidence on the effect of hypnotics on patients’ risk for accidents such as traffic accidents, falls and hip fractures (i.e. end-points for residual effects). Information on the duration and severity of residual effects of 11 hypnotics (flunitrazepam, flurazepam, loprazolam, lormetazepam, midazolam, nitrazepam, temazepam, triazolam, zaleplon, zolpidem and zopiclone) was derived from expert ratings, a meta-analysis and actual driving studies. Epidemiological studies show that the risks of an accident increase with increasing half-life of the hypnotic, but that the use of hypnotics with a short half-life, such as triazolam, zopiclone and zolpidem, can also be associated with increased risks. A summary of results from experimental studies should enable prescribing clinicians to compare residual effects of the various hypnotics at different doses and select the one considered most favourable in this respect for the individual patient. This information should also enable them to inform patients more adequately about the likelihood and duration of residual effects of a specific hypnotic dose.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Residual daytime sleepiness and associated impairment of psychomotor and cognitive functioning the day after bedtime administration, sometimes called ‘hangover’ effects, constitute one of the main problems associated with the use of hypnotics. Reduced alertness and slowed reactions increase a person’s risk of becoming involved in accidents at home and work or while driving. In the elderly, ataxia and impaired motor co-ordination may increase the risk of falling and sustaining hip fractures. The latter is of particular concern, since hip fractures constitute a major cause of placement in nursing homes. A number of epidemiological studies have shown that the use of benzodiazepines is associated with an increased risk of injury from car accidents,[1–3] falling and hip or femur fractures.[4–10]
The avoidance of residual effects of hypnotics seems, therefore, particularly relevant for the elderly and persons whose activity the next morning involves skilled work and where impairment of performance could be a danger to themselves or others, such as driving a car. Since experimental studies have shown that there are substantial differences between hypnotics in their potential to produce residual effects, avoidance of residual effects can be achieved by selecting a drug and dose shown to have no effects >8 hours after bedtime ingestion.
The problem is, however, that information on the duration and severity of residual effects of hypnotics is not available in a way that allows clinicians to easily compare and choose from the many drugs and doses available. All prescription hypnotics have a comparable mechanism of action, e.g. agonism of benzodiazepine receptors and a rapid onset of action. The half-life of hypnotics differs substantially, however, ranging from 1–75 hours (table I). Since package inserts contain non-specific warnings about residual effects that are the same for all hypnotics, selection is usually based on the half-life of the drug. In general, hypnotics with a long half-life are associated with more residual effects compared with those that have a short half-life (see section 1). Although half-life is a strong predictor of duration of action and hence residual effects, the relationship is not always consistent. Hypnotics with a short half-life can have residual effects, depending on the dose and rate of absorption, whereas those with a long half-life may have a relatively short duration of action and few residual effects when the drug is largely redistributed to peripheral tissue.
The duration and severity of residual effects should therefore be determined empirically, most suitably by experimental performance studies. Many such studies have been carried out; however, differences between studies with respect to experimental design, performance tests and subjects make it extremely difficult to reach any firm conclusions concerning the duration and degree of behavioural impairment attributable to particular drugs and doses. This lack of agreement was noted by several investigators in the field of experimental human psychopharmacology and led to methodological guidelines to remedy the situation.[11,12] Most studies assessing residual effects of benzodiazepine hypnotics were performed before the introduction of the guidelines, however. Fortunately, it was demonstrated that consensus among experts was sufficient to allow some form of categorisation of the residual effects of most of these older hypnotics, although the results are only available in a technical report and have not been updated.[13] Probably as a consequence of these difficulties, no recent reviews comparing the residual effects of hypnotics are available. The objective of this paper is, therefore, to review and compare the residual effects of currently used hypnotics.
This objective is achieved by reviewing empirical data from epidemiological and experimental studies of the residual effects of currently available benzodiazepine and benzodiazepine-like hypnotics. Data from epidemiological studies of accident risks associated with the use of hypnotics are reviewed to establish the clinical relevance of residual effects on daily life. These studies may also shed some light on drug and patient characteristics that may increase the risk of accidents. Results from experimental performance studies will be used to compare the severity and duration of residual effects of various hypnotics. Residual effects on psychomotor performance and attention have been studied most frequently and systematically, as they constitute an important hazard potential for traffic safety and work-related accidents. Consequently, effects on these behavioural and mental functions will be the focus of this review.
It is impossible to review the results of every study that has been performed on all the hypnotics at their various doses. As mentioned above, many studies were performed in the 1980s and are of varying methodological quality. However, selecting only those that meet certain quality criteria would leave some important drugs with almost no data. It was, therefore, decided to use primarily the results from an expert survey,[13] a meta-analysis of experimental studies[14] and experimental studies using a standardised and calibrated test.[15,16] These data will be summarised for: flunitrazepam 1 and 2mg; flurazepam 15 and 30mg; loprazolam 1 and 2mg; lormetazepam 1 and 2mg; nitrazepam 5 and 10mg; temazepam 20mg; triazolam 0.125, 0.25 and 0.5mg; zaleplon 10 and 20mg; zolpidem 10 and 20mg; and zopiclone 7.5mg. These drugs and doses constitute the most frequently prescribed hypnotics in Western societies. Since oxazepam, lorazepam and diazepam are primarily indicated for anxiety disorders, yet widely used as hypnotics too, the effects of these drugs will also be discussed to a limited extent.
The review of study data will be preceded by a brief overview of the results and conclusions of previous reviews, some background information on the prevalence of insomnia and the use of hypnotics, recent discoveries on the function of the benzodiazepine receptor subtypes, and the pharmacodynamics of hypnotics and pharmacokinetic aspects related to their duration of action. The review will conclude with a discussion of the implications for accident prevention and patient education.
It is hoped that the findings of this review will convince clinicians of the importance of taking residual effects into consideration when prescribing a hypnotic. Most importantly, the findings should enable clinicians to compare the residual effects of various hypnotics at different doses and select the one considered most favourable in this respect for the individual patient. Furthermore, if a prescribed hypnotic is likely to produce residual effects, this review should enable clinicians to inform their patients more specifically about the severity and duration of the risks and provide them with some practical advice on how to minimise them.
1. Results of Previous Reviews
Most reviews on the effects of hypnotics summarise the literature on the efficacy and adverse effects of an individual drug, such as zaleplon,[17] zopiclone[18–21] or zolpidem.[22,23] Only a few review and compare data on multiple hypnotics.[24–26] All agree that hypnotics with a longer half-life tend to produce more residual effects than drugs with a shorter half-life, but they also add that this relationship is not straightforward.
Johnson and Chernik[24] published an extensive and critical review of 52 placebo-controlled studies assessing the residual effects of 11 benzodiazepine hypnotics on performance. They found that these drugs generally improved sleep, but not the quality of daytime performance, as would be expected when effects of poor sleep are normalised by the use of hypnotics. Moreover, all hypnotics were likely to be associated with residual effects at higher doses. Although long-acting drugs generally showed more performance decrement than short-acting ones, half-life did not adequately explain the data. For example, across dose levels, they found that flurazepam produced less decrement than nitrazepam, and that temazepam produced less decrement than triazolam, in spite of the respective longer half-life of flurazepam and temazepam. Tasks showing the largest decrement were those involving speeded performance and memory for information presented during the night, indicating that the drugs mainly produced psychomotor slowing and anterograde amnesia. Johnson and Chernik[24] concluded that the dose level was the most important factor in determining performance decrement; large doses increase impairment at any given time after administration and extend the effect over time.
Roth and Roehrs[25] critically discussed the methodologies used in studies assessing the residual effects of hypnotics, since they believed that any particular study can produce any effects desired: “Given the right dose and the right task, one can demonstrate a decrement or no effect on performance”.[25] They pointed out several methodological issues that were largely neglected in the majority of studies, such as power calculations and external validity of the tests used. Furthermore, they emphasised the importance of dose equivalence with respect to the hypnotic effects when comparing relative risks for residual effects between different drugs and the need to assess residual effects in different populations, notably in high risk populations such as the elderly. At that time, they concluded that little was known about the effects of pharmacological and behavioural tolerance; however, 5 years later they tentatively concluded that behavioural tolerance to the residual effects of hypnotics develops over time.[27]
Recently, Van Laar and Volkerts[28] reviewed the effects of benzodiazepines on driving. Epidemiological studies show that the use of these drugs in general is related to an increased risk of traffic accidents. No distinction was made between hypnotics and anxiolytics, however. Although the risk was not considered extraordinarily high, it appeared to increase with increasing dose, multiple drug use and recency of drug use. According to these authors, experimental driving and laboratory studies have shown that single bedtime doses of diazepam 10mg, flunitrazepam 1mg, loprazolam 1mg, lormetazepam 1mg, nitrazepam 5mg, oxazepam 30mg, temazepam 20mg or triazolam 0.25mg may be relatively safe when used occasionally. Changes with continued use are unclear. Theoretically, benzodiazepines with a long half-life may accumulate and produce increasing sedation; at the same time, tolerance to benzodiazepines may develop, which diminishes these effects. However, data on this issue were found to be limited and conflicting. Finally, the authors stressed the importance of individual differences in response to drugs, resulting in the need to monitor each patient carefully.
In conclusion, most reviews lack a comparison of the effects of individual drugs and doses. Barely any specific information is provided on the duration of residual effects of individual drugs and doses. Instead, the reviews extensively discuss the problems involved in comparing results from different studies due to methodological differences. The only advice that can be deduced from these reviews is that patients who need to be alert in the morning should use the lowest dose possible of a hypnotic drug with a short half-life. The present review will not discuss these methodological issues again.
2. Overview of Insomnia
2.1 Prevalence
Since 1980, more than 20 studies have assessed the prevalence of insomnia.[29] Most of them found that approximately one-third of the general adult population complains of occasional poor sleep, and that about one in ten people experience moderate-to-severe symptoms of insomnia on a long-term basis.[29–33]
A European telephone survey among nearly 25 000 adults showed that the most frequent insomnia symptom was disrupted sleep occurring at least three times a week (18%), followed by early morning awakening (11%), difficulties initiating sleep (10%) and nonrestorative sleep (9%).[34] A similar pattern of complaints was found among Americans.[35]
Typically, the prevalence of insomnia is higher in women than in men (1.5 : 1) and increases with age. More than 30% of persons >65 years of age complain of poor sleep.[36]
Insomnia is strongly associated with psychiatric problems, such as anxiety, depression and substance use or abuse (especially with caffeine, nicotine, alcohol), and with the use of a number of prescription drugs, such as stimulants, β-adrenoceptor antagonists, oral contraceptives and monamine oxidase inhibitors.[37] In addition, there are several medical conditions associated with disturbed sleep, such as chronic pain, cardiovascular diseases, sleep apnoea, and periodic leg movement disorder and restless legs syndrome. Patients with at least one insomnia symptom were shown to be 12 times more likely to have a sleep or mental disorder diagnosis than patients without insomnia symptoms.[34]
Although it is generally assumed that people complaining of insomnia are sleep deprived, the effects of sleep deprivation in healthy volunteers do not necessarily resemble the symptoms of insomniac patients.[38] For example, many patients with chronic insomnia show signs of hyper-arousal and have normal to prolonged sleep latencies in the multiple sleep latency test (MSLT), whereas sleep-deprived healthy controls show signs of hypo-arousal and shortened sleep latencies in the MSLT. In performance testing, patients with insomnia were more like healthy controls than sleep-deprived controls, making it difficult to assume specific functional impairments due to poor sleep per se.
2.2 Pharmacological Treatment
Before the introduction of benzodiazepines, insomnia was treated with bromide and barbiturates. Flurazepam was the first benzodiazepine introduced for the treatment of insomnia, in the US in 1971. Although benzodiazepines have several advantages over barbiturates, it soon became clear that they were not ideal agents for treating insomnia, as their generally long half-lives resulted in significant residual effects.[39–43] The introduction of a variety of short-acting benzodiazepines during the 1980s reduced this problem.[44,45] However, these drugs also had drawbacks, such as the development of tolerance and problems with abrupt withdrawal.[46–48] These effects seemed to be of a lower frequency, however, for the benzodiazepine receptor agonists from other chemical classes with short half-lives that were introduced around 1990, such as zopiclone and zolpidem.[18,22] At the end of the 1990s, these agents were followed by zaleplon, which has a half-life of only 1 hour.[49]
Although the newer hypnotics seem to have a more favourable safety profile than their older counterparts, the use of hypnotic drugs has decreased considerably in the US and Europe since the 1980s.[50,51] In contrast, the use of antidepressants, such as trazodone, for the treatment of insomnia has grown substantially (see section 2.2.2).[51] Furthermore, there is now consensus among clinicians that hypnotics should be used at their lowest possible dose for limited durations and preferably on an as-needed basis.[52,53]
2.2.1 Prevalence of Hypnotic Use
Population surveys show that between 0.7 and 7% of all adults report current use of sleep-enhancing medication. Ohayon and Caulet[54–56] found a current usage of 3.8% in Montreal, Canada and 3.5% in the UK, with 5.3% of the latter respondents reporting that they had used sleep-enhancing medication in the last year. Results of a recent survey in four European countries revealed lower rates of current use however: 2.5% in France, 1.6% in the UK and 0.7% in Germany and Italy.[57] Lapeyre-Mestre et al.[50] reported a current usage of 6.2% in 1996 in Toulouse, France, and Pallesen et al.[32] found a current usage of prescription hypnotics of 6.9% in the winter of 1999-2000 in Norway.
Not all people experiencing poor sleep try to solve this problem by using hypnotic drugs, although a substantial majority does. Of the people experiencing poor sleep in the surveys mentioned above,[32,50,54–57] 20–30%, mostly women and the elderly, report using some form of sleep-enhancing medication. Simon and VonKorff[33] found that 28% of patients reporting major current insomnia used a psychotropic drug. Of those, 14% received a benzodiazepine and 19% received antidepressants, as compared with 1 and 9%, respectively, of patients without insomnia.
Not surprisingly, the use of hypnotics is most frequent among persons with serious sleep complaints.[58,59] Although sleep-initiation problems are less frequent than sleep-maintenance problems, hypnotic use has been found to be higher in patients complaining of sleep-initiation difficulties than in patients with sleep-maintenance problems.[60]
2.2.2 Medications Used
Sleep-enhancing medication may not only be prescription hypnotics, but also anxiolytics, antidepressants and non-prescription (over-the-counter [OTC]) drugs. In the US, the drugs most frequently prescribed for the treatment of insomnia changed dramatically between 1987 and 1996.[51] The two most frequently prescribed drugs in 1987 were triazolam and flurazepam, and in 1996 trazodone and zolpidem. The use of temazepam was stable over the years. The frequent prescribing of trazodone indicates a shift towards the use of antidepressants in the treatment of insomnia. The forces underlying this shift were most likely to be the increasing evidence for and concern about drug dependence and adverse effects of benzodiazepines and the increased recognition of depression in those reporting insomnia.[51]
A similar decrease in the use of hypnotics was found in France between 1986 and 1996.[50] However, in France it was accompanied by an increase in the use of analgesics, probably reflecting the effects of drug information programmes for the better management of pain and a change in the OTC use of analgesic drugs in this period.
OTC sleeping pills are mainly used by younger patients and by those with milder sleep complaints.[58] These drugs were also found to be used less frequently and for shorter periods than prescription hypnotics.
The most frequently used hypnotics differ depending on the country and the drugs registered. The five most prescribed hypnotics in four major European countries between 1993 and 1997 were temazepam (16%), zopiclone (16%), nitrazepam (14%), zolpidem (13%) and flunitrazepam (11%).[61] Other frequently used hypnotics are flurazepam, loprazolam, lormetazepam and triazolam.[17,61,62]
In spite of the recommendation and consensus among clinicians that hypnotics should be used at their lowest possible dose for limited durations, Ohayon et al.[56] found that 76% of hypnotic users in their sample (n = 4972, aged >15 years) reported using the drug at least 3 days a week and 61% had been using hypnotics for at least 1 year. A recent survey[61] supports the finding that most users take hypnotics for >1 year.
2.2.3 Mechanism of Action of Hypnotics
Pharmacodynamics
Currently available prescription hypnotics are all benzodiazepine receptor agonists. They act on the GABAA receptor complex to enhance the effects of GABA, the main inhibitory transmitter in the brain. GABAA receptors consist of five subunits, forming a rosette around a central channel that is selectively permeable to chloride ions. The subunits are proteins from three principal families designated α, β and γ. Whereas GABA binds to the β subunit, hypnotics interact with the α subunit. The binding of GABA to its receptor induces a conformational change that opens the ion channel whereupon chloride ions can enter the cell producing slight, short lasting hyperpolarisation and, thus, a reduction in the excitability of the neuron. Hypnotics increase the affinity of the receptors for GABA and enhance the effects of GABA on the neuron.
Since 1987 many different subtypes of the α, β and γ subunits have been identified, which has significantly progressed insights into the mechanism of action of hypnotics.[63–65] There are at least six different α subtypes, four β subtypes and three γ subtypes. Yet, only certain combinations of subtypes seem to exist and appear to be localised in specific cell types. It seems that >50% of all GABA receptors in the brain are composed of α-1, β-2 and γ-2, and correspond to what was previously defined as a benzodiazepine type-I or ω-1 receptor. They are sensitive to all modern hypnotics (zaleplon, zolpidem, zopiclone and benzodiazepines) and are present throughout the brain, particularly on hippocampal and cortical interneurons. Receptors containing α-2, -3 and -5 subunits correspond to benzodiazepine type-II or ω-2 receptors and are also sensitive to zopiclone and benzodiazepines, but much less so to zolpidem and zaleplon. Receptors containing α-2 and -3 subunits are enriched in the limbic structures (amygdala) and spinal motor neurons.
Benzodiazepine receptor agonists have multiple actions. The most prominent central effects are sedation, hypnosis, decreased anxiety, muscle relaxation, anterograde amnesia and anticonvulsant activity. It is now assumed that the sedative/hypnotic effects are linked to the α-1 subunit and anxiolytic effects to the α-2 subunit, although distinguishing between these behaviours is problematic.[65,66] In addition, the α-1 subunit seems to have an important role in the amnesic effects produced by benzodiazepines and the α-2 subunit in the myorelaxant effects.[65] This could explain why zolpidem and zaleplon are effective hypnotics, but have fewer adverse effects than benzodiazepines.
Benzodiazepines mainly shorten sleep latency and diminish the number and duration of awakenings during sleep, thus increasing the total time spent asleep during the night. However, the extra time asleep is mostly spent in stage 2 or light sleep. Compared with natural sleep, the percentage of time spent in the most restorative stages of sleep, i.e. deep sleep (stage 3 and 4) and rapid eye movement (REM) sleep, is decreased following administration of a benzodiazepine. In contrast, zolpidem does not suppress REM sleep to the same extent as benzodiazepines.[67] With continued use, tolerance seems to develop to the effects of benzodiazepines on sleep stages, but rebound occurs when such use is discontinued. The increase in REM sleep after discontinuation may be especially prominent during the first nights.
Duration of Effects
Under most circumstances, the choice of drug is determined by the onset of action and duration of effect. All hypnotics have a rapid onset of action (between 30 and 90 minutes), whereas duration of action differs greatly. Both are dose dependent.
The onset of action is largely determined by the formulation and the rate of absorption of the drug from the gastrointestinal tract after oral administration. Time to peak plasma concentration (tmax) is often taken to indicate the onset of action for rapidly absorbed benzodiazepines, such as diazepam and flurazepam. In the case of the slowly absorbed drug loprazolam, however, tmax is delayed with respect to onset of action. The pharmaceutical preparation (formulation) can influence the rate of absorption, e.g. temazepam is much more slowly absorbed from hard gelatine capsules (HGCs) than from soft gelatine capsules (SGCs). Once they reach the blood, all benzodiazepines quickly reach their site of action, since all are lipophilic substances that easily traverse the blood-brain barrier.
The duration of effect is often equated to the half-life, as indicated by the fact that hypnotics are often divided into categories based on their half-life, as short-acting (half-life <6 hours), intermediate-acting (half-life between 6 and 24 hours) or long-acting (half-life >24 hours) drugs. The action of a drug may be terminated by at least three mechanisms however: (i)disappearance from the receptor site by redistribution from the brain to peripheral tissue; (ii)biotransformation by the liver to inactive metabolites; and (iii)short-term tolerance of the receptors.
In addition, dose is considered one of the most important determinants of duration of effect. It will take longer for drug concentrations to drop below effective levels after administration of twice the recommended dose and a shorter duration after only half the recommended dose. The relationship between half-life and duration of effect is, therefore, not straightforward.
3. Epidemiology of Accidents Associated with Hypnotic Use
Epidemiological studies probably provide the most important information concerning the effects of medication on performance, namely the actual accident risks associated with their use. The unquestioned strength of these studies is, therefore, the relevance of their outcomes. Their weakness is, however, that they can only assess the risks associated with drugs that have been on the market for some time and that are commonly prescribed. New or rarely prescribed drugs have a low probability of showing up in epidemiological statistics and related research papers. Another limitation is that users and nonusers may differ in ways other than their drug use that could affect their performance, such as the indication for the drug. Despite these limitations, epidemiological studies provide important information and criteria to validate the conclusions from experimental studies.
Most epidemiologists have assessed the risks of benzodiazepines as a group, rather than individually. For the purpose of this review it is necessary, however, to distinguish the risks associated with the use of anxiolytic benzodiazepines from those of hypnotic benzodiazepines. Sedative anxiolytics, which are generally administered during the day, are likely to affect daytime performance and increase accident risks, whereas hypnotics, which are administered at bedtime, can only affect performance when they are active at the time patients get out of bed. Few authors actually make this distinction.[1–3,7,68] Instead, benzodiazepines are often classified by their half-life irrespective of their therapeutic use.[5,9,10,69–72] Occasionally, authors provide information on specific drugs.[2,3,7,68] This review mainly focuses on those studies providing direct or indirect information on the risks associated with hypnotics.
Three types of accidents have been studied in association with benzodiazepine use: traffic accidents, falls and hip fractures. Traffic accidents are relatively rare events, but can occur in any user 18 years and older, whereas falls and hip fractures are more common but mainly occur in the elderly (e.g. the average annual incidence of falls is 36% in community dwelling elderly patients[73] ). Falls are a predominant cause of injuries in the elderly and a strong predictor of placement in a nursing home.[74]
3.1 Traffic Accidents
The role of alcohol in traffic accidents is firmly established, but there are relatively few epidemiological data on the role of psychoactive medication in traffic accidents. Studies to provide such data are not easy to perform. Crashes are infrequent events for individual drivers and those that cause injury even more so. Moreover, there are a large number of drugs available that can be taken in a variety of doses and whose metabolic fate and behavioural effects show great variability between individuals. This makes it necessary to carry out a large number of studies in order to establish relative risks associated with specific drugs and doses.
A relatively simple epidemiological method to study drugs and driving is to assess the prevalence of drugs in blood or urine of injured or killed drivers. De Gier[75] recently reviewed such studies carried out in Europe and concluded that benzodiazepines are the most frequently detected licit drugs other than alcohol in drivers. In studies of drivers stopped for suspicion of driving under the influence, the prevalences of benzodiazepines varied between 13 and 75%; lower prevalences (2–13%) were found in drivers involved in crashes.
Similar findings have been reported for Australia.[76,77] Blood samples from 2500 injured drivers tested positive for alcohol (8.6%), tetrahydrocannabinol [THC] (7.1%) and benzodiazepines (1.8%). About half the drivers tested positive for one benzodiazepine only, whereas about a third tested positive for two benzodiazepines. Since the majority of plasma concentrations were at sub-therapeutic or therapeutic levels, it may be assumed that the drugs were used therapeutically, rather than being abused.
Results such as these reveal little about the importance of benzodiazepines as a cause of accidents. Without comparison to a control group it is difficult to determine whether a particular drug is over-represented in accidents. Case-control and cohort studies are better able to establish the relationship between drug use and accidents. In case-control studies, the frequency of medication use in accident victims is compared with that in non-injured controls, whereas in cohort studies groups of users and nonusers are identified and followed over time to compare the rate of accidents in each group.
A number of case-control and cohort studies have studied the risk of traffic accidents after the use of psychoactive medication. Some of the more recent studies made use of large electronic databases to collect information on specific drugs and doses, for example, by linking prescription records of pharmacies to records of hospitalisation or police records of motor vehicle crashes (table II).
Two studies compared the risks associated with the use of hypnotics and anxiolytics separately, in drivers aged 20 years and older.[1–3] The first study was performed by Neutel[2,3] in Canada. Her group assessed several medical events, such as falling and traffic accidents, within 2 months after filling a prescription for a benzodiazepine by patients registered between 1979 and 1986 in Saskatchewan health databases.[7] Data were collected for 78 070 and 147 726 patients who filled a prescription for a hypnotic (triazolam, flurazepam) or an anxiolytic (diazepam, lorazepam or oxazepam), respectively, and for 97 862 persons in a control group. Analysis of data as a function of time after prescription showed that risks during the first week were extremely high. The odds ratio (OR) for traffic accidents for anxiolytics was 13.5 and for hypnotics was 9.1, although confidence limits were wide. Risks were highest in the first week after the prescription was filled. The OR for a traffic accident within 2 weeks after the prescription of a hypnotic was filled was 6.5 and for anxiolytics was 5.6, and within 4 weeks after the prescription was filled was 3.9 for hypnotics and was 2.5 for anxiolytics. During the second month, ORs had dropped to 1.4 for hypnotics and 1.2 for anxiolytics. These data suggest that some form of tolerance to the adverse effects of benzodiazepines on driving performance may occur. Alternatively, actual exposure to the drugs might diminish over time, because patients stop taking the medication. Further analyses, however, showed that risks were also reduced after a third prescription for a hypnotic as compared with the first prescription (OR 1.4 versus 3.4, respectively). This supports the idea that some form of tolerance might develop.[3]
Factors that were shown to modify these risks were half-life and the dose of a particular drug, and age and gender of the patient.[2,3] Among hypnotics and anxiolytics, risks generally increased with dose and were increased the most for drugs with the longest half-lives, such as flurazepam and diazepam (see table II). It is noteworthy that men had risks 3.6 times higher than those of women, and that the risks for those <60 years of age were higher than for those aged ≥60 years (the ORs were: triazolam 3.5 versus 2.9; flurazepam 6.1 versus 3.4, respectively).
The second study was conducted in the UK.[1] This group linked prescription records of 410 306 individuals to police records of road accidents and identified a total of 19 386 drivers involved in their first traffic accident between 1992 and 1995, of which 916 were users of benzodiazepines. For each patient, periods of drug exposure were calculated from prescription data and the odds of having an accident while exposed and unexposed were compared. The OR for a car accident for benzodiazepine use was 1.62. No significant increase in risks was found for the use of hypnotics (OR 1.2). The only exception was zopiclone (OR 4.0), which was the only hypnotic in this study with a half-life <6 hours. All other hypnotics in this study had intermediate or long half-lives (i.e. flunitrazepam, flurazepam, loprazolam, lormetazepam, nitrazepam and temazepam). Most of the risks associated with benzodiazepines in this study were attributable to anxiolytics with a long half-life (OR 2.2). Both hypnotics and anxiolytics showed a dose-response relationship for risks of traffic accidents and, similar to the findings by Neutel,[2,3] the risks were highest among younger drivers (<45 years) and decreased with increasing age. No significant difference was found between men and women (OR 1.7 versus 1.5, respectively).
The difference in risks found by Barbone et al.[1] and Neutel[2,3] may partly be attributable to differences in study designs. Barbone et al.[1] note that case-crossover studies are likely to underestimate risks in people treated long-term, because exposure would be the same at the time of the accident and during control periods. Since long-term treatment increased with age, in particular for hypnotics, risks associated with these drugs in the elderly may have been underestimated. However, this explanation is not supported by the data. Although risks in younger age groups (30–65 years) were somewhat elevated (OR 1.4) the increase was not significant.
Although the high risk associated with the use of zopiclone is surprising, the data are supported by results from experimental studies showing significant residual impairment of driving performance between 10 and 11 hours after bedtime administration of zopiclone 7.5mg.[82–84] In addition, there are indications that zopiclone may be misused or abused. A forensic toxicology study in Norway revealed that of suspected intoxicated drivers who tested positive for zopiclone, 80% had blood concentrations higher than those expected 8 hours after intake of therapeutic doses.[85]
Three studies assessed the relationship between the use of benzodiazepines and traffic accidents in the elderly.[72,80,81] Leveille et al.[81] did not find an increased risk of traffic accidents for elderly users of benzodiazepine hypnotics, whereas Ray et al.[72] did find increased risks for elderly users of benzodiazepine anxiolytics (relative risk [RR] 1.5). The most frequently used hypnotics in the study by Leveille et al.[81] were triazolam (50%) and flurazepam (27%), which were both associated with significantly increased risks in the elderly in the study by Neutel.[3] The inability to find an effect might be related to the lack of a clear distinction between exposure and non-exposure in the study by Leveille et al.,[81] since 42% of all prescriptions were indicated as ‘take as needed’ and no daily recommended dose could be determined. Later, Hemmelgarn et al.[80] found significantly increased risks for elderly users of benzodiazepines with a long half-life (OR 1.45; clonazepam, diazepam, clorazepate, chlordiazepoxide, flurazepam and nitrazepam), but not for users of benzodiazepines with a half-life <24 hours (alprazolam, bromazepam, lorazepam, oxazepam, temazepam and triazolam). Risks were highest in the first week of use and decreased thereafter.
In summary, the use of benzodiazepines in general is associated with increased risks for traffic accidents in the young and the elderly, yet these effects seem most pronounced in drivers younger than 60 years. Whereas anxiolytics increased risks in the young and the elderly, the effects of hypnotics were less consistent. Nevertheless, there is sufficient evidence that the use of hypnotics can increase the risks for traffic accidents, depending on the drug, dose and patients characteristics.
Risk of Falling and Hip Fracture
There is a substantial body of literature showing that the use of benzodiazepines is a consistent risk factor for falls (table III) and hip fractures (table IV) in the elderly, in both community and clinical settings. A meta-analysis of 40 epidemiological studies assessing risk factors associated with falling in the elderly yielded pooled ORs of 1.54 for the use of sedatives/hypnotics and 1.48 for the use of benzodiazepines.[73] However, the risks varied considerably between studies. In a review of epidemiological studies on hip fractures, Cumming and Le Couteur[86] encountered a similar inconsistency between results, and concluded that it could almost entirely be explained by differences in study designs. In their review, studies that did not show an association between the use of benzodiazepines and hip fracture were nearly all hospital-based case-control studies. The difficulty of finding an appropriate control group in hospitals might bias the results. Excluding those studies, they found that risks of hip fracture in the elderly increased by 50 to 110% with the use of benzodiazepines. Nevertheless, risks may also vary depending on half-life, dose and metabolic pathway of the drug, and tolerance and polydrug use by the patient.
3.2.1 Half-Life
It is generally assumed that risks of falling and hip fracture are higher after the use of benzodiazepines with a long half-life (≥24 hours) than those with a short half-life (<24 hours), which is supported by at least four studies.[9,10,88,91] Ray et al.[9,10,88] conducted three of the studies. In the first study, it was found that elderly patients treated with flurazepam, diazepam and chlordiazepoxide had an increased risk of hip fracture (OR 1.8), whereas no increased risk was found for those using diphenhydramine, hydroxyzine and chloral hydrate.[9] In the second study, the RR of hip fracture was significantly increased within 30 days after filling a prescription for chlordiazepoxide (RR 2.3), flurazepam (RR 1.9) and diazepam (RR 1.5), but not for triazolam, lorazepam and oxazepam.[10] In the third study, falls instead of hip fractures were assessed and a similar lack of increased risks was found with the use of benzodiazepines with a short half-life (mainly temazepam, zolpidem and oxazepam) and significantly increased risks with the use of drugs with intermediate and long half-lives (mainly lorazepam, alprazolam and diazepam).[88] Finally, Lord et al.[91] found significantly increased risks for multiple falls after the use of flunitrazepam (OR 5.2) and nitrazepam (OR 3.9), but not after the use of temazepam (OR 0.7).
However, in the first three studies, it cannot be excluded that the differences in risks associated with the short and long half-life drugs are due to factors other than half-life. In the first study, the drugs belong to very different classes of drugs (the short half-life drugs were mainly sedating antihistamines, whereas the long half-life drugs were all benzodiazepines). Similarly, in the third study, the drugs differed in their dose regimen since the short half-life drugs were predominantly hypnotics, whereas the intermediate and long half-life drugs were predominantly anxiolytics. Only the fourth study comprised drugs from only one therapeutic class (e.g. hypnotics).
Does this mean that hypnotics with a short half-life are not associated with an increased risk of falling and hip fractures in the elderly? Not necessarily. In the third study by Ray et al.[88] further analysis of the data revealed that although short half-life drugs were not associated with a significant increase in the risk of falling, this was only true during the daytime. During the evening and at night, i.e. between 20.00 and 07.00h, the risk for falling associated with the use of benzodiazepines increased in general, and was highest for short half-life benzodiazepines (OR 2.2).
Additionally, a number of studies have shown that hypnotics with short and intermediate half-lives can also be associated with significantly increased risks, for example, triazolam,[68,70] temazepam[4,5,90] and zolpidem.[95] Moreover, two studies have even found what seems to be a negative association between half-life and risks, i.e. increased risks with short half-life drugs, but no increased risks associated with long half-life drugs. Cumming and Klineberg[4] found temazepam to be the only benzodiazepine associated with significantly increased risks for hip fractures (OR 3.8); nitrazepam was not associated with the same risk, despite its longer half-life. Similarly, Passaro et al.[70] found significantly increased risks associated with short (OR 1.9) and intermediate (OR 1.8), but not with long (OR 0.8), half-life benzodiazepines in hospitalised patients of all ages.
The significant finding for short and intermediate half-life drugs might be explained by the fact that the vast majority of all prescriptions in these classes were for triazolam and lorazepam, respectively, both previously found to be associated with increased risk of falling and hip fractures.[5,68,71,74,90] Most surprising was the inability to find increased risks for long half-life benzodiazepines. According to Passaro et al.,[70] this could be explained by the fact that users of long half-life drugs, in their study, were younger and healthier than the controls. This suggests that clinicians were more likely to prescribe long-acting benzodiazepines to patients in better clinical condition, presumably because of the established risks associated with this class of drugs (i.e. confounding by indication).
In conclusion, these findings indicate that although the risks of falling and hip fracture may increase with increasing half-life, the relationship is not clear cut. Risks clearly depend on other factors as well. The above findings illustrated the role of time since administration and sensitivity of the patient. The role of dose, metabolic pathway, tolerance and the interaction with other drugs will be considered in sections 3.2.2 to 3.2.5.
3.2.2 Dose
Two studies showed that the risks of falling and hip fracture increase with increasing dose.[5,88] In addition to the association with half-life, Ray et al.[88] found that risks for falling increased with dose; from an adjusted rate ratio (ARR) of 1.3 after the use of benzodiazepines in doses equivalent to diazepam 2mg, to an ARR of 2.2 after doses equivalent to diazepam 8mg or more.
Herings et al.[5] were unable to find a relationship between the risk of femur fracture and benzodiazepine half-life, but they did show a highly significant relationship between the dose of hypnotics that was administered and the risk of fractures. The benzodiazepines most frequently used by patients in this study were the hypnotics nitrazepam (27%) and temazepam (23%), and the anxiolytics oxazepam (18%) and lorazepam (11%), all of which were classified by Herings et al.[5] as having a ‘short’ half life, defined by the authors as ≤24 hours. Of these drugs, temazepam and lorazepam were associated with significantly increased risks of femur fracture (OR 1.8 and 3.8, respectively), whereas nitrazepam and oxazepam were not associated with these risks. Of the long acting benzodiazepines only chlordiazepoxide and flunitrazepam were found to be associated with increased risks (OR 6.5 and 2.7, respectively), showing that a long half-life is not consistently associated with increased risks. Risks did increase systematically with dose, however; ORs increased from 1.0 for those exposed to daily doses of <0.75 of the defined daily dose (DDD), to 1.9 for those exposed to daily doses between 0.75 and 1.24 DDD, to 2.3 for those exposed to daily doses of 1.25 DDD or more.
3.2.3 Metabolic Pathway
The way that drugs are metabolised is related to half-life. All benzodiazepines are metabolised by conjugation, some directly but most needing to undergo oxidation first. The rate of oxidative reactions in the liver decreases with advancing age, thus resulting in higher blood concentrations and longer half-life. It is, therefore, often proposed that benzodiazepines undergoing direct conjugation such as temazepam, lormetazepam, oxazepam and lorazepam (i.e. nonoxidative benzodiazepines) could be safer for the elderly than oxidative benzodiazepines.
A study by Sgadari et al.[96] specifically addressed this issue and found only partial support for this hypothesis. The risk of femur fracture associated with the use of oxidative and nonoxidative benzodiazepines was compared between groups of elderly patients aged 65–74 years, 75–84 years and 85 years and older. On average, the use of benzodiazepines was associated with a slight but significant increase in the risk for femur fracture (OR 1.1), which seemed mainly due to high doses of oxidative benzodiazepines with long half-lives (>24 hours). Overall, oxidative benzodiazepines were not more harmful than nonoxidative agents. Age dependent increases in the risk of femur fractures were only found among elderly patients receiving high or as-needed doses of oxidative hypnotics. This suggests that in patients younger than 85 years and in those receiving low doses of oxidative hypnotics, oxidative hepatic processes were still adequate to metabolise oxidative benzodiazepines, whereas oxidative capacity may have been exceeded in patients aged 85 years or older and in those receiving high doses of these drugs. It was concluded that oxidative benzodiazepines are only more harmful than nonoxidative benzodiazepines when they are given in high doses to very old individuals. It should be noted, however, that most oxidative benzodiazepines (63%) in this study had short (triazolam) or intermediate (alprazolam, estazolam) half-lives.
3.2.4 Tolerance
The results from a number of studies suggest that some form of tolerance may develop to the effects of benzodiazepines involved in falling. Neutel[68] found that risks for falling were highest at the initiation of treatment. After the use of hypnotics (triazolam and flurazepam), the risk of hospitalisation for fall-related injuries decreased from an OR of 3.6 in the first 2 weeks to 2.3 in weeks 3 and 4, and to 1.4 in the second month after filling the prescription. A similar trend was found for anxiolytics (OR 2.6, 1.4 and 1.1, respectively).
These data are supported by those of Ray et al.,[88] who found that risks for falling among nursing home residents decreased from an ARR of 3.0 in the first week of treatment, to 2.2 in weeks 2–4, to 1.3 in the second month of treatment. Furthermore, Herings et al.[5] report higher risks for femur fracture in incidental users than continuous users (OR 2.5 versus 1.6) of benzodiazepines. In addition, Maxwell et al.[8] demonstrated that the risks for fall-related injuries were lower after a third prescription for benzodiazepines than after the first one. Stratification of their data by age, however, showed that the risks only decreased (from OR 2.9 to 2.4) with repeated use in persons aged 70 years and older; risks for new and repeat users younger than 70 years were identical (OR 2.6).
Together, these data strongly suggest that pharmacokinetic or pharmacodynamic changes account for the development of tolerance. It is also possible, however, that patients change their behaviour in response to noticeable drug effects on their balance, e.g. become more careful when they stand or walk (behavioural tolerance). However, this explanation is not supported by the finding that incidental use is associated with higher risks than continuous use.[5] This issue deserves further study in the light of the recent trend to recommend ‘as needed’ use of hypnotics.
3.2.5 Polydrug Use
The use of combinations of different drugs consistently and substantially increases the risks of falling and hip fracture.[5,70,71,74,87,92,94,97] For example, Granek et al.[94] found that the risks of falling more than doubled in elderly patients using sedatives/hypnotics in combination with antidepressants, NSAIDs, cardiac drugs or diuretics. Mendelson[90] found that the effect is not limited to the elderly. In their study of falls in hospitalised patients of all ages, those who fell were 3.7 times more likely to have received two psychotropic drugs, and 9.5 times more likely to have received three psychotropic drugs, than patients who did not fall.
Neutel et al.[74] recently compared risks of falling associated with various drug classes. Although few classes were by themselves associated with an increased risk, the use of five or more different drugs increased the risks by at least 4-fold as compared with the use of four drugs or less. For more specific drug classes, the highest risk (OR 11.4) was found for combinations of benzodiazepines with antipsychotic drugs. The authors conclude that the number of different drugs taken is a more important risk factor than the class of drugs taken.
3.3 Discussion
Epidemiological studies have shown that the use of hypnotics and anxiolytics is associated with an increased risk of traffic accidents, particularly in younger patients, and with an increased risk of falling and hip fractures in the elderly. The hypotheses that short half-life benzodiazepines are safer than long half-life benzodiazepines is partially supported by epidemiological data. In general, the risks increase with increasing half-life, but a short elimination half-life offers no guarantee that a particular drug will be safe. For example, temazepam, triazolam, zolpidem and zopiclone were found to be associated with increased risks, despite their relatively short half-lives. Other important factors determining risk are dose, tolerance and polydrug use. Risks were shown to increase with increasing dose and the number of drugs used simultaneously, and to decrease after repeated administration. Finally, the hypothesis that nonoxidative benzodiazepines are safer for use in elderly patients than oxidative benzodiazepines could not be supported by epidemiological data. The use of nonoxidative benzodiazepines, lorazepam, oxazepam and temazepam, has been found to be associated with increased risks.
A problem with the results of these studies is the great variation in risks found. Some studies even failed to find significantly increased risks. It has been argued that in some studies the risks may be underestimated because actual drug exposure is overestimated.[89] A frequently used measure of drug exposure in prospective studies is drug use at the time of baseline measurement. If drugs are actually used intermittently during the period of follow up, exposure is overestimated and risks may consequently be underestimated. Similarly, when risks are in fact only increased during the first days following the start of the new drug treatment, risks averaged over longer periods will be much lower.[74] Likewise, many studies grouped all benzodiazepines with half-lives of ≤24 hours as ‘short half-life’ benzodiazepines. This may average out the risks associated with specific drugs and administration regimens (i.e. anxiolytics). Half-lives of 10–24 hours can hardly be considered as short with respect to residual effects of hypnotics.
Another problem is that epidemiological studies can only detect significantly increased risks for drugs that have been commonly prescribed, such as temazepam, triazolam, zolpidem, oxazepam, lorazepam and flurazepam. The fact that a drug was not found to be associated with a significantly increased risk may be due to a lack of power. Sample sizes of most studies are too small to detect new or rarely prescribed drugs. For example, Pierfitte et al.[71] only found a significant risk associated with lorazepam; other drugs, such as temazepam, nitrazepam and loprazolam, had similar ORs, but the confidence intervals were wide as a result of the small number of observations.
Furthermore, it should be kept in mind that epidemiological data reflect statistical associations and do not demonstrate causality. It is extremely difficult to distinguish the effects of disease from those of medication. Moreover, if clinicians, for whatever reason, prescribe drugs considered as most safe only to relatively fragile patients, and drugs considered less safe to stronger patients, the risks found in epidemiological studies may be comparable.
Finally, the finding that the use of hypnotics is associated with higher risks of falling during the night than the day, raises questions with respect to the validity of many epidemiological studies for establishing the relationship between residual effects of hypnotics and falling or hip fracture in the elderly.[88] It seems that, despite the use of hypnotics, the elderly may wake up and get out of bed during the night, thus running the risk of falling. Such accidents cannot be attributed to the residual effects of a drug. It seems highly unlikely, however, that patients with insomnia will drive a car in the middle of the night after using a sleeping pill. For this reason, epidemiological data on traffic accidents after the use of hypnotics may be more valid indications of their residual effects than data on falls and hip fractures after the use of these drugs.
4. Experimental Studies of the Residual Effects of Hypnotics
Whereas epidemiological studies can show that the use of hypnotics is associated with increased risks for injuries, they are not the best way to compare the effects of specific drugs and doses. This is more appropriately done in experimental performance studies, in which the effects of drugs and doses of drugs can be studied systematically in homogeneous groups of subjects under constant circumstances.
As already indicated in the introduction, many such studies have been carried out, yet the experimental designs, performance tests and subjects used differ widely between studies, making it extremely difficult to review and compare them all. Therefore, three other sources of information that compare, or allow comparison of, the effects of various hypnotics in different doses will be used. One is a survey among experts rating the severity of residual effects of hypnotics at different doses and at different times after administration.[13] Another source is a meta-analysis of experimental studies[14] and the last is residual effects as assessed using a standard highway driving test.
4.1 Expert Ratings
In 1989 and 1990, a worldwide survey was conducted among a group of 45 experts engaged in research on the effects of drugs on traffic safety to determine whether a consensus existed concerning the severity of drug effects.[13] Experts were requested to rate the impairment produced by those drugs and doses with which they had research experience. The main result of this survey was a proposal for a new package-label warning system allowing categorisation of one or more doses of drugs as likely to produce no, minor, moderate and severe impairment (table V). This system was introduced in the European Community in January 1994.
Categorisation system for drugs affecting psychomotor performance[13]
Additionally, the survey resulted in a list of drugs categorised for their impairing effects on driving performance. From this, rank scores were assigned (i.e. 0, 1, 2 and 3 for no, minor, moderate and severe impairment, respectively) and average rank scores were calculated for different drugs, doses and formulations. For hypnotics, the system was extended to include three time intervals; 8–12 hours (morning), 12–16 hours (afternoon) and 16–22 hours (evening) after administration. The results for 11 hypnotics and three anxiolytics often used as hypnotics are presented in table VI.
Summary of expert categorisations of the residual effects on psychomotor performance of hypnotics and anxiolytic-hypnotics.[13] Indicated are mean rating scores: 0 unlikely to produce effects, 1 minor effects, 2 moderate effects and 3 severe effects
Results showed that immediately upon arising after 8 hours of sleep, the only hypnotic rated as unlikely to produce residual effects was zolpidem 10mg. Low doses of short and intermediate half-life hypnotics (i.e. brotizolam 0.125 and 0.25mg, lormetazepam 0.5 and 1mg, midazolam 7.5 and 15mg, temazepam 10 and 20mg, triazolam 0.125mg and zopiclone 7.5mg) were generally rated as likely to produce only minor impairment at that time, whereas the residual effects of higher doses and longer half-life hypnotics or anxiolytic hypnotics were on average rated as moderate-to-severe upon arising. According to the experts, the residual effects of flunitrazepam 2mg, flurazepam 30mg, loprazolam 2mg and lorazepam 5mg were most persistent (the residual effects of these drugs and doses were rated to be severe or moderate the entire day after bedtime use). Although experts sometimes disagreed regarding the degree of impairment of a drug (e.g. whether the effect was moderate or severe), they generally did agree upon the rank order of effects between drugs and doses (e.g. triazolam 0.25mg produces more severe effects than temazepam 10mg between 8 and 12 hours after ingestion). Wolschrijn et al.[13] therefore concluded that the resulting rank order should at least enable clinicians to choose the least impairing drug within a group.
4.2 Meta-Analysis
Berghaus[14] conducted a meta-analysis of 812 experimental studies assessing the effects of 248 medicinal drugs on driving, including 11 hypnotics. Drugs and doses were evaluated for the percentage of performance measures that were found to be significantly different from placebo at various times after ingestion. Performance measures included assessments of psychomotor performance, visual perception, attention, information processing and driving. Table VII shows the percentages of impairment found between 8 and 18 hours after ingestion of hypnotics and anxiolytic-hypnotics.
Summary of results for hypnotics from a meta-analysis of experimental studies assessing medicinal drug effects on driving performance.[14] Indicated are the percentage of performance parameters found to be significantly different from placebo between 8 and 12 hours, and at 15 and 18 hours after ingestion of various hypnotics and doses of hypnotics
To determine what percentage of significant differences could be considered as clinically relevant, the effects of blood alcohol concentrations (BAC) of 0.03 g/L were used as a criterion. It was estimated that on average 20% of the performance measures would be significantly affected by this BAC. Therefore, time intervals during which consistently 20% or more of all performance measures were significantly affected were defined as the duration of significant impairment. According to this criterion, lormetazepam 2mg, nitrazepam 10mg, flunitrazepam 2mg, flurazepam 30mg, diazepam 5 and 10mg, and lorazepam 2mg would produce clinically significant impairment ≥8 hours after ingestion. It should be noted, however, that the power of many studies to detect minor or moderate impairment may not have been high, since >70% of all studies included in the meta-analysis were conducted with ≤12 patients, who were usually healthy young men.
Standardised Highway Driving Test
A few performance tests have been applied unchanged for several years, providing comparable data on the effects of a variety of drugs and doses. One such test is a highway driving test, which was standardised in the early 1980s[15,16] and subsequently used in over 75 studies. The test evolved from studies on driver fatigue conducted in the US during the early 1970s. It involves subjects driving a specially instrumented car over a 100km (61 miles) primary highway circuit while maintaining a constant speed and a steady lateral position between the boundaries of the slower traffic lane. Subjects are accompanied by a licensed driving instructor, who has access to dual controls. Speed and lateral position relative to the lane delineation are continuously recorded during the 1-hour drive by apparatus aboard the vehicle. After completion of the test the data are reduced to yield several measures, including the primary performance parameter, the standard deviation of lateral position (SDLP, in cm). SDLP can be interpreted as an index of weaving or road tracking error. It is a reliable characteristic of individual driving performance (test retest; r = 0.7–0.9) and has proven sensitive to many sedating drugs.[98–100]
The test was calibrated for the effects of alcohol in a closed circuit study wherein 24 social drinkers were tested sober and after controlled drinking to raise BACs in steps of 0.3 g/L to a maximum of 1.2 g/L.[101] In line with the relationship between BAC and accident risk as estimated in a large epidemiological study by Borkenstein,[102] the relationship between BAC and SDLP was shown to be an exponential function. Based on this relationship, BACs of 0.5, 0.8 and 1.0 g/L were associated with mean changes in SDLP of 2.4, 4.2 and 5.1cm. Mean changes in driving performance under the influence of hypnotic drugs can thus be compared with those associated with BACs at various legal limits.
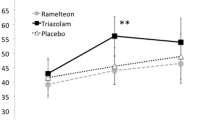
Figure 1, figure 2 and figure 3 show the results from eleven studies employing comparable procedures for assessing the residual effects of hypnotic drugs on driving performance. Results are presented separately for hypnotics with short (figure 1), intermediate (figure 2) and long (figure 3) half-lives.
Residual effects of short half-life hypnotics on driving performance as measured in a standard highway driving test. Indicated are mean changes from placebo in standard deviation of lateral position (in cm; an index of weaving) in morning and afternoon tests. Tests in the morning started 10 hours after bedtime administration or 4 or 5 hours after administration in the middle-of-the-night. Tests in the afternoon started 16 hours after bedtime administration. Also shown by vertical broken lines are mean changes produced by blood alcohol concentrations (BAC) of 0.5, 0.8 and 1.0 g/L.[82–84,103,104]
Residual effects of intermediate half-life hypnotics on driving performance as measured in a standard highway driving test. Indicated are mean changes from placebo in standard deviation of lateral position (in cm; an index of weaving) in morning (i.e. 10–11 hours post-administration [hpa] at bedtime) and afternoon (i.e. 16 hpa at bedtime) tests. Also shown by vertical broken lines are mean changes produced by blood alcohol concentrations (BAC) of 0.5, 0.8 and 1.0 g/L. SGC = soft gelatine capsule.[105–108]
Residual effects of long half-life hypnotics on driving performance as measured in a standard highway driving test. Indicated are mean changes from placebo in standard deviation of lateral position (in cm; an index of weaving) in morning (i.e. 10–11 hours post-administration [hpa] at bedtime) and afternoon (i.e. 16 hpa at bedtime) tests. Also shown by vertical broken lines are mean changes produced by blood alcohol concentrations (BAC) of 0.5, 0.8 and 1.0 g/L.[103,105,107–110]
Five studies assessed the residual effects after two nights of treatment with hypnotics in women complaining of insomnia and who formerly used hypnotics.[84,105,108,109,111] In the other studies, testing occurred after a single night of treatment, or subjects were healthy men or women volunteers.[82,83,103,104,106,110] In all studies, driving tests were undertaken in the morning between 10 and 11 hours after intake; in six studies a second driving test was performed in the afternoon between 16 and 17 hours after ingestion. The effects of zaleplon, zolpidem and zopiclone were also assessed after administration of these drugs in the middle of the night, i.e. 4[104] or 5 hours[82] before testing.
The most severe residual effects in the morning and afternoon tests were found for flurazepam 30mg and loprazolam 2mg.[108–110] The average degrees of impairment were worse than those associated with a BAC of 1.0 g/L in the morning or afternoon, and equivalent to 0.8 g/L in the afternoon. Drugs that had residual effects in the morning or afternoon equivalent to BACs between 0.5 and 0.8 g/L were nitrazepam 10mg, flunitrazepam 2mg, zopiclone 7.5mg, oxazepam 50mg, lormetazepam 2mg (capsules) and flurazepam 15mg. In the afternoon, these effects rapidly declined for hypnotics with short and intermediate half-lives (zopiclone 7.5mg, lormetazepam 2mg and oxazepam 50mg), but remained significant or even increased for hypnotics with long half-lives (flunitrazepam 2mg and nitrazepam 10mg, respectively). Drugs that had no significant residual effects in the morning and afternoon were zaleplon 10 and 20mg, zolpidem 10mg, lormetazepam 1mg (capsules), temazepam SGC 20mg and nitrazepam 5mg. Moreover, zaleplon 10 and 20mg had no significant effects on driving in the morning even after middle-of-the-night administration.[82,104]
Not shown in figure 1 and figure 2 are the results of a study comparing the residual effects of triazolam 0.5mg, midazolam 15mg and temazepam SGC 20mg after daytime sleep in shift workers.[112] Results are not comparable to those of the other studies because the procedures differed: the driving test was performed in the afternoon, between 7.5 and 8.5 hours after morning ingestion of the drugs or placebo. Nonetheless, results confirmed previous findings suggesting that temazepam SGC 20mg is unlikely to produce residual effects on driving. In contrast, triazolam 0.5mg produced residual impairment equivalent to a BAC over 1.0 g/L after the first treatment and equivalent to a BAC of 0.8 g/L after the fifth consecutive treatment. Midazolam 15mg had minor effects on the fifth day of treatment, but none on the first day.
A number of results are noteworthy as illustrations that dose and absorption rates may sometimes be more important than half-life in determining residual effects. The repeated finding that zopiclone 7.5mg has significant moderate-to-severe residual effects clearly illustrates that a short half-life does not guarantee a drug is devoid of residual effects, whereas the lack of effect of nitrazepam 5mg illustrates the reverse is also not true (i.e. a long half-life does not necessarily mean that a drug will have a long duration of effect). Both findings suggest that dose is a crucial factor for these drugs.
The importance of a slow absorption rate is illustrated by the residual effects of temazepam in HGCs (tmax 2.5 hours), lormetazepam tablets (tmax 3 hours) and loprazolam (tmax 5 hours, range 1–12 hours[113] ). Half-lives of these drugs are roughly equivalent, but residual effects clearly increase with an increasing tmax. Since these drugs should induce sleep well before they reach peak plasma concentrations, the latter are probably considerably higher than the threshold for inducing sleep. Consequently, it may take a relatively long time before they will have dropped below this threshold.
4.4 Summary of Results
For most hypnotics and doses, results of expert ratings, the meta-analysis and the driving test are in agreement. Results are summarised for a number of hypnotics in table VIII. Anxiolytic hypnotics will only briefly be discussed.
4.4.1 Short Half-Life Hypnotics
Zaleplon
It is clear that zaleplon in doses of 10 and 20mg is the hypnotic drug least likely to produce residual effects as a result of its extremely short half-life (1 hour). Zaleplon had no significant residual effects on psychomotor performance, attention and actual driving, regardless of the dose and time of administration in the driving studies.[82,83,104] These findings are supported by other studies failing to find significant effects of zaleplon on performance >2 hours after administration.[114–116] The only significant findings after middle-of-the-night administration of zaleplon were minor impairments of memory within the first 4 hours.[82,116,117] O’Hanlon[118] and Patat et al.[17] have recently written reviews of the effects of zaleplon on performance.
Zolpidem
Zolpidem 10mg is generally considered free of residual effects when taken at bedtime before 8 hours of sleep.[23,119–121] However, it may have moderate-to-severely impairing effects within 5 hours post-administration, that may be detectable until 7 hours post-administration.[17,104,114,115,122,123] A double therapeutic dose administered in the middle-of-the-night, 4 hours before driving, produced effects that were more severe than a BAC of 1.0 g/L.[104] Residual impairment has been reported in some studies with higher doses.[116]
Zopiclone
In the review by Nicholson[20] of the residual effects of zopiclone, the author concludes that overnight ingestion of zopiclone 5mg is free of residual effects, whereas 10mg is associated with marked impairment. Results are less consistent for 7.5mg. The latter is also illustrated by the findings that the experts considered its effects as minor-to-moderate, whereas results from the driving tests have repeatedly suggested that they are moderate-to-severe, at least until 12 hours after bedtime administration.[13,82–84] Two reviews conclude, however, that the inconsistencies in results are most likely due to differences in the study designs and the sensitivity of the tests and procedures.[19,20] Nicholson,[20] therefore, concludes that zopiclone 7.5mg should be avoided by those whose activity the next day involves skilled work and where impairment of performance could be a danger to themselves or others. This recommendation is supported by epidemiological findings showing a significantly increased risk for traffic accidents associated with the use of zopiclone.[1]
Triazolam
Triazolam 0.5mg can produce marked residual effects. For this reason the recommended dose was lowered to 0.25mg in the US in 1987. The residual effects of triazolam 0.25mg on psychomotor performance seem to be mostly confined to the first hour after arising.[116,124] No studies were found that assessed the residual effects of triazolam 0.125mg after 8 hours of sleep, but studies of daytime administration suggest that the severity and duration of impairment following administration of this dose is equivalent to that produced by zolpidem 5mg.[125–127] This suggests that triazolam 0.125mg is unlikely to produce residual impairment the morning after bedtime use.
It is clear that the residual effects of triazolam are strongly dose dependent. Perhaps differences in the dose used might partly explain why some epidemiological studies found significantly increased accident risks associated with the use of triazolam,[3,70] while others have not.[10,81]
Midazolam
Relatively few studies have assessed the residual effects of midazolam after bedtime administration as a hypnotic, which may be a result of the fact that this drug is mainly used as premedication for minor surgery. Most studies failed to find significant residual effects 8 hours or more after bedtime administration; however, two studies demonstrated minor, but significant, residual effects on driving and divided attention at 8 and 9 hours after administration.[112,128] At 10 hours after ingestion the only significant impairment found was on memory.[129,130] It may, therefore, be concluded that the risk for residual effects 8 hours or more after bedtime use of midazolam 15mg is low, but not absent. Residual effects of midazolam 7.5mg are unlikely.
4.4.2 Intermediate Half-Life Hypnotics
Temazepam
Temazepam is available in two formulations: SGCs and HGCs. The latter have a slower rate of absorption, resulting in an increase in the duration of effect. Numerous investigators have assessed the residual effects of temazepam on laboratory tasks, and almost none of them found significant effects with a 20mg dose in SGCs, after 8 hours, whereas higher doses in HGCs were occasionally found to produce significant residual effects.[131–135] The meta-analysis showed a very low incidence of significant residual effects and no dose dependent increase in effects of temazepam in doses of 10, 20 and 30mg. No difference was found between formulations. No significant residual effects of temazepam SGC 20mg were found in the driving test.[105,112] It may, therefore, be concluded that temazepam SGC 20mg is unlikely to produce residual effects after a normal night of sleep (i.e. >8 hours).
Lormetazepam
Lormetazepam is also available in two formulations (capsules and tablets), of which the tablets result in a slower rate of absorption. Lormetazepam 1mg capsules can be categorised as unlikely to produce residual effects >8 hours after administration based on expert ratings, the meta-analysis and a driving study.[107] The same dose in tablets was shown to have minor, yet significant, effects on driving between 8 and 12 hours after administration.[136] The effects of lormetazepam 2mg capsules seem minor-to-moderate. They were rated as moderate between 8 and 12 hours after administration by the experts, and found to be approximately equivalent to that of a BAC of 0.5 g/L on driving performance in the morning.[107] The effects on driving had disappeared in the afternoon. The meta-analysis comprised very few studies assessing the effects of a 2mg dose and no distinction was made between formulations.
Loprazolam
Loprazolam is characterised by a steep dose-response curve. Residual effects of loprazolam 1mg on driving were nearly equivalent to those produced by a BAC of 0.5 g/L, but the effects of 2mg 10 hours after administration were more severe than those produced by a BAC of 1.0 g/L (i.e. at least a 3-fold increase) [see figure 2].[108] Furthermore, the reduction of effects over the course of the day was found to be small for both doses. Few other studies have assessed the residual effects of loprazolam, resulting in few expert ratings and a lack of differentiation between doses in the meta-analysis. Nonetheless, the residual effects of loprazolam 2mg can be categorised as severe and slowly diminishing. The residual effects of loprazolam 1mg seem minor-to-moderate between 8 and 12 hours after administration.
4.4.3 Long Half-Life Hypnotics
Nitrazepam
The residual effects of nitrazepam 10mg can be categorised as moderate-to-severe and persisting over the entire day, whereas those of nitrazepam 5mg seem to be minor >8 hours after administration. The residual effects of nitrazepam 10mg on driving in the morning and afternoon tests were equivalent to those of BACs between 0.5 and 0.8 g/L and lasted throughout 8 days of consecutive treatment.[105] The meta-analysis confirmed that nitrazepam 10mg is likely to impair performance for the entire day after bedtime administration and experts rated the effects initially as severe and declining to moderate.[13,14] Nitrazepam 5mg was occasionally found to produce significant residual impairment between 8 and 12 hours after administration,[130,137–140] but not on performance in the driving test.[108] There was no consensus among experts with respect to the severity of the residual effects of nitrazepam 5mg.
Flunitrazepam
Flunitrazepam 2mg is likely to produce moderate residual effects at 8–12 hours post-administration that persist over the course of the day.[13,14] The effects of flunitrazepam 1mg were rated as similarly impairing in the morning, but more rapidly declining. Contrary to expert opinion, however, results from the meta-analysis suggest that the residual effects of flunitrazepam 1mg are unlikely or minor. Two out of three studies assessing the residual effects of flunitrazepam 2mg on driving showed effects that were approximately equivalent to those associated with a BAC of 0.5 g/L and persisted at least until 16 hours after administration.[108] A third study was unable to find significant residual effects on driving, which might be related to the fact that subjects were tested after the first night of treatment instead of after the second night as in the earlier studies.[103] The effects of the lower dose have not been assessed in the driving model. Woods and Winger[141] have conducted an extensive review of flunitrazepam, including its effects on psychomotor performance, driving and memory.
Flurazepam
Flurazepam 30mg is likely to produce severe residual effects that persist over the day.[13,14] Residual effects of flurazepam 15mg are somewhat less pronounced in the morning, but also[110] persist over the day. Flurazepam 30mg was repeatedly found to have residual effects on driving 10 hours after administration that were more severe than the effects of alcohol while BAC was 1.0 g/L.[107,109,110] Furthermore, the effects were found to increase during 8 nights of continuous use, indicating accumulation of the drug in the plasma.[107] The residual effects of flurazepam 15mg on driving were found to be equivalent to a BAC between 0.5 and 0.8 g/L.[109]
4.4.4 Anxiolytic Hypnotics
Experts rated the residual effects of oxazepam 10–50mg, lorazepam 1mg and diazepam 10mg on average as moderate, and those of lorazepam 2.5–5mg and diazepam 15–20mg as severe.[13] The meta-analysis found that lorazepam 2mg and diazepam 5 and 10mg produced significant residual impairment. No information is available for higher doses of lorazepam as a result of a lack of studies assessing their effects.[14] Oxazepam 50mg was the only anxiolytic hypnotic assessed in the driving model. In the morning, it was found to produce residual effects comparable to those associated with a BAC of 0.5 g/L, but these effects had completely disappeared by the afternoon.[106]
4.4.5 Tolerance
It is often assumed that tolerance will develop to the residual impairing effects of hypnotics. However, relatively few investigators have actually tested this assumption.
Two studies assessed the effects on performance in the driving test during a week of continued use of temazepam SGC 20mg, lormetazepam SGC 1 and 2mg, nitrazepam 10mg and flurazepam 30mg.[105,107] Residual impairment following flurazepam 30mg slightly increased over 7 nights of continued use and persisted until the morning after the first washout night, suggesting accumulation rather than the development of tolerance within the first week of treatment. The residual effects of nitrazepam 10mg did not diminish from the second to the seventh night of treatment, but had disappeared after one washout night. The residual effects of lormetazepam SGC 2mg were most pronounced after 4 nights of treatment, suggesting initial accumulation followed by the development of tolerance within the first week of treatment. Lormetazepam SGC 1mg and temazepam SGC 20mg showed no residual effects throughout a week of treatment.
According to Berghaus,[14] their meta-analysis showed that the overall percentage of performance parameters showing significant impairment for various hypnotics (averaged over dose and time after administration) is reduced with repeated use and that this reduction appears to be more rapid for hypnotics with a shorter half-life. These data suggest that tolerance to residual performance impairment may develop to some extent, but it may not be complete and may take longer than a week depending on the drug and dose.
It has also been argued that if a drug continues to have sleep-enhancing effects during the night, there is no reason to assume that tolerance will be complete for its residual effects on performance; unless, perhaps, behavioural adaptations are made in the way tasks are performed, so-called ‘behavioural tolerance’. Behavioural tolerance is defined as “a reduction in the potency of a drug secondary to an intentional change in the behaviour of the user based on cognitive anticipation of the possibility of adverse effects”.[142] When a drug produces predictable effects on a users’ performance, they may adapt their behaviour so that the effects of the drug are less pronounced. For example, alcohol-dependent individuals learn to reduce stumbling and falling by avoiding rapid movements and walking next to solid objects that they can hold on to for postural stabilisation. Something similar may occur for users of hypnotics. For example, Roehrs et al.[143] found that tolerance developed within 9 days to the residual effects of flurazepam 30mg on performance in divided attention and vigilance tasks, but not to residual sedation as measured by the MSLT. Since the results from the MSLT indicated that the pharmacological activity of the drug had not changed, the authors concluded that the adaptation to the effects on performance must be accounted for by mechanisms of behavioural tolerance.
5. Clinical Implications and Concluding Remarks
The aim of this paper was to review the evidence that residual effects of hypnotics increase patients’ risk for injurious accidents, and to provide information on how to reduce these risks. For this, empirical data from epidemiological and experimental studies on the residual effects of currently available benzodiazepine and benzodiazepine-like hypnotics were reviewed.
Experimental studies show that hypnotics can produce residual sedation and impairing effects on psychomotor performance, attention and memory the day after bedtime use. These residual effects on performance impair the patients’ quality and safety of activities of daily living and increase patients’ risks of becoming involved in accidents. Epidemiological studies confirm this by showing that the use of hypnotics is associated with excess risks of accidents, such as falling, hip fractures and traffic accidents. It is likely that these drugs also increase the risk of a variety of occupational accidents, although studies on this are scarce. Both experimental and epidemiological studies show that the excess risk varies with treatment-related factors, such as drug, dose, time after administration and frequency of administration, and with patient-related factors such as age and gender.
It seems worthwhile to select the safest alternative possible. Menzin et al.[144] recently developed a model to calculate the potential impact of the use of safer hypnotics on the numbers of motor vehicle accidents and their associated costs. According to their model, hypnotics that produce residual effects on driving equivalent to those associated with a BAC of 0.5 g/L would increase motor vehicle accidents by 25%; whereas, hypnotics that produce impairment comparable to that associated with a BAC of 0.8 or 1.2 g/L would increase the risk by two to five times, respectively. The model was applied to a hypothetical cohort of 100 000 drivers with insomnia who were treated either with a hypnotic that would not increase risks or one that produces effects comparable to a BAC of 0.8 g/L. Use of the latter over 14 days in France was expected to result in 503 excess accidents per 100 000 drivers, of which 16 would involve injuries in addition to property damage.
Obviously, the treatment of insomnia should preferably be accomplished without medication, for example, by increasing sleep hygiene. However, if the use of sleep-enhancing medication cannot be avoided, its effects should ideally be confined to the night that the drug is taken. Clinicians who prescribe hypnotics should recognise that there are considerable differences between hypnotics and doses in their potential to produce residual effects. Although the choice of a drug is primarily determined by clinical efficacy, the risk of accidents due to residual effects should be taken into consideration in selecting a hypnotic. Selection of the safest drug and dose possible among those available is a first step towards minimising the risks. A second step is to adequately inform patients about any risks and ways to minimise them by adjusting their behaviour.
The review of information derived from experimental studies should enable clinicians to compare the residual effects of various hypnotics in different doses and select the one considered most favourable in this respect for the individual patient. Although the information is not new, it was not available in a convenient form. The summary of results provided in table VIII is intended as a quick reference guide for the duration and severity of residual effects on psychomotor performance of a number of commonly prescribed hypnotics. It can be used to select hypnotics and doses generally considered unlikely to produce residual effects.
Before prescribing a hypnotic, clinicians should determine whether and how often patients’ activities the day after the use of a hypnotic require optimal alertness, for example, when driving a car. Active consideration should also be given to whether the patient has a history of injurious accidents, such as falls or hip fractures. Clinicians should further determine whether the hypnotic in the dose they intend to prescribe by itself or in combination with other drugs or patient characteristics is likely to produce residual effects and, if so, consider safer alternatives. Since residual effects strongly depend on the dose administered, it is recommended to start with the lowest dose possible, particularly in the elderly. However, when efficacy of this dose is not sufficient, the dose should not be doubled without consideration of the consequences of residual effects, as doubling the dose might produce a disproportional increase in the severity of the residual effects. Patients should be informed of this too. Since the use of hypnotics in combination with other psychoactive drugs largely increases the risks of residual effects, use of multiple drugs should be avoided. For example, some antidepressants can inhibit the metabolism of some benzodiazepines and, thus, lead to increased plasma hypnotic concentrations and prolonged duration of action.
Clinicians should not rely upon the package insert to inform their patients about drug effects on performance. Warnings are often stated in very general terms and not taken seriously by the majority of patients. The advice to patients to be very careful after using a hypnotic would probably have more impact if it were more specific with respect to time. Patients should, therefore, be adequately informed of the duration and severity of residual effects of the hypnotic in the dose prescribed. If a hypnotic that is likely to produce moderate or severe effects cannot be avoided, the recommendation to prohibit driving completely at the start of treatment should be considered. Patients should always be advised not to drive when they feel sleepy, dizzy or are experiencing a lack of concentrated. However, it should also be made clear to them that the absence of such feelings does not mean their performance is normal. Finally, clinicians should keep a careful eye on the patient after prescription of a hypnotic, in particular at the start of treatment.
References
Barbone F, McMahon AD, Davey PG, et al. Association of road-traffic accidents with benzodiazepine use. Lancet 1998; 352(9137): 1331–6
Neutel CI. Risk of traffic accident injury after a prescription for a benzodiazepine. Ann Epidemiol 1995; 5(3): 239–44
Neutel I. Benzodiazepine-related traffic accidents in young and elderly drivers. Hum Psychopharmacol 1998; 13Suppl. 2: S115–23
Cumming RG, Klineberg RJ. Psychotropics, thiazide diuretics and hip fractures in the elderly. Med J Aust 1993; 158: 414–7
Herings RMC, Stricker BH, De Boer A, et al. Benzodiazepines and the risk of falling leading to femur fractures: dosage more mportant than half-life. Arch Intern Med 1995; 155: 1801–7
Lichtenstein MJ, Griffin MR, Cornell JE, et al. Risk factors for hip fractures occurring in the hospital. Am J Epidemiol 1994; 140(9): 830–8
Neutel CI, Downey W, Senet D. Medical events after a prescription for a benzodiazepine. Pharmacoepidemiol Drug Saf 1995; 4: 63–73
Maxwell CJ, Neutel CI, Hirdes JP. A prospective study of falls after benzodiazepine use: a comparison between new and repeat use. Pharmacoepidemiol Drug Saf 1997; 6: 27–35
Ray WA, Griffin MR, Schaffner W, et al. Psychotropic drug use and the risk of hip fracture. N Engl J Med 1987; 316(7): 363–9
Ray WA, Griffin MR, Downey W. Benzodiazepines of long and short elimination half-life and the risk of hip fracture. JAMA 1989; 262(23): 3303–7
ICADTS. Guidelines on experimental studies undertaken to determine a medicinal drug’s effect on driving or skills related to driving. Koeln: Bundes Anstalt fuer Strassenwesen (BASt), 1999 Jun
Vermeeren A, De Gier JJ, O’Hanlon JF. Methodological guidelines for experimental research on medicinal drugs affecting riving performance: an international expert survey. Maastricht: Institute for Human Psychopharmacology, 1993. Report No.: IHP 93-127
Wolschrijn H, De Gier JJ, De Smet PAGM. Drugs and driving new categorization system for medicinal drugs. Maastricht: Institute for Drugs, Safety and Behavior, 1991. Report No.: IGVG 91-124
Berghaus G. Arzneimittel und Fahrtuechtigkeit: Metaanalyse experimenteller Studien. Bergisch Gladbach: Bundesanstalt fuer Strassenwesen, 1998. Report No.: FP 2.9108
O’Hanlon JF, Haak TW, Blaauw GJ, et al. Diazepam impairs lateral position control in highway driving. Science 1982; 217(4554): 79–81
O’Hanlon JF. Driving performance under the influence of drugs: rationale for, and application of, a new test. Br J Clin Pharmacol 1984; 18Suppl. 1: 121s–9
Patat A, Paty I, Hindmarch I. Pharmacodynamic profile of zaleplon, a new non-benzodiazepine hypnotic agent. Hum Psychopharmacol 2001; 16(5): 369–92
Goa KL, Heel RC. Zopiclone: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy as a hypnotic [update of 1986]. Drugs 1991; 32: 48–65
O’Hanlon JF. Zopiclone’s residual effects on psychomotor and information processing skills involved in complex tasks such as car driving: a critical review. Eur Psychiatry 1995; 10Suppl. 3: 137s–43
Nicholson AN. Residual sequelae of zopiclone. Rev Contemp Pharmacother 1998; 9(2): 123–9
Noble S, Langtry HD, Lamb HM. Zopiclone: an update of its pharmacology, clinical efficacy and tolerability in the treatment of insomnia. Drugs 1998; 55(2): 277–302
Langtry HD, Benfield P. Zolpidem: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential. Drugs 1990; 40(2): 291–313
Rush CR. Behavioral pharmacology of zolpidem relative to benzodiazepines: a review. Pharmacol Biochem Behav 1998; 61(3): 253–69
Johnson LC, Chernik DA. Sedative-hypnotics and human performance. Psychopharmacology 1982; 76: 101–13
Roth T, Roehrs T. Determinants of residual effects of hypnotics. Accid Anal Prev 1985; 17(4): 291–6
Roth T, Roehrs TA. A review of the safety profiles of benzodiazepinehypnotics. J Clin Psychiatry 1991; 52 Suppl.: 38–41
Roth T, Roehrs TA, Stepanski EJ, et al. Hypnotics and behavior.Am J Med 1990; 88(3a): 43s–6
Van Laar MW, Volkerts ER. Driving and benzodiazepine use:evidence that they do not mix. CNS Drugs 1998; 10(5):383–96
Leger D, Guilleminault C, Dreyfus JP, et al. Prevalence ofnsomnia in a survey of 12,778 adults in France. J Sleep Res 2000; 9(1): 35–42
Baiter MB, Manheimer DI, Mellinger GD, et al. A cross-nationalcomparison of anti-anxiety/sedative drug use. Curr Med Res Opin 1984; 8 Suppl. 4: 5–20
Mellinger GD, Balter MB, Uhlenhuth EH. Insomnia and itstreatment: prevalence and correlates. Arch Gen Psychiatry 1985; 42(3): 225–32
Pallesen S, Nordhus IH, Nielsen GH, et al. Prevalence ofinsomnia in the adult Norwegian population. Sleep 2001; 24(7): 771–9
Simon GE, VonKorff M. Prevalence, burden, and treatment ofinsomnia in primary care. Am J Psychiatry 1997; 154(10):1417–23
Ohayon MM, Roth T. What are the contributing factors forinsomnia in the general population? J Psychosom Res 2001; 51(6): 745–55
Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. I. Sleep 1999; 22Suppl. 2: S347–53
Christer GM, Hublin CGM, Partinen MM. The extent andimpact of insomnia as a public health problem. Primary Care Companion J Clin Psychiatry 2002; 4 Suppl. 1: 8–12
Reite MD. Treatment of insomnia. In: Schatzberg AF, Nemeroff CB, editors. Textbook of psychopharmacology. 2nd ed. Washington,DC: American Psychiatric Press Inc., 1998: 997–1014
Benca RM. Consequences of insomnia and its therapies. J Clin Psychiatry 2001; 62 Suppl. 10: 33–8
Bond AJ, Lader MH. Residual effects of hypnotics. Psychopharmacology 1972; 25(2): 117–32
Bond AJ, Lader MH. The residual effects of flurazepam.Psychopharmacology 1973; 32(3): 223–35
Bond AJ, Lader MH. Residual effects of flunitrazepam. Br J Clin Pharmacol 1975; 2(2): 143–50
Borland RG, Nicholson AN. Comparison of the residual effects of two benzodiazepines (nitrazepam and flurazepam hydrochloride) and pentobarbitone sodium on human performance. Br J Clin Pharmacol 1975; 2(1): 9–17
Hindmarch I. A repeated dose comparison of three benzodiazepine derivative (nitrazepam, flurazepam and flunitrazepam) on subjective appraisals of sleep and measures of psychomotor performance the morning following night-time medication.Acta Psychiatr Scand 1977; 56(5): 373–81
Hindmarch I. Effects of hypnotic and sleep-inducing drugs on objective assessments of human psychomotor performanceand subjective appraisals of sleep and early morning behaviour.Br J Clin Pharmacol 1979; 8(1): 43s–6s
Hindmarch I, Clyde CA. The effects of triazolam and nitrazepamon sleep quality, morning vigilance and psychomotorperformance. Arzneimittel Forschung 1980; 30(7): 1163–6
Aranko K, Mattila MJ, Seppala T. Development of toleranceand cross-tolerance to the psychomotor actions of lorazepamand diazepam in man. Br J Clin Pharmacol 1983; 15(5):545–52
Gillin JC, Spinweber CL, Johnson LC. Rebound insomnia: acritical review. J Clin Psychopharmacol 1989; 9(3): 161–72
Kales A, Soldatos CR, Bixler EO, et al. Rebound insomnia andrebound anxiety: a review. Pharmacology 1983; 26(3): 121–37
Hurst M, Noble S. Zaleplon. CNS Drugs 1999; 11(5): 387–92
Lapeyre-Mestre M, Chastan E, Louis A, et al. Drug consumptionin workers in France: a comparative study at a 10-yearinterval (1996 versus 1986). J Clin Epidemiol 1999; 52(5):471–8
Walsh JK, Schweitzer PK. Ten-year trends in the pharmacologicaltreatment of insomnia. Sleep 1999; 22(3): 371–5
Doghramji K. The need for flexibility in dosing of hypnoticagents. Sleep 2000; 23Suppl. 1: s16–20
Kirkwood CK. Management of insomnia. J Am Pharm Assoc 1999; 39(5): 688–96
Ohayon MM, Caulet M. Insomnia and psychotropic drug consumption.Prog Neuropsychopharmacol Biol Psychiatry 1995;19(3): 421–31
Ohayon MM, Caulet M, Guilleminault C. How a general populationperceives its sleep and how this relates to the complaintof insomnia. Sleep 1997; 20(9): 715–23
Ohayon MM, Caulet M, Priest RG, et al. Psychotropic medicationconsumption patterns in the UK general population. J Clin Epidemiol 1998; 51(3): 273–83
Ohayon MM, Vecchierini MF. Daytime sleepiness and cognitiveimpairment in the elderly population. Arch Intern Med 2002; 162(2): 201–8
Roehrs T, Hollebeek E, Drake C, et al. Substance use forinsomnia in Metropolitan Detroit. J Psychosom Res 2002; 53(1): 571–6
Weyerer S, Dilling H. Prevalence and treatment of insomnia in the community: results from the Upper Bavarian Field Study.Sleep 1991; 14(5): 392–8
Bader G, Ohayon MM. Insomnia in the general population ofSweden. J Sleep Res 2002; 11 Suppl. 1: 10–1
Ohayon MM, Lader MH. Use of psychotropic medication in thegeneral population of France, Germany, Italy, and the United Kingdom. J Clin Psychiatry 2002; 63(9): 817–25
Nowell PD, Buysse DJ, Reynolds 3rd CF, et al. Clinical factorscontributing to the differential diagnosis of primary insomniaand insomnia related to mental disorders. Am J Psychiatry 1997; 154(10): 1412–6
Doble A. New insights into the mechanism of action of hypnotics.J Psychopharmacol 1999; 13(4 Suppl. 1): S11–20
Landolt H-P, Gillin JC. GABA-A1a receptors: involvement insleep regulation and potential of selective agonists in thetreatment of insomnia. CNS Drugs 2000; 13(3): 185–99
Moehler H, Fritschy JM, Rudolph U. A new benzodiazepinepharmacology. J Pharmacol Exp Ther 2002; 300(1): 2–8
McKernan RM, Rosahl TW, Reynolds DS, et al. Sedative butnot anxiolytic properties of benzodiazepines are mediated bythe GABA (A) receptor alphal subtype. Nat Neurosci 2000; 3(6): 587–92
Hardman JG, Limbird LE, Gilman A. Goodman and Gillman’s:the pharmacological basis of therapeutics. 10th ed. New York: MacMillan, 2001
Neutel CI, Hirdes JP, Maxwell CJ. New evidence on benzodiazepineuse and falls: the time factor. Age Ageing 1996; 25: 273–8
Herings RMC. Geneesmiddelen als determinant van ongevallen.Utrecht: Universiteit Utrecht, Faculteit Farmacie, 1994
Passaro A, Volpato S, Romagnoni F, et al. Benzodiazepineswith different half-life and falling in a hospitalized population:The GIFA study. Gruppo Italiano di Farmacovigilanza nell’Anziano.J Clin Epidemiol 2000; 53(12): 1222–9
Pierfitte C, Macouillard G, Thicoipe M, et al. Benzodiazepinesand hip fractures in elderly people: case-control study. BMJ 2001; 322(7288): 704–8
Ray WA, Fought RL, Decker MD. Psychoactive drugs and therisk of injurious motor vehicle crashes in elderly drivers. Am JEpidemiol 1992; 136: 873–83
Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in olderpeople: a systematic review and meta-analysis: I. psychotropicdrugs. J Am Geriatr Soc 1999; 47(1): 30–9
Neutel CI, Perry S, Maxwell C. Medication use and risk of falls.Pharmacoepidemiol Drug Saf 2002; 11(2): 97–104
De Gier JJ. Review of investigations of prevalence of illicitdrugs in road traffic in diffrent European countries. In: Group P, editor. Road traffic and drugs; 1999. Strasbourg: Council ofEurope, 1999: 13–60
Longo MC, Hunter CE, Lokan RJ, et al. The prevalence ofalcohol, cannabinoids, benzodiazepines and stimulantsamongst injured drivers and their role in driver culpability: parti: the prevalence of drug use in drive the drug-positive group.Accid Anal Prev 2000; 32(5): 613–22
Longo MC, Hunter CE, Lokan RJ, et al. The prevalence ofalcohol, cannabinoids, benzodiazepines and stimulantsamongst injured drivers and their role in driver culpability: partII: the relationship between drug prevalence and drug concentration,and driver culpability. Accid Anal Prev 2000; 32(5):623–32
Honkanen R, Ertama L, Linnoila M, et al. Role of drugs intraffic accidents. BMJ 1980; 281: 1309–12
Skegg DCG, Richards SM, Doll R. Minor tranquilisers and roadaccidents. BMJ 1979; 1(6168): 917–9
Hemmelgarn B, Suissa S, Huang A, et al. Benzodiazepine useand the risk of motor vehicle crash in the elderly. JAMA 1997;278(1): 27–31
Leveille SG, Buchner DM, Koepsell TD, et al. Psychoactivemedications and injurious motor vehicle collisions involvingolder drivers. Epidemiology 1994; 5(6): 591–8
Vermeeren A, Danjou PE, O’Hanlon JF. Residual effects ofevening and middle-of-the-night administration of zaleplon 10and 20mg on memory and actual driving performance. Hum Psychopharmacol 1998; 13 Suppl. 2: S98–107
Vermeeren A, Riedel WJ, Van Boxtel CJ, et al. Differentialresidual effects of zaleplon and zopiclone on actual driving: acomparison with a low dose of alcohol. Sleep 2002; 25(2):224–31
Volkerts ER, O’Hanlon JF. Residu effecten op de actuele rijprestatie:zopiclone versus flunitrazepam en nitrazepam (Residualeffects on real car driving performance: zopiclone versus flunitrazepamand nitrazepam; Dutch with English summary andlegends). J Drug Ther Res 1988; 13: 111–4
Bramness JG, Skurtveit S, Morland J. Zopiklonfunn hos mangebilforere-tegn pa feilbruk og misbruk. Tidsskr Nor Laegeforen 1999; 119(19): 2820–1
Cumming RG, Le Couteur DG. Benzodiazepines and risk of hipfractures in older people: a review of the evidence. CNS Drugs 2003; 17: 825–37
Tromp AM, Pluijm SM, Smit JH, et al. Fall-risk screening test:a prospective study on predictors for falls in community-dwellingelderly. J Clin Epidemiol 2001; 54(8): 837–44
Ray WA, Thapa PB, Gideon P. Benzodiazepines and the risk offalls in nursing home residents. J Am Geriatr Soc 2000; 48(6):682–5
Ray WA, Thapa PB, Gideon P. Misclassification of currentbenzodiazepine exposure by use of single baseline measurementand its effects upon studies of injuries.Pharmacoepidemiol Drug Saf 2002; 11(11): 1–7
Mendelson WB. The use of sedative/hypnotic medication andits correlation with falling down in the hospital. Sleep 1996; 19(9): 698–701
Lord SR, Anstey KJ, Williams P, et al. Psychoactive medicationuse, sensori-motor function and falls in older women.Br J Clin Pharmacol 1995; 39(3): 227–34
Cumming RG, Miller JP, Kelsey JL, et al. Medications andmultiple falls in elderly people: the St Louis OASIS Study.Age Ageing 1991; 20: 455–61
Campbell AJ, Borrie MJ, Spears GF. Risk factors for falls in acommunity-based prospective study of people 70 years andolder. J Gerontol A Biol Sci Med Sci 1989; 44(4): 112–7
Granek E, Baker SP, Abbey H, et al. Medications and diagnosesin relation to falls in a long-term care facility. J Am Geriatr Soc 1987; 35(6): 503–11
Wang PS, Bohn RL, Glynn RJ, et al. Zolpidem use and hipfractures in older people. J Am Geriatr Soc 2001; 49(12):1685–90
Sgadari A, Lapane KL, Mor V, et al. Oxidative and nonoxidative benzodiazepines and the risk of femur fracture: the Systematic Assessment of Geriatric Drug Use Via Epidemiology Study Group. J Clin Psychopharmacol 2000; 20(2): 234–9
Blake AJ, Morgan K, Bendall MJ, et al. Falls by elderly peopleat home: prevalence and associated factors. Age Ageing 1988;17(6): 365–72
Riedel WJ, Vermeeren A, Van Boxtel MPJ, et al. Mechanismsof drug-induced driving impairment: a dimensional approach.Hum Psychopharmacol 1998; 13Suppl. 2: S49–63
O’Hanlon JF, Ramaekers JG. Antihistamine effects on actualdriving performance in a standard test: a summary of Dutchexperience, 1989–94. Allergy 1995; 50(3): 234–42
O’Hanlon JF, Vermeeren A, Uiterwijk MM, et al. Anxiolytics’effects on the actual driving performance of patients andhealthy volunteers in a standardized test: an integration ofthree studies. Neuropsychobiol 1995; 31(2): 81–8
Louwerens JW, Gloerich ABM, De Vries G, et al. The relationshipbetween drivers’ blood alcohol concentration (BAC) andactual driving performance during high speed travel. In:Noordzij PC, Roszbach R, editors. International congress onalcohol, drugs and traffic safety, T86; 1987. Amsterdam: ExerptaMedica, 1987: 183–6
Borkenstein RF. The role of the drinking driver in traffic accidents,the Grand Rapids study. 2nd ed. Blutalcohol 1974; 11Suppl. 1: 1–131
Vermeeren A, O’Hanlon JF, Declerck AC, et al. Acute effectsof zolpidem and flunitrazepam on sleep, memory and drivingperformance, compared to those of partial sleep deprivationand placebo. Acta Ther 1995; 21: 47–64
Verster JC, Volkerts ER, Schreuder AH, et al. Residual effectsof middle-of-the-night administration of zaleplon and zolpidemon driving ability, memory functions, and psychomotorperformance. J Clin Psychopharmacol 2002; 22: 576–83
O’Hanlon JF, Volkerts ER. Hypnotics and actual driving performance.Acta Psychiatr Scand Suppl 1986; 332: 95–104
Volkerts ER, Van Laar MW, Van Willigenburg AP, et al. Acomparative study of on-the-road and simulated driving performanceafter nocturnal treatment with lormetazepam 1mgand oxazepam 50mg. Hum Psychopharmacol 1992; 7(5):297–309
Brookhuis KA, Volkerts ER, O’Hanlon JF. Repeated dose effectsof lormetazepam and flurazepam upon driving performance.Eur J Clin Pharmacol 1990; 39(1): 83–7
Volkerts ER, O’Hanlon JF. Hypnotics’ residual effects on drivingperformance. In: O’Hanlon JF, De Gier JJ, editors. Drugsand driving. London: Taylor Francis, 1986
O’Hanlon JF. Alcohol and hypnotic hangovers as an influenceon driving performance. Trav Traf Med Int 1983; 1(3): 147–52
Vermeeren A, Ramaekers JG, Van Leeuwen CJ, et al. Residualeffects on actual car driving of evening dosing ofchlorpheniramine 8 and 12mg when use with terfenadine 60mgin the morning. Hum Psychopharmacol 1998; 13Suppl. 2:S79–86
Brookhuis KA, Volkerts ER, O’Hanlon JF. Repeated dose effectsof lormetazepam 1 and 2mg (in soft gelatine capsules)and flurazepam 30mg upon driving performance [Report VK86-18]. Haren: Traffic Research Centre, 1986. Report No.: VK86-18
Riedel WJ, Quasten R, Hausen C, et al. A study comparing thehypnotic efficacies and residual effects on actual driving performanceof midazolam 15mg, triazolam 0.5mg, temazepam20mg and placebo in shiftworkers on night duty. Technicalreport. Maastricht: Institute for Drugs Safety and Behavior,1988. Report No.: IGVG 88-01
Mclnnes GT, Bunting EA, Ings RM, et al. Pharmacokineticsand pharmacodynamics following single and repeated nightlyadministrations of loprazolam, a new benzodiazepine hypnotic.Br J Clin Pharmacol 1985; 19(5): 649–56
Danjou P, Paty I, Fruncillo R, et al. A comparison of theresidual effects of zaleplon and zolpidem following administration5 to 2h before awakening. Br J Clin Pharmacol 1999; 48(3): 367–74
Hindmarch I, Patat A, Stanley N, et al. Residual effects ofzaleplon and zolpidem following middle of the night administrationfive hours to one hour before awakening. Hum Psychopharmacol 2001; 16: 159–67
Troy SM, Lucki I, Unruh MA, et al. Comparison of the effectsof zaleplon, zolpidem, and triazolam on memory, learning, andpsychomotor performance. J Clin Psychopharmacol 2000; 20(3): 328–37
Stone BM, Turner C, Mills SL, et al. Sleep in a noisy environment:hypnotic and residual effects of zaleplon [abstract]. JSleep Res 2000; 9 Suppl. 1: 183
O’Hanlon JF. Residual effects on memory and psychomotorperformance of zaleplon and other hypnotic drugs. Prim CareCompanion J Clin Psychiatry 2002; 4 Suppl. 1: 38–44
Darcourt G, Pringuey D, Salliere D, et al. The safety andtolerability of zolpidem: an update. J Psychopharmacol 1999;3(1): 81–93
Lader M, Hindmarch I. Memory, psychomotor and cognitivefunctions after treatment with zolpidem. In: Freeman H, Puech AJ, Roth T, editors. Zolpidem: un update of its pharmacologicalproperties and therapeutic place in management of insomnia.Paris: Elsevier, 1996
Unden M, Roth Schechter B. Next day effects after nighttimetreatment with zolpidem: a review. Eur Psychiatry 1996; 11Suppl. 1: S21–30
Erman MK, Erwin CW, Gengo FM, et al. Comparative efficacyof zolpidem and temazepam in transient insomnia. Hum Psychopharmacol 2001; 16: 169–76
Zammit G. Zaleplon vs zolpidem: differences in next-dayresidual sedation after middle-of-the-night administration [abstract].J Sleep Res 2000; 9Suppl. 1: 214
Cluydts R, De Roeck J, Schotte C, et al. Comparative study onthe residual daytime effects of flunitrazepam, triazolam, andplacebo in healthy volunteers. Acta Ther 1986; 12: 205–17
Lobo BL, Greene WL. Insomnia. N Engl J Med 1997; 336(26):1919–20
Mintzer MZ, Frey JM, Yingling JE, et al. Triazolam andzolpidem: a comparison of their psychomotor, cognitive, andsubjective effects in healthy volunteers. Behav Pharmacol1997; 8(6–7): 561–74
Rush CR, Griffiths RR. Zolpidem, triazolam, and temazepam:behavioral and subject-rated effects in normal volunteers. JClin Psychopharmacol 1996; 16(2): 146–57
Moskowitz H, Linnoila M, Roehrs T. Psychomotor performancein chronic insomniacs during 14-day use of flurazepam andmidazolam. J Clin Psychopharmacol 1990; 104Suppl. 4: 44s–55
Jackson JL, Louwerens JW, Cnossen F, et al. Testing the effectsof hypnotics on memory via the telephone: fact or fiction? Psychopharmacology 1993; 111(2): 127–33
Godtlibsen OB, Jerko D, Gordeladze JO, et al. Residual effectof single and repeated doses of midazolam and nitrazepam inrelation to their plasma concentrations. Eur J Clin Pharmacol 1986; 29: 595–600
Borbely AA, Mattmann P, Loepfe M. Hypnotic action andresidual effects of a single bedtime dose of temazepam.Arzneimittel Forschung 1984; 34(1): 101–3
Hemmeter U, Muller M, Bischof R, et al. Effect of zopicloneand temazepam on sleep EEG parameters, psychomotor andmemory functions in healthy elderly volunteers. Psychopharmacology 2000; 147(4): 384–96
Hindmarch I. Hypnotics and residual sequalae. In: Nicholson AN, editor. Hypnotics in clinical practice. Edinburgh: MedicinePublishing Foundation, 1982: 7–16
Porcu S, Bellatreccia A, Ferrara M, et al. Performance, abilityto stay awake, and tendency to fall asleep during the night aftera diurnal sleep with temazepam or placebo. Sleep 1997; 20(7):535–41
Warburton DM, Wesnes K. A comparison of temazepam andflurazepam in terms of sleep quality and residual changes inactivation and performance. Arzneimittel Forschung 1984; 34(11): 1601–4
Volkerts ER, Abbink F. Kater-effecten op de rijvaardigheid naslaapmiddelgebruik? Lormetazepam en oxazepam versus placebo(Hangover effects on driving performance following useof hypnotics?). J Drug Ther Res 1990; 15(1): 22–6
Bourin M, Hubert C, Colombel MC, et al. Residual effects oftemazepam versus nitrazepam on memory and vigilance in thenormal subject. Hum Psychopharmacol 1987; 2: 185–9
Laurell H, Tornros J. The carry-over effects of triazolam comparedwith nitrazepam and placebo in acute emergency drivingsituations and in monotonous simulated driving. Acta Pharmacol Toxicol (Copenh) 1986; 58(3): 182–6
Morgan K. Effects of repeated dose nitrazepam andlormetazepam on psychomotor performance in the elderly.Psychopharmacology 1985; 86(1–2): 209–11
Subhan Z, Hindmarch I. Asessing residual effects of benzodiazepineson short term memory. Pharm Med 1984; 1: 27–32
Woods JH, Winger G. Abuse liability of flunitrazepam. J Clin Psychopharmacol 1997; 17(3 Suppl. 2): 1s–57
Carvey PM. Drug action in the central nervous system. NewYork: Oxford University Press, 1998
Roehrs T, Kribbs N, Zorick F, et al. Hypnotic residual effects ofbenzodiazepines with repeated administration. Sleep 1986; 9(2): 309–16
Menzin J, Lang KM, Levy P, et al. A general model of theeffects of sleep medications on the risk and cost of motorvehicle accidents and its application to France. Pharmacoeconomics 2001; 19(1): 69–78
Acknowledgements
The author has provided no information on sources of funding or on conflicts of interest directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vermeeren, A. Residual Effects of Hypnotics. CNS Drugs 18, 297–328 (2004). https://doi.org/10.2165/00023210-200418050-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00023210-200418050-00003