Abstract
Rationale
The effects of hypnotics on automobile driving have been attracting increasing attention. However, few driving simulators (DSs) have been confirmed to have acceptable reliability and validity for assessing the next-day residual effects of zopiclone as a positive control on driving performance.
Objective
To investigate whether a new DS could permit detection of the next-day residual effects of zopiclone on driving performance.
Methods
In this double-blind, randomized, placebo-controlled crossover trial, 28 healthy males received zopiclone 7.5 mg at bedtime on days 1 and 8 and placebo on the other days over a period of 16 days. The participants took part in three driving tasks—road-tracking, car-following, and harsh-braking—using a DS on days 2 and 9 at 9-h post-dosing. Scores on the Karolinska Sleepiness Scale and Profile of Mood States-Second Edition were then assessed, as was the serum concentration of zopiclone.
Results
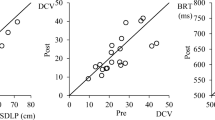
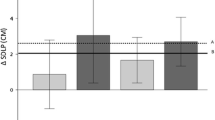
The estimated differences in the standard deviation of lateral position (cm) in the road-tracking task between the zopiclone and placebo groups on days 2 and 9 were 3.75 cm (90% confidence interval (CI): 1.71–5.79) and 4.07 cm (90% CI: 2.02–6.11), respectively. The estimated differences in the distance coefficient of variation in the car-following task and in the brake reaction time in the harsh-braking task between the zopiclone and placebo groups on day 2 were 4.31 (90% CI: 1.94–6.69) and 24.6 ms (90% CI: 12.7–36.4), respectively.
Conclusions
The DS used in this study has sufficient sensitivity to detect the next-day residual effects of zopiclone on driving performance.



Similar content being viewed by others
References
Akerstedt T, Gillberg M (1990) Subjective and objective sleepiness in the active individual. Int J Neurosci 52:29–37
American Psychiatric Association (2013) Diagnostic and Statistical Manual for Mental Disorders (DSM-5), 5th edn. American Psychiatric Association Publishing, Washington, DC
Anderson GD (2008) Gender differences in pharmacological response. Int Rev Neurobiol 83:1–10
Barbone F, McMahon AD, Davey PG, Morris AD, Reid IC, McDevitt DG, MacDonald TM (1998) Association of road-traffic accidents with benzodiazepine use. Lancet 352:1331–1336
Berthelon C, Bocca ML, Denise P, Pottier A (2003) Do zopiclone, zolpidem and flunitrazepam have residual effects on simulated task of collision anticipation? J Psychopharmacol 17:324–331
Bocca ML, Marie S, Lelong-Boulouard V, Bertran F, Couque C, Desfemmes T, Berthelon C, Amato JN, Moessinger M, Paillet-Loilier M, Coquerel A, Denise P (2011) Zolpidem and zopiclone impair similarly monotonous driving performance after a single nighttime intake in aged subjects. Psychopharmacology 214:699–706
Boyle J, Trick L, Johnsen S, Roach J, Rubens R (2008) Next-day cognition, psychomotor function, and driving-related skills following nighttime administration of eszopiclone. Hum Psychopharmacol 23:385–397
Chang CM, Wu EC, Chen CY, Wu KY, Liang HY, Chau YL, Wu CS, Lin KM, Tsai HJ (2013) Psychotropic drugs and risk of motor vehicle accidents: a population-based case-control study. Br J Clin Pharmacol 75:1125–1133
World Health Organization (2016) Drug use and road safety: a policy brief. World Health Organization. https://apps.who.int/iris/handle/10665/249533. Accessed 4 Sept 2021
Fernandez C, Martin C, Gimenez F, Farinotti R (1995) Clinical pharmacokinetics of zopiclone. Clin Pharmacokinet 29:431–441
Gaillot J, Heusse D, Hougton GW, Marc Aurele J, Dreyfus JF (1983) Pharmacokinetics and metabolism of zopiclone. Pharmacology 27(Suppl 2):76–91
Hoiseth G, Hjelmeland K, Morland J (2021) A comparison of driving related skills impaired by ethanol and zopiclone. Traffic Inj Prev 22:26–31
Huizinga CR, Zuiker RG, de Kam ML, Ziagkos D, Kuipers J, Mejia Y, van Gerven JM, Cohen AF (2019) Evaluation of simulated driving in comparison to laboratory-based tests to assess the pharmacodynamics of alprazolam and alcohol. J Psychopharmacol 33:791–800
Irwin C, Iudakhina E, Desbrow B, McCartney D (2017) Effects of acute alcohol consumption on measures of simulated driving: a systematic review and meta-analysis. Accid Anal Prev 102:248–266
Iwata M, Iwamoto K, Kawano N, Kawaue T, Ozaki N (2018) Evaluation method regarding the effect of psychotropic drugs on driving performance: a literature review. Psychiatry Clin Neurosci 72:747–773. https://doi.org/10.1097/MD.0000000000019395
Iwata M, Iwamoto K, Kitajima I, Nogi T, Onishi K, Kajiyama Y, Nishino I, Ando M, Ozaki N (2021) Validity and reliability of a driving simulator for evaluating the influence of medicinal drugs on driving performance. Psychopharmacology 238:775–786
Iwata M, Iwamoto K, Kambe D, Tachibana N, Ando M, Ozaki N (2020) Development and validation of a driving simulator for evaluating the residual effects of drugs on driving performance - sensitivity analysis using zopiclone as a positive control: Study Protocol Clinical Trial (SPIRIT Compliant). Medicine (Baltimore) 99: e19395.
Jongen S, Vuurman E, Ramaekers JG, Vermeeren A (2018) Comparing the effects of oxazepam and diazepam in actual highway driving and neurocognitive test performance: a validation study. Psychopharmacology 235:1283–1294
Kay GG, Hochadel T, Sicard E, Natarajan KK, Kim NN (2017) Next-day residual effects of flibanserin on simulated driving performance in premenopausal women. Hum Psychopharmacol 32:e2603. https://doi.org/10.1002/hup.2603
Leufkens TR, Vermeeren A (2009) Highway driving in the elderly the morning after bedtime use of hypnotics: a comparison between temazepam 20 mg, zopiclone 7.5 mg, and placebo. J Clin Psychopharmacol 29:432–438
Leufkens TR, Vermeeren A (2014) Zopiclone’s residual effects on actual driving performance in a standardized test: a pooled analysis of age and sex effects in 4 placebo-controlled studies. Clin Ther 36:141–150
Leufkens TR, Lund JS, Vermeeren A (2009) Highway driving performance and cognitive functioning the morning after bedtime and middle-of-the-night use of gaboxadol, zopiclone and zolpidem. J Sleep Res 18:387–396
Leufkens TR, Ramaekers JG, de Weerd AW, Riedel WJ, Vermeeren A (2014) Residual effects of zopiclone 7.5 mg on highway driving performance in insomnia patients and healthy controls: a placebo controlled crossover study. Psychopharmacology 231:2785–2798
McNair DM, Lorr M, Droppeleman LF (1971) EITS Manual for the Profile of Mood States. Educational and Industrial Testing Services, San Diego
Mets MA, de Vries JM, de Senerpont Domis LM, Volkerts ER, Olivier B, Verster JC (2011) Next-day effects of ramelteon (8 mg), zopiclone (7.5 mg), and placebo on highway driving performance, memory functioning, psychomotor performance, and mood in healthy adult subjects. Sleep 34:1327–1334
National Highway Traffic Safety Administration (2019) Traffic Safety Facts 2017. National Highway Traffic Safety Administration, Washington, DC
National Police Agency (2018) Statistics about Road Traffic annual report 2018. National Public Safety Commision and National Police Agency, Tokyo
Ogden EJ, Moskowitz H (2004) Effects of alcohol and other drugs on driver performance. Traffic Inj Prev 5:185–198
Orriols L, Philip P, Moore N, Castot A, Gadegbeku B, Delorme B, Mallaret M, Lagarde E, Group CR (2011) Benzodiazepine-like hypnotics and the associated risk of road traffic accidents. Clin Pharmacol Ther 89:595–601
Orriols L, Queinec R, Philip P, Gadegbeku B, Delorme B, Moore N, Suissa S, Lagarde E, Group CR (2012) Risk of injurious road traffic crash after prescription of antidepressants. J Clin Psychiatry 73:1088–1094
Ramaekers JG, Conen S, de Kam PJ, Braat S, Peeters P, Theunissen EL, Ivgy-May N (2011) Residual effects of esmirtazapine on actual driving performance: overall findings and an exploratory analysis into the role of CYP2D6 phenotype. Psychopharmacology 215:321–332
Ravera S, van Rein N, de Gier JJ, de Jong-van den Berg LT, (2011) Road traffic accidents and psychotropic medication use in The Netherlands: a case-control study. Br J Clin Pharmacol 72:505–513
Ravera S, Monteiro SP, de Gier JJ, van der Linden T, Gomez-Talegon T, Alvarez FJ, Partners DPW (2012) A European approach to categorizing medicines for fitness to drive: outcomes of the DRUID project. Br J Clin Pharmacol 74:920–931
Simen AA, Gargano C, Cha JH, Drexel M, Bautmans A, Heirman I, Laethem T, Hochadel T, Gheyle L, Bleys K, Beals C, Stoch A, Kay GG, Struyk A (2015) A randomized, crossover, placebo-controlled clinical trial to assess the sensitivity of the CRCDS Mini-Sim to the next-day residual effects of zopiclone. Ther Adv Drug Saf 6:86–97
Stranks EK, Crowe SF (2014) The acute cognitive effects of zopiclone, zolpidem, zaleplon, and eszopiclone: a systematic review and meta-analysis. J Clin Exp Neuropsychol 36:691–700
US Food and Drug Administration (2013) FDA drug safety communication: risk of next-morning impairment after use of insomnia drugs; FDA requires lower recommended doses for certain drugs containing zolpidem (Ambien, Ambien CR, Edluar, and Zolpimist). https://www.fda.gov/media/84992/download. Accessed 4 Sept 2021
US Food and Drug Administration (2017) Evaluating drug effects on the ability to operate a motor vehicle guidance for industry. https://www.fda.gov/media/90670/download. Accessed 4 Sept 2021
Vermeeren A (2004) Residual effects of hypnotics: epidemiology and clinical implications. CNS Drugs 18:297–328
Vermeeren A, Riedel WJ, van Boxtel MP, Darwish M, Paty I, Patat A (2002) Differential residual effects of zaleplon and zopiclone on actual driving: a comparison with a low dose of alcohol. Sleep 25:224–231
Vermeeren A, Vuurman EF, Leufkens TR, Van Leeuwen CJ, Van Oers AC, Laska E, Rico S, Steinberg F, Roth T (2014) Residual effects of low-dose sublingual zolpidem on highway driving performance the morning after middle-of-the-night use. Sleep 37:489–496
Vermeeren A, Sun H, Vuurman EF, Jongen S, Van Leeuwen CJ, Van Oers AC, Palcza J, Li X, Laethem T, Heirman I, Bautmans A, Troyer MD, Wrishko R, McCrea J (2015) On-the-road driving performance the morning after bedtime use of suvorexant 20 and 40 mg: a study in non-elderly healthy volunteers. Sleep 38:1803–1813
Vermeeren A, Vets E, Vuurman EF, Van Oers AC, Jongen S, Laethem T, Heirman I, Bautmans A, Palcza J, Li X, Troyer MD, Wrishko R, McCrea J, Sun H (2016) On-the-road driving performance the morning after bedtime use of suvorexant 15 and 30 mg in healthy elderly. Psychopharmacology 233:3341–3351
Vermeeren A, Jongen S, Murphy P, Moline M, Filippov G, Pinner K, Perdomo C, Landry I, Majid O, Van Oers ACM, Van Leeuwen CJ, Ramaekers JG, Vuurman E (2019) On-the-road driving performance the morning after bedtime administration of lemborexant in healthy adult and elderly volunteers. Sleep 42:zsy260. https://doi.org/10.1093/sleep/zsy260
Verster JC, Roth T (2011) Standard operation procedures for conducting the on-the-road driving test, and measurement of the standard deviation of lateral position (SDLP). Int J Gen Med 4:359–371
Verster JC, Spence DW, Shahid A, Pandi-Perumal SR, Roth T (2011) Zopiclone as positive control in studies examining the residual effects of hypnotic drugs on driving ability. Curr Drug Saf 6:209–218
Volkerts ER, Louwerens JW, Gloerich ABM, Brookhuis KA, O’Hanlon JF (1984) Zopiclone’s residual effect upon actual driving performance versus those of nitrazepam and flunitrazepam. Technical report VK-84-10. Traffic Research Centre, University of Groningen, Groningen.
Funding
This trial was supported by Taisho Pharmaceutical Company, Ltd.; Research on Regulatory Science of Pharmaceuticals and Medical Devices from the Japan Agency for Medical Research and Development (JP21mk0101137h0003); research grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan; the Ministry of Health, Labour and Welfare of Japan; and a Grant-in-Aid from the “Center of Innovation for Personalized and Diverse Society” carried out under the Center of Innovation Program from the Japan Science and Technology Agency.
Research on Regulatory Science of Pharmaceuticals and Medical Devices from the Japan Agency for Medical Research and Development,JP21mk0101137h0003,Kunihiro Iwamoto,Ministry of Education,Culture,Sports,Science and Technology of Japan,Ministry of Health,Labour and Welfare,Grant-in-Aid from the “Center of Innovation for Personalized and Diverse Society” carried out under the Center of Innovation Program from the Japan Science and Technology Agency
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
KI has received speakers’ honoraria from Eisai, Kyowa, Meiji Seika Pharma, Otsuka, Sumitomo Dainippon, Taisho, Takeda, and Towa. MI has no conflicts of interest to declare. DK, YI, KN, and YK are employees of Taisho Pharmaceutical Co., Ltd., Japan. MA has received subsidies from Kyowa Kirin. NO has received research support or speakers’ honoraria from, or has served as a consultant to, Sumitomo Dainippon, Eisai, Otsuka, KAITEKI, Mitsubishi Tanabe, Shionogi, Eli Lilly, Mochida, DAIICHI SANKYO, Nihon Medi-Physics, Takeda, Meiji Seika Pharma, EA Pharma, Pfizer, MSD, Lundbeck Japan, Taisho Pharma, Janssen, UCB, Shionogi, Nihon Medi-Physics, Tsumura, Novartis, and Astellas.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Iwamoto, K., Iwata, M., Kambe, D. et al. Residual effects of zopiclone on driving performance using a standardized driving simulator among healthy volunteers. Psychopharmacology 239, 841–850 (2022). https://doi.org/10.1007/s00213-022-06075-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-022-06075-y