Abstract
-
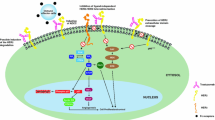
▲ Trastuzumab is a humanised IgG1 monoclonal antibody, which selectively binds to the human epidermal growth factor receptor 2 (HER2), inhibiting cell proliferation and survival in HER2-dependent tumours.
-
▲ In a randomised phase III trial in postmenopausal women with HER2 and hormone receptor (HR) copositive metastatic breast cancer, median progression-free survival (primary endpoint) was significantly longer in patients receiving intravenous trastuzumab plus oral anastrozole than in those receiving anastrozole alone.
-
▲ Overall response rate and clinical benefit rate were also significantly higher, and median time to disease progression was significantly longer with trastuzumab plus anastrozole versus anastrozole alone.
-
▲ There were no reports of new or unexpected adverse events with trastuzumab plus anastrozole combination therapy in the randomised phase III trial.
-
▲ In a noncomparative phase II study of trastuzumab plus letrozole in postmenopausal women with HER2 and HR co-positive metastatic breast cancer, the majority of adverse events were mild or moderate in severity.

Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
American Cancer Society. Breast cancer facts and figures 2005–2006 [online]. Available from URL: http://www.cancer.org [Accessed 2007 Nov 30]
European Society for Medical Oncology. Recurrent or metastatic breast cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2007; 18 Suppl. 2: ii9–11
Plosker GL, Keam SJ. Trastuzumab: a review of its use in the management of HER2-positive metastatic and early-stage breast cancer. Drugs 2006; 66(4): 449–75
McKeage K, Perry CM. Trastuzumab: a review of its use in the treatment of metastatic breast cancer overexpressing HER2. Drugs 2002; 62(1): 209–43
Jackisch C. HER-2-positive metastatic breast cancer: optimizing trastuzumab-based therapy [online]. Available from URL: http://www.TheOncologist.com [Accessed 2007 Aug 8]
Committee for Medicinal Products for Human Use. Scientific discussion (of the extension of the indication for the use in combination with an aromatase inhitor for the treatment of patients with HER2-positive and hormone receptor positive metastatic breast cancer, not previously treated with trastuzumab) [online]. Available from URL: http://www.emea.europa.eu [Accessed 2007 Aug 10]
Ponzone R, Montemurro F, Maggiorotto F, et al. Clinical outcome of adjuvant endocrine treatment according to PR and HER-2 status in early breast cancer. Ann Oncol 2006 Nov; 17(11): 1631–6
Schiff R, Massarweh S, Shou J, et al. Breast cancer endocrine resistance: how growth factor signaling and estrogen receptor coregulators modulate response. Clin Cancer Res 2003 Jan; 9 Suppl. 1: 447–54S
Wong ZW, Isaacs C, Harris L, et al. A phase II trial of letrozole and trastuzumab for ER and/or PgR and HER2 positive metastatic breast cancer [abstract no. 144]. 26th Annual San Antonio Breast Cancer Symposium; 2003 Dec 3–6; San Antonio (TX)
Albaneil J, Codony J, Rovira A, et al. Mechanism of action of anti-HER2 monoclonal antibodies: scientific update on trastuzumab and 2C4. Adv Exp Med Biol 2003; 532: 253–68
Hudis CA. Trastuzumab: mechanism of action and use in clinical practice. N Engl J Med 2007 Jul 5; 357(1): 39–51
Ocana A, Cruz JJ, Pandiella A. Trastuzumab and antiestrogen therapy: focus on mechanisms of action and resistance. Am J Clin Oncol 2006 Feb; 29(1): 90–5
Mohsin SK, Weiss HL, Gutierrez MC, et al. Neoadjuvant trastuzumab induces apoptosis in primary breast cancers. J Clin Oncol 2005 Apr 10; 23(11): 2460–8
Molina MA, Codony-Servat J, Albaneil J, et al. Trastuzumab (Herceptin), a humanized anti-HER2 receptor monoclonal antibody, inhibits basal and activated HER2 ectodomain cleavage in breast cancer cells. Cancer Res 2001 Jun; 61: 4744–9
Izumi Y, Xu L, di Tomaso E., et al. Tumour biology: herceptin acts as an anti-angiogenic cocktail. Nature 2002; 416: 279–80
Yakes FM, Chinratanalab W, Ritter CA, et al. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and akt is required for antibody-mediated effects on p27, cyclin D1, and antitumour action. Cancer Res 2002 Jul 15; 62: 4132–41
Lane H, Beuvink I, Motoyama AB, et al. ErB2 potentiates breast tumor proliferation through modulation of p27Kip1-Cdk2 complex formation: receptor occupation does not determine growth dependency. Mol Cell Biol 2000 May; 20(9): 3210–23
Lane HA, Motoyama A, Beuvink I, et al. Modulation of p27/ Cdk2 complex formation through 4D5-mediated inhibition of HER2 receptor signaling. Ann Oncol 2001; 12 Suppl. 1: S21–2
Valabrega G, Montemurro F, Aglietta M. Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann Oncol 2007 Jan 17; 18(6): 977–84
Gennari R, Menard S, Fagnoni F, et al. Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clin Cancer Res 2004 Sep 1; 10(17): 5650–5
Herceptin® (trastuzumab) prescribing information. San Francisco (CA): Genentech Inc., 2006 Nov
Carter P, Presta L, Gorman CM, et al. Humanization of an anti-pl85HER2 antibody for human cancer therapy. Proc Natl Acad Sci U S A 1992 May; 89: 4285–9
Nahta R, Takahashi T, Ueno NT, et al. P27kiPl down-regulation is associated with trastuzumab resistance in breast cancer cells. Cancer Res 2004 Jun 1; 64: 3981–6
Nagata Y, Lan K-H, Zhou X, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell 2004 Aug; 6: 117–27
Lu Y, Zi X, Pollak M, et al. Molecular mechanisms underlying IGF-I-induced attenuation of the growth-inhibitory activity of trastuzumab (herceptin) on SKBR3 breast cancer cells. Int J Cancer 2004; 108: 334–41
Valabrega G, Montemurro F, Sarotto I, et al. TGFα expression impairs trastuzumab-induced HER2 downregulation. Onco-gene 2005; 24: 3002–10
Harris K, Washington CB, Lieberman J-F. A population pharmacokinetic (PK) model for trastuzumab (Herceptin) and implications for clinical dosing [abstract no. 488; plus poster]. 38th Annual Meeting of the American Society of Clinical Oncology; 2002 May 18; Orlando (FL)
Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 1999; 17(9): 2639–48
Baselga J, Carbonell X, Castaneda-Soto NJ, et al. Phase II study of efficacy, safety, and pharmacokinetics of trastuzumab monotherapy administered on a 3-weekly schedule. J Clin Oncol 2005 Apr 1; 23(10): 2162–71
Leyland-Jones B, Gelmon K, Ayoub JP, et al. Pharmacokinetics, safety, and efficacy of trastuzumab administered every three weeks in combination with paclitaxel. J Clin Oncol 2003 Nov 1; 21(21): 3965–71
European Medicines Agency. Herceptin: summary of product characteristics [online]. Available from URL: http://www.emea.europa.eu [Accessed 2007 Aug 8]
Kaufman B, Mackey J, Clemens M, et al. Trastuzumab plus anastrozole prolongs progression-free survival in postmenopausal women with HER2-positive, hormone-dependent metastatic breast cancer [abstract no. LBA2]. Ann Oncol 2006 Sep; 17 Suppl. 9: i
Mackey JR, Kaufman B, Clemens M, et al. Trastuzumab prolongs progression-free survival in hormone-dependent and HER2-positive metastatic breast cancer [abstract; plus slide presentation at the 29th Annual San Antonio Breast Cancer Symposium 2006 Dec 14–17]. Breast Cancer Res Treat 2006 Dec; 100 Suppl. 1: S5–6
Marcom PK, Isaacs C, Harris L, et al. The combination of letrozole and trastuzumab as first or second-line biological therapy produces durable responses in a subset of HER2 positive and ER positive advanced breast cancers. Breast Cancer Res Treat 2007; 102(1): 43–9
Clemens M, Kaufman B, Mackey J, et al. Trastuzumab [Herceptin®] plus anastrozole may prolong overall survival in post-menopausal women with HER2-positive, hormone-dependent metastatic breast cancer: results of a post hoc analysis from the TAnDEM study [poster]. ASCO Breast Cancer Symposium; 2007 Sept 7–8; San Francisco (CA)
Suter TM, Procter M, van Veldhuisen DJ, et al. Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial. J Clin Oncol 2007 Jul 23; 25: 3859–65
Acknowledgements and Disclosure
The manuscript was reviewed by: M. Clemens, Klinikum Mutterhaus der Borromäerinnen, Trier, Germany; B. Kaufman, Breast Cancer Unit, Sheba Medical Center, Israel.
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Orman, J.S., Perry, C.M. Trastuzumab. Drugs 67, 2781–2789 (2007). https://doi.org/10.2165/00003495-200767180-00009
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200767180-00009