Abstract
Background
Estrogen receptor (ER) and/or progesterone receptor expression occurs in ∼50% HER2 positive (HER2+) breast cancers and cross-talk between the estrogen and HER2 pathways promotes endocrine therapy resistance. The efficacy of the aromatase inhibitor letrozole in combination with trastuzumab was therefore tested in a Phase 2 study.
Methods
Patients with ER+ and/or PgR+ and HER2+ (IHC 2+ or 3+ or FISH+) advanced breast cancer were treated with trastuzumab plus letrozole until disease progression or unacceptable toxicity.
Results
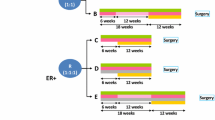
Thirty-three patients were enrolled, of which thirty one were considered evaluable. The majority of patients (82%) had received tamoxifen and 82% had HER2 FISH+ and/or IHC 3+ tumors. Eight patients responded (1 CR and 7 PR) for an overall response rate (ORR) of 26% and a clinical benefit rate (CBR) of 52%. The median time to progression (TTP) was 5.8 months and the median duration of response (DOR) was 20.6+ months. Excluding IHC 2+, FISH− tumors, the OR was 24%, CBR 44%, TTP 5.5 months and DOR 17+ months. The combination was well tolerated with only two toxicity events requiring termination of study medication.
Conclusions
Combined trastuzumab and letrozole treatment for patients with HER2+ and ER+ advanced breast cancer produced durable responses consistently lasting at least 1 year in one quarter of the patients. While these data are promising for a subgroup, for half the patients, trastuzumab plus letrozole was inactive. This finding demonstrates ER+ HER2+ advanced disease is heterogeneous and additional agents will be required for optimal management based on targeted therapeutics alone.
Similar content being viewed by others
References
Slamon DJ, Godolphin W, Jones LA et al (1989) Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244:707–712
Marty M, Cognetti F, Maraninchi D et al (2005) Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol 23:4265–4274
Slamon DJ, Leyland-Jones B, Shak S et al (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783–792
Piccart-Gebhart MJ, Procter M, Leyland-Jones B et al (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353:1659–1672
Ellis M (2004) Overcoming endocrine therapy resistance by signal transduction inhibition. Oncologist 9 Suppl 3:20–26
Lipton A, Ali SM, Leitzel K et al (2002) Elevated serum Her-2/neu level predicts decreased response to hormone therapy in metastatic breast cancer. J Clin Oncol 20:1467–1472
Lipton A, Ali SM, Leitzel K et al (2003) Serum HER-2/neu and response to the aromatase inhibitor letrozole versus tamoxifen. J Clin Oncol 21:1967–1972
De Laurentiis M, Arpino G, Massarelli E et al (2005) A meta-analysis on the interaction between HER-2 expression and response to endocrine treatment in advanced breast cancer. Clin Cancer Res 11:4741–4748
Hu JC, Mokbel K: (2001) Does c-erbB2/HER2 overexpression predict adjuvant tamoxifen failure in patients with early breast cancer? Eur J Surg Oncol 27:335–337
Viale G, Dell’Orto P, Del Curto B et al (2005) Central review of ER, PgR and HER-2 in BIG 1–98 evaluating letrozole vs. tamoxifen as adjuvant endocrine therapy for postmenopausal women with receptor-positive breast cancer. Breast Cancer Res Treat 94 (Abstract 44)
Dowsett M, Harper-Wynne C, Boeddinghaus I et al (2001) HER-2 amplification impedes the antiproliferative effects of hormone therapy in estrogen receptor-positive primary breast cancer. Cancer Res 61:8452–8458
Ellis MJ, Tao Y, Young O et al (2006) Estrogen independent proliferation is present in the majority of estrogen receptor positive (ER+) HER2 gene amplified primary breast cancers after letrozole exposure despite frequent tumor regressions during neoadjuvant treatment. J Clin Oncol (in press)
Ellis MJ, Coop A, Singh B et al (2001) Letrozole is more effective neoadjuvant endocrine therapy than tamoxifen for ErbB-1- and/or ErbB-2-positive, estrogen receptor-positive primary breast cancer: evidence from a phase III randomized trial. J Clin Oncol 19:3808–3816
Benz CC, Scott GK, Sarup JC et al (1993) Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res Treat 24:85–95
Shou J, Massarweh S, Osborne CK et al (2004) Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst 96:926–935
Witters LM, Kumar R, Chinchilli VM et al (1997) Enhanced anti-proliferative activity of the combination of tamoxifen plus HER-2-neu antibody. Breast Cancer Res Treat 42:1–5
Leyland-Jones B, Gelmon K, Ayoub JP et al (2003) Pharmacokinetics, safety, and efficacy of trastuzumab administered every three weeks in combination with paclitaxel. J Clin Oncol 21:3965–3971
Vogel CL, Cobleigh MA, Tripathy D et al (2002) Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 20:719–726
Author information
Authors and Affiliations
Corresponding author
Additional information
Note: Aspects of this work were presented at the ASCO Annual Meeting 2005.
Disclosure: Washington University conflicts of interest committee requires the following disclosures. Novartis Pharma and Genentech provided financial support for this clinical trial. Dr␣M.J. Ellis is a paid consultant for Novartis and Genentech and has also received speaking honoraria.
Rights and permissions
About this article
Cite this article
Marcom, P.K., Isaacs, C., Harris, L. et al. The combination of letrozole and trastuzumab as first or second-line biological therapy produces durable responses in a subset of HER2 positive and ER positive advanced breast cancers. Breast Cancer Res Treat 102, 43–49 (2007). https://doi.org/10.1007/s10549-006-9307-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-006-9307-8