Abstract
Meconium aspiration syndrome (MAS) is an important cause of respiratory distress in the term infant. Therapy for the disease remains problematic, and newer treatments such as high-frequency ventilation and inhaled nitric oxide are being applied with increasing frequency. There is a significant disturbance of the pulmonary surfactant system in MAS, with a wealth of experimental data indicating that inhibition of surfactant function in the alveolar space is an important element of the pathophysiology of the disease. This inhibition may be mediated by meconium, plasma proteins, haemoglobin and oedema fluid, and, at least in vitro, can be overcome by increasing surfactant phospholipid concentration. These observations have served as the rationale for administration of exogenous surfactant preparations in MAS, initially as standard bolus therapy and, more recently, in association with therapeutic lung lavage.
Bolus surfactant therapy in ventilated infants with MAS has been found to improve oxygenation in most studies, although there are a significant proportion of nonresponders and in many cases the effect is transient. Pooled data from randomised controlled trials of surfactant therapy suggest a benefit in terms of a reduction in the requirement for extracorporeal membrane oxygenation (relative risk 0.48 in surfactant-treated infants) but no diminution of air leak or ventilator days. Current evidence would support the use of bolus surfactant therapy on a case by case basis in nurseries with a relatively high mortality associated with MAS, or the lack of availability of other forms of respiratory support such as high-frequency ventilation or nitric oxide. If used, bolus surfactant should be administered as early as practicable to infants who exhibit significant parenchymal disease, at a phospholipid dose of at least 100 mg/kg, rapidly instilled into the rachea. Natural surfactant or a third-generation synthetic surfactant should be used and the dosage repeated every 6 hours until oxygenation has improved.
Lung lavage with dilute surfactant has recently emerged as an alternative to bolus therapy in MAS, which has the advantage of removing surfactant inhibitors from the alveolar space in addition to augmenting surfactant phospholipid concentration. Combined animal and human data suggest that lung lavage can remove significant amounts of meconium and alveolar debris, and thereby improve oxygenation and pulmonary mechanics. Arterial oxygen saturation inevitably falls during lavage but has been noted to recover relatively rapidly, even in infants with severe disease. Several randomised controlled trials of surfactant lavage in MAS are underway, and until the results are known, lavage must be considered an unproven and experimental therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Meconium aspiration syndrome (MAS) is an important cause of respiratory distress in the mature infant, which in the developed world accounts for approximately 10% of all cases of respiratory failure requiring intubation at or near full term. MAS is a disease of many elements, which coalesce into a problematic respiratory syndrome that is frequently difficult to treat by conventional means. For this reason, numerous adjunctive or alternative therapies have been applied in MAS, including, in the surfactant era, exogenous surfactant either by bolus administration or in the form of lung lavage. The role of exogenous surfactant therapy in MAS is the subject of this review. We seek to establish the main pathophysiological disturbances in MAS, in particular those involving the surfactant system, the scientific basis for the use of exogenous surfactant, the outcomes of clinical application of bolus surfactant therapy in MAS, and the evidence in support of surfactant lavage therapy in MAS.
1. Meconium Aspiration Syndrome (MAS): the Disease
The epidemiology, clinical features, conventional therapy and outcome of MAS have been extensively reviewed,[1–5] and are only briefly discussed here. MAS is a disease of the term and post-term infant, and is rare before 37 weeks gestation. The incidence of MAS in the developed world currently stands around 2 per 1000 live births,[2,6] and may be considerably higher in under-served inner urban populations[7,8] and in the developing world.[9] Clinically, MAS presents a relatively stereotypical picture, with early onset of respiratory distress in the context of meconium-stained amniotic fluid and, usually, fetal compromise of some degree. Radiographically the features of aspiration are regional atelectasis and/or hyperinflation, with widespread patchy opacification, although these appearances are neither specific to meconium aspiration[10,11] nor present in all cases.[12]
Approximately one-third of infants with MAS require intubation and mechanical ventilation.[4] Those that do usually exhibit hypoxaemic respiratory failure, frequently accompanied by respiratory acidosis, persistent pulmonary hypertension or both. Management of such infants remains a significant challenge for the neonatologist, and is one in which newer technologies and therapies for newborn respiratory failure are frequently brought into play, including high-frequency ventilation, inhaled nitric oxide and bolus surfactant therapy. Mortality for this condition is estimated to be at least 4%,[4] and approximately 10% of ventilated infants have an air leak.[6] Infants with significant MAS frequently require oxygen for relatively long time periods, and a small proportion are discharged home still receiving oxygen and have ongoing respiratory morbidity in infancy.[13]
2. Pathogenesis and Pathophysiology of MAS
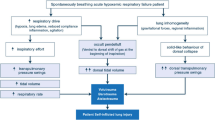
The lung dysfunction of MAS is a variable interplay of several elements, chief amongst which are airflow obstruction, decreased alveolar gas exchange, impaired pulmonary mechanics, surfactant inhibition and persistent pulmonary hypertension. Figure 1 shows a proposed pathogenesis of MAS, indicating the antecedents to these physiological disturbances, the critical one being entry of meconium into the lung.
A proposed pathogenesis of meconium aspiration syndrome. Shaded boxes with bolded text denote obligatory elements, shaded boxes with normal text denote usual elements, and no shading denotes occasional elements.[5]
Meconium is the viscid, pigmented secretion of the fetal gastrointestinal tract, to which is added elements derived from swallowed amniotic fluid, including fetal squames and lanugo hair. It is a noxious substance when inhaled, producing one of the worst forms of aspiration pneumonitis encountered in humans. In vitro, meconium exhibits several adverse biophysical properties, including high tenacity (stickiness),[14] extremely high surface tension (215 mN/m)[14] and potent inhibition of surfactant function (see section 3.3). It is directly toxic to type II pneumocytes,[15,16] chemotactic to neutrophils[17] and possibly vasoactive.[18,19] These adverse properties of meconium are reflected in many of the known pathophysiological features of MAS (figure 1), supporting the notion that the presence of meconium in the lung is crucial in the pathogenesis of the disease.
The universal disturbances of lung function in MAS are hypoxaemia and decreased lung compliance. Poor oxygenation is attributable to a combination of ventilation-perfusion mismatching, intrapulmonary shunting related to regional atelectasis, and extrapulmonary shunting related to pulmonary hypertension. Many infants exhibit regional hyperinflation related to increased expiratory resistance in meconium-plugged airways, and a smaller subgroup have global hyperinflation and a high functional residual capacity secondary to extensive gas trapping.[20]
3. Disturbances of the Surfactant System in MAS
Pulmonary surfactant is a mixture of phospholipid and protein vital for the normal function of the lungs.[21–24] The major phospholipid component, dipalmitoyl phosphatidylcholine (DPPC) forms a monolayer on the alveolar surface that lowers surface tension, thereby maintaining alveolar volume in expiration and conferring favourable pulmonary mechanics during inspiration from low lung volume.[21] Several other components of surfactant assist in the formation of the DPPC monolayer, including other phospholipids (e.g. phosphatidylglycerol) and surfactant-associated proteins A, B and C (SP-A, SP-B, SP-C). All elements of surfactant are synthesised, stored, secreted and recycled by the alveolar type II cell.[24]
The physiological importance of surfactant is highlighted by the plight of the premature newborn infant with hyaline membrane disease (HMD), where primary surfactant deficiency is associated with low end-expiratory lung volume and decreased pulmonary compliance.[22] All elements of surfactant are known to be deficient in this condition, with a much reduced surfactant pool size[25] and measurable deficiency of DPPC,[26] SP-A,[27,28] SP-B[27,28] and SP-C[28] in the airspaces. The introduction of exogenous surfactant therapy for HMD, in which animal-derived or synthetic surfactant mixtures are instilled into the trachea, has led to a considerable reduction in mortality and risk of pneumothorax.[29,30] The rapid improvement in oxygenation and pulmonary mechanics frequently noted soon after surfactant administration in these infants reinforces how critically important an intact surfactant system is to the normal function of the lung. A summary of the commercially available surfactant preparations is presented in table I.
There is good evidence that the function of pulmonary surfactant is also perturbed during MAS. Such evidence comes from in vitro studies of surfactant-meconium mixtures, and from in vivo studies of various aspects of surfactant in animal models of MAS and in human infants with the disease.
3.1 Airspace Concentration of Surfactant Phospholipid in MAS
In direct contrast to HMD, the available data suggest that MAS may not involve significant deficiency of surfactant phospholipid. Indeed, at least in vitro, exposure of cultured alveolar type II cells to meconium leads to a dose-dependent increase in phosphatidylcholine secretion.[15] At concentrations >1% meconium becomes directly toxic to type II cells,[15] at least in part related to the increased cellular permeability and intracellular calcium accumulation induced by bile salts.[16] Data from animal models of MAS are conflicting regarding alveolar concentration of surfactant phospholipid, with several studies showing total phospholipid and/or DPPC content similar to controls for up to 72 hours after initiation of meconium injury,[41,42] and yet others finding a lower concentration of total phospholipid[43] or DPPC[44] in the meconium-injured lung. Variations in the amount of meconium instilled, the duration of the experiment, and the method of obtaining and analysing lung fluid may explain the differences in the findings of these studies.
Information regarding surfactant phospholipid status in human infants with MAS comes entirely from analysis of lung fluid retrieved using one or other method of diagnostic bronchoalveolar lavage, where small aliquots of saline are instilled into the lung and then recovered by suction.[45] Analysis of tracheal aspirate fluid from infants with severe MAS requiring extracorporeal membrane oxygenation (ECMO) found an apparent increase in phospholipid and/or DPPC concentration during the course of the ECMO support, suggesting that there may have been a deficiency of surfactant phospholipid at the outset.[46,47] Interpretation of these results is limited in both cases by the lack of pre-ECMO samples or data from controls with normal lungs. In a group of ventilated infants with MAS not requiring ECMO, our research group noted no difference in total phospholipid or DPPC concentration in bronchoalveolar lavage fluid compared with samples from the normal lung.[48]
3.2 Airspace Concentration of Surfactant Protein in MAS
The available evidence suggests that the concentration of surfactant proteins, in particular SP-A and SP-B, may be reduced in MAS. In the rat model of MAS, Cleary et al.[41] demonstrated a time-dependent decrease in SP-A and SP-B in large surfactant aggregates from whole lung lavage (maximum effect at 24–48 hours), although the lung tissue concentration of SP-B was normal throughout. In human infants with MAS requiring ECMO, sequential tracheal aspirate samples have shown a progressive increase in SP-A concentration during resolution of lung disease, suggesting recovery of surfactant synthesis.[46,49] In contrast, we found no difference in the airspace concentration of SP-A in ventilated infants with MAS compared with that in healthy infants with no lung disease.[48]
3.3 Surfactant Inhibition in MAS
Apart from the actual concentration of surfactant components within the alveolus, there is evidence that the biophysical function of surfactant is significantly affected in MAS. In vitro, meconium causes concentration-dependent inhibition of surfactant function,[31,50–52] which can be overcome by increasing surfactant concentration in the mixture under study.[31,50,52] Moses and co-workers[50] found that meconium, even at a 1 : 6500 dilution (20 μg/mL), caused significant inhibition of a surfactant preparation with phospholipid concentration 1.5 mg/mL, with an increase in the minimum surface tension achievable by the mixture. In contrast, Bae et al.[52] found that a meconium concentration of at least 1 mg/mL was necessary to cause significant inhibition of a 1.5 mg/mL surfactant mixture. At phospholipid concentrations >5 mg/mL, surfactant appears to be resistant to meconium-related inhibition.[31,50,52] Clark and co-workers[53] found that inhibitory activity of meconium resided in the ether-extractable fraction of meconium, and hypothesised that it was related to the high proportion of oleic and stearic acids known to be present.[54] More recently, several studies have identified inhibitory activity in both the water- and chloroform-soluble fractions of meconium,[50,51] with the chloroform fraction, containing free fatty acids, triglycerides and cholesterol, having greater inhibitory potency.[51] The inhibitory effect of meconium is associated with a marked alteration in the morphology of surfactant phospholipid ultrastructure, with the loosely stacked layers of natural surfactant changed to a spherical lamellar structure.[52]
Surfactant inhibition in MAS may also come about through mechanisms other than a direct effect of meconium. Both in experimental animals and in human infants, MAS is associated with a marked exudation of plasma protein,[42–44,46,55] alveolar oedema[55,56] and accumulation of debris including hyaline membranes.[55,56] Additionally, MAS is associated with haemorrhagic alveolitis[45] and occasionally results in overt pulmonary haemorrhage.[57,58] Accumulation of any or all of these elements within the alveolar space may result in surfactant inhibition. Surfactant function is demonstrably compromised by serum proteins,[59–61] in particular fibrinogen,[33,59] and, to a lesser extent, albumin.[59,62,63] As with meconium, plasma protein-induced inhibition is dependent on the concentration of both inhibitor and surfactant, and can be largely overcome by increasing the phospholipid concentration to >5 mg/mL.[59] Apart from plasma proteins, both haemoglobin and cell membrane phospholipids also impair the biophysical activity of surfactant,[64] as do the breakdown products of hyaline membranes.[65]
While inhibition of surfactant preparations by meconium, plasma proteins and other alveolar debris is clearly demonstrable in vitro, the degree to which endogenous surfactant function is impaired in vivo is more difficult to ascertain. The concentrations of either inhibitor or surfactant in the alveolar space are not known with any great precision. Even if they were, the degree of surfactant dysfunction may be difficult to predict because much of the in vitro data examining the stoichiometry of inhibition come from studies using surfactant preparations that are much more susceptible to inhibition than endogenous surfactant.[31] However, there are several in vivo functional studies that do suggest a significant component of surfactant inhibition in MAS. Instillation of meconium solutions into isolated canine lungs results in significant and immediate changes to static pressure volume deflation curves, suggestive of a change in surfactant function.[66] Using a different approach, Lu and co-workers demonstrated improvement in lung function in the rat MAS model by addition of polyethylene glycol,[67] an agent with known anti-inhibitory properties.[68,69] These studies give credence to the notion that surfactant inhibition makes an important contribution to the lung dysfunction that occurs in MAS.
Evidence for a functional disturbance of the surfactant system in MAS also comes from animal and human data regarding physiological responses to exogenous surfactant administration. In animal models, bolus surfactant administration has been seen to improve oxygenation in most studies (see section 4.1) but the effect is often transient,[70] with gradual return to the baseline state within 3–4 hours, and persistence of pulmonary atelectasis and inflammatory changes both macroscopically and histologically.[55,70] These observations suggest that the main effect of exogenous surfactant therapy is a temporary alleviation of surfactant inhibition.
3.4 Inhibition of Exogenous Surfactant Preparations
Commercially available surfactant preparations show variable resistance to inhibition by meconium and plasma protein. Even in the absence of inhibitors, synthetic surfactants lacking SP-B or SP-C (pumactant [ALEC® {artificial lung expanding compound}]Footnote 1, colfosceril palmitate [Exosurf®]) have inferior biophysical properties in vitro,[31,71] and the few data examining their susceptibility to inhibition are conflicting and relatively meaningless given the difficulty of testing for inactivation when the surfactant is already comparatively inactive.[31] Several investigators have examined the relative resistance of natural and third-generation synthetic surfactants to inhibition by protein and meconium. Natural surfactant preparations derived from whole lung lavage (bovactant [Alveofact®], calfactant [Infasurf®]) have been found more resistant than lung mince surfactants (beractant [Survanta®], poractant alfa [Curosurf®]) to the inhibitory effects of fibrinogen and albumin,[33] almost certainly related to their higher concentration of SP-B.[72] Lung lavage surfactant (bovactant) was also noted to be more resistant to meconium-related inactivation than beractant in one study[73] but not another,[31] with the disparity being at least in part explicable by different phospholipid : meconium ratios used in the two studies (2 : 1 vs 1 : 1). Both studies found that at a phospholipid concentration of 5 mg/mL, bovactant achieved a lower minimum surface tension than beractant in the presence of meconium 2.5 mg/mL. The third-generation synthetic surfactants, sinapultide (lucinactant) [Surfaxin®] and lusupultide (rSP-C-surfactant) [Venticute®], have some protection against inactivation related to the presence of SP-B-like activity and recombinant SP-C, respectively. Sinapultide appears to be more resistant to the effects of fibrinogen than beractant in vitro[74] but more susceptible to albumin-induced inhibition in vivo.[75] Lusupultide also resists fibrinogen-related inhibition, but not to the same degree as calfactant.[76] Where tested, both synthetic preparations show considerable resistance to meconium, with better biophysical function than beractant, poractant alfa or bovactant at a phospholipid : meconium ratio of 1 : 1. This difference became less evident with a doubling of the phospholipid concentration to 5 mg/mL.
4. Bolus Surfactant Therapy in MAS
4.1 Animal Studies
The physiological effect of exogenous surfactant therapy using a variety of different surfactant preparations has been investigated in animal models of MAS.[43,55,67,70,77–86] Better oxygenation has been noted after bolus surfactant administration in most studies,[55,67,70,77–86] with the maximal effect in some cases requiring the application of another therapy in addition to surfactant, such as high-frequency ventilation,[78] nitric oxide[80,82] or polyethylene glycol.[67] In several cases the oxygenation effect was transient[55,70] or absent.[43] A more pronounced physiological response was noted with a 200 mg/kg surfactant dose (compared with 100 mg/kg) in the rat MAS model[55] but not in the rabbit model.[81] The improvements in gas exchange have been associated with concomitant improvements in pulmonary mechanics[55,70,77–80,85,86] and, where measured, epithelial injury and proteinaceous exudation.[55,78,80]
Several reports of bolus surfactant therapy in animal models of MAS have emphasised the heterogeneity of distribution of surfactant in this condition.[87,88] Hudson and co-workers[87] noted a more uneven distribution of surfactant phospholipid after intratracheal instillation of a standard dose of surfactant in the meconium-injured piglet lung than in the normal lung. Another study in the rat MAS model found that the maldistribution of surfactant could be improved using partial liquid ventilation.[88] Most investigators have noted that more rapid administration of surfactant to animals with MAS produces more even phospholipid distribution,[89,90] particularly when compared with a slow infusion over 45 minutes.[89] At odds with these findings is a single report in the rabbit MAS model where better pulmonary mechanics were noted after an infusion of beractant over 60 minutes than with standard bolus administration.[83]
4.2 Bolus Surfactant Administration in Humans
Initial uncontrolled studies suggested that bolus surfactant therapy may be of benefit in MAS. Auten and co-workers[91] were the first to report on a small series of seven infants with MAS treated with calfactant 90 mg/kg, all of whom demonstrated improvements in oxygenation after administration. In another cohort of 20 infants with MAS receiving 100 mg/kg of bovine lung extract surfactant, improvement in arterial/alveolar oxygen ratio within 6 hours was noted in 75% of cases, and none of the treated infants required ECMO.[92] Other groups have documented more modest benefits, with a survey of European neonatologists reporting no response to treatment in 44% of 54 infants treated with a median dose of poractant alfa 100 mg/kg,[93] and a similar proportion of nonresponders noted by Blanke and Jorch.[94] in a further 10 infants. The uncontrolled use of bolus surfactant therapy in combination with other rescue treatments in MAS has also been documented, including infants treated while receiving ECMO,[95] and infants receiving high-frequency jet ventilation,[96] with the suggestion of physiological improvement in both cases.
Two randomised trials of bolus surfactant therapy have been conducted in patients with MAS (see table II),[97,98] and the pooled trial data have been subjected to meta-analysis.[99] Findlay and co-workers[97] conducted a single-centre randomised controlled trial of surfactant therapy in 40 ventilated infants with MAS. Entry criteria included a mean airway pressure (PAW) >7cm H2O, and an alveolar-arterial oxygen ratio of <0.22, with enrolment occurring in all cases by 6 hours of age. A large dose of beractant 150 mg/kg was administered as an infusion into the lung over 20 minutes via a sideport on the endotracheal tube. This mode of administration was chosen to avoid the deleterious effects associated with rapid instillation of the relatively large fluid volume required for each dose (6 mL/kg). All infants received three surfactant doses 6 hours apart. Compared with controls, treated infants showed a marked and sustained improvement in oxygenation after administration of the second surfactant dose (figure 2), in association with echocardiographic evidence of resolution of pulmonary hypertension. Surfactant-treated infants had a reduced risk of air leak (0% vs 25%) and a reduction in the need for ECMO support (5% vs 30%). There were no deaths in either arm of the trial. Duration of ventilation was 29% less in the surfactant-treated infants than in controls. There were no reported deleterious effects during or immediately after the infusion of surfactant.
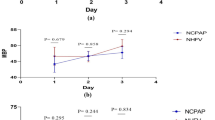
Change in oxygenation after exogenous surfactant therapy in ventilated infants with meconium aspiration syndrome. Mean and standard error for oxygenation index (OI) over time in infants treated with repeat doses of beractant 150 mg/kg delivered as an infusion over 20 minutes, and controls receiving standard care (reproduced from Findlay et al.,[97] with permission). * Denotes a significant difference in OI between the two groups. A clear improvement is noted in oxygenation after the second surfactant dose. OI = (PAW × FiO2 × 100)/PaO2 (where FiO2 = fraction of inspired oxygen and PaO2 = arterial partial pressure of oxygen).
In a methodologically distinct trial, Lotze et al.[98] studied ventilated term infants up to 120 hours of age with respiratory failure needing 100% oxygen and having an oxygenation index (OI) of between 15 and 40 (OI = [PAW × FiO2 × 100]/PaO2) [where FiO2 = fraction of inspired oxygen and PaO2 = arterial partial pressure of oxygen]. Of the 328 infants enrolled, 168 had a primary diagnosis of MAS. Infants randomised to surfactant therapy received four bolus doses of beractant 100 mg/kg, administered in the conventional manner as mini-boluses instilled down the endotracheal tube. Compared with controls, surfactant-treated infants showed a decrease in the requirement for ECMO (37% vs 52%), but no reduction in pulmonary complications, and no difference in ventilator days, duration of oxygen therapy or length of hospital stay. Endotracheal tube obstruction and/or hypoxia were noted in 33% of surfactant-treated infants compared with 8% in the sham-treated control group. In no case was there precipitous deterioration causing death or immediate transition to ECMO after administration of surfactant.
Pooled together, the data from these trials suggest that repeated dose administration with exogenous surfactant in ventilated infants with MAS may be associated with a reduction in the need for ECMO, with a relative risk of 0.48 (95% CI 0.27, 0.84).[99] However, in other respects the trials showed rather disparate outcomes, with infants in the trial of Lotze et al.[98] showing no benefit in terms of air leak or duration of ventilation. These differences may best be explained by the timing of surfactant administration — surfactant was given in all cases within 6 hours of delivery in the trial by Findlay et al.,[97] whereas in that of Lotze et al.[98] the mean age of first surfactant therapy was 31 hours. Clearly, infants in the latter study would have had greater opportunity for development of lung injury and proteinaceous oedema, thus rendering them less likely to respond favourably to exogenous surfactant therapy.
While the pooled controlled trial data are somewhat equivocal, bolus surfactant therapy has found a place in the treatment of MAS in many newborn intensive care units. In Australia and New Zealand, up to 45% of ventilated newborns with the disease receive at least one dose of surfactant.[6] In a cohort of 34 outborn infants with severe MAS managed at the Royal Children’s Hospital, Melbourne, Australia, 14 (41%) were treated with surfactant, although the timing and dosage varied considerably.[100] The treatment was generally used as ‘late rescue’ therapy, and in some cases the dose was restricted to one vial of surfactant, that is, a lower dose per kg bodyweight than is used in HMD. Repeat dose administration was unusual.
5. Practical Considerations Regarding Bolus Surfactant Therapy in MAS
The following sections are a synthesis of the evidence in relation to important questions of practice in the use of bolus surfactant therapy in MAS.
5.1 Should Surfactant Therapy Be Used in MAS?
Mortality risk in MAS is low, and the justification for the use of bolus surfactant therapy lies in the potential to stabilise the condition of the infant and thereby avoid ECMO, reduce the risk of air leak and decrease the duration of all forms of respiratory support. As has been seen, the available data support the proposition that bolus surfactant therapy can help reduce the requirement for ECMO but are equivocal regarding air leak and duration of ventilation. ECMO is now required very infrequently in infants with MAS,[101] and at this time accounts for a much smaller proportion of neonatal ECMO candidates.[102,103] It is unlikely that this is a direct result of the use of bolus surfactant therapy; more likely, it is related to general improvements in care, and the application of a number of newer therapies, including high-frequency ventilation and inhaled nitric oxide, in addition to surfactant.[103] For an individual nursery, the decision to use surfactant in MAS should be taken with the full knowledge of local outcomes for this condition, in particular the frequency of ECMO therapy, if available.
5.2 Which Infants with MAS Should Receive Surfactant Therapy?
Given the relatively high cost of bolus surfactant therapy in term infants with MAS, we consider that it should be used selectively, targeting those who stand to gain the most benefit. Some ventilated infants with MAS pose no real management problems and are easily stabilised by conventional means. Bolus surfactant administration in these infants is difficult to justify, as the benefit of surfactant therapy in terms of reductions in risk of air leak or duration of ventilation may be minimal in this group. It would seem appropriate to reserve surfactant therapy for infants in whom there is evidence of substantial aspiration of meconium, with marked impairment of gas exchange. Such infants would be expected to have an OI >15, and an alveolar-arterial oxygen difference (AaDO2) >450mm Hg (AaDO2 = PiO2 − PaCO2/0.8 − PaO2) [where PiO2 = partial pressure of inspired oxygen and PaCO2 = arterial partial pressure of carbon dioxide]. These infants usually have coexistent pulmonary hypertension, and often receive newer therapies such as high-frequency ventilation and/or inhaled nitric oxide. Our experience is that this group represents around one-half of all infants who are intubated and ventilated with MAS.
5.3 When Should Surfactant Be Administered?
MAS is a progressive lung disease evolving over time after the initial inhalation. Given this, if surfactant is to be used in MAS it should be given as early as possible after intubation and stabilisation of the infant. The available clinical trial data support this view, with the trial in which surfactant was given early (always before 6 hours) being the one in which the therapy resulted in the most benefit.[97] Conversely, it is doubtful whether there is much to be gained by administration of surfactant beyond the first 24 hours of life. As with HMD, measures to stabilise the infant take precedence over surfactant administration, and in MAS the time period for stabilisation may be relatively long, particularly where there is severe parenchymal disease or pulmonary hypertension.
5.4 Which Surfactant Should Be Used?
There is good evidence that the currently available surfactant preparations have variable resistance to inhibition by meconium and plasma protein (see sections 3.3 and 3.4). Artificial preparations containing no proteolipids are highly susceptible to inactivation and should not be used in MAS. There are differences in resistance to inhibition induced by meconium and plasma protein among the other surfactant preparations, which are apparent in vitro but may be of lesser importance in vivo. To date, there have been few comparative studies of surfactant preparations in animal models of MAS and none in human infants. More data are needed before any further recommendations can be made.
5.5 What Dose of Surfactant Should Be Used and How Should it Be Administered?
In the preliminary reports of surfactant therapy in MAS[91–96] and the clinical trial of Lotze et al.,[98] surfactant phospholipid dose was generally equivalent to that used in HMD (i.e. around 80–100 mg/kg). The trial showing the greatest benefit from surfactant therapy used a very large surfactant phospholipid dose (150 mg/kg, i.e. 50% greater than the dose used in HMD).[97] We contend that the improvements in gas exchange in surfactant-treated infants in this trial were much more related to the repeated dose administration than the amount of phospholipid in any one dose. Furthermore, the relatively large volume of surfactant (6 mL/kg) was administered as an infusion over 20 minutes; administration of surfactant by infusion may be associated with relatively poor distribution of surfactant,[89] already known to be suboptimal in MAS.[87] Administration of a standard dose of surfactant (i.e. 100 mg/kg) as small boluses in the usual manner may be a more effective way of distributing the drug in this condition.
5.6 Should Repeated Surfactant Doses Be Used and, If So, How Frequently?
In both randomised controlled trials, repeated doses of surfactant were administered every 6 hours as long as oxygenation criteria were continually met. In the trial conducted by Findlay et al.,[97] improvement in oxygenation was seen only after the second surfactant dose, which was necessary in all cases. All infants in the trial of Lotze et al.[98] received four doses. Both these investigations, and the transient oxygenation improvements seen in other cases, support the use of repeated doses of surfactant 6 hours apart until oxygenation has improved and FiO2 can be reduced.
These findings can be synthesised into the following recommendations about bolus surfactant therapy in MAS.
-
1.
A decision to use surfactant should be made based around knowledge of local outcomes and availability of newer therapies, including high-frequency ventilation and inhaled nitric oxide.
-
2.
If it is to be used, surfactant therapy should be administered selectively to infants with severe disease, with OI >15 and/or AaDO2 >450mm Hg.
-
3.
Dose administration should begin as soon as practicable after intubation and stabilisation.
-
4.
A surfactant known to have some anti-inhibitory properties should be used, either a natural surfactant or a third-generation artificial surfactant.
-
5.
The surfactant dose should be equivalent to that used in HMD, delivered as small boluses directly instilled into the trachea.
-
6.
Repeated doses should be used while oxygenation remains compromised.
6. Therapeutic Lung Lavage in MAS
Therapeutic lung lavage may be defined as any procedure in which fluid is instilled into the lung, followed by an attempt to remove it by suctioning and/or postural drainage. It has been applied in a number of lung diseases in humans, including pulmonary alveolar proteinosis,[104] acute respiratory distress syndrome,[105] cystic fibrosis[106] and lipoid pneumonia,[107] as well as MAS. Lung lavage using dilute surfactant is now emerging as a promising new treatment for MAS, which may well overcome many of the shortcomings of conventionally administered surfactant.[1] By virtue of removing meconium from the airspaces, lung lavage has the potential to alter the natural history of MAS in a way other therapies, including bolus surfactant administration, do not.
Lavage therapy of one form or another has been used in MAS for >30 years, with the earliest reports documenting the use of saline lavage in the delivery room to improve clearance of meconium from the airways of meconium-stained babies.[108] This technique was largely abandoned as a result of the increased number of infants with transient tachypnoea after saline lavage, ascribed to lavage fluid retention in the lung.[109] More recently there has been renewed interest in the possibility of cleansing the lung with fluids with more favourable biophysical properties than saline, including surfactant and perfluorocarbon. Evidence for the potential efficacy of lavage therapy in MAS comes from animal experimentation, case reports and case series in humans, and from two small randomised controlled trials.
6.1 Lung Lavage in Animal Models of MAS
The potential therapeutic benefits of various forms of lung lavage have been investigated in animal models of MAS.[44,70,84,110–123] Several different lavage fluids have been used, including saline,[70,84,112,115,117–119,121,123] perfluorocarbon,[44,111,113,115,120,122,123] and full-strength[119] or diluted[44,70,84,110,114,116–118,120,121,123] exogenous surfactant. Total lavage volume (the amount of fluid instilled into the lung during the entire lavage procedure) has varied from 5 to 80 mL/kg, with aliquot volume (the amount of fluid instilled into the lung at one time) ranging from as little as 2mL up to 15 mL/kg. The therapeutic lavage has been undertaken between 10 minutes and 3 hours after introduction of meconium into the lung to create the MAS model. In all cases, recovery of lavage fluid during suctioning was incomplete, being anywhere from 31% to 75% of the amount of the total lavage fluid volume instilled into the lung.
Several important conclusions can be drawn from the studies of lavage in animals with MAS. Lung lavage using surfactant has been found to produce better gas exchange and/or pulmonary mechanics than either no lavage[44,70,114,116–120,123] or bolus surfactant therapy.[70] Both surfactant[70,84,114,117–119,123] and perfluorocarbon[115] have been found to be better lavage fluids than saline. Despite considerable enthusiasm for perfluorocarbon as a lavage fluid in MAS, neither pure[120,123] nor emulsified[44] perfluorocarbon have shown any advantage over dilute surfactant. This may be due to the relatively high density of perfluorocarbon and/or the relative immiscibility of meconium with perfluorochemicals.
Where measured, lung lavage has been found to recover a significant proportion of the meconium introduced into the lung in the creation of the model of MAS.[44,70,114,117,118] Ohama and co-workers[117] recovered 57% of the introduced meconium solids using a 10 mL/kg total lavage volume, Cochrane et al.[70] recovered around 40% of meconium pigment with a 48 mL/kg lavage and Dargaville et al.[44] recovered 35% and 37% of solids and pigment, respectively, using a 30 mL/kg dilute surfactant lavage. Proteinaceous exudate was also recovered from the lung in the return fluid and alveolar protein content was lower after dilute surfactant lavage.[44] Compared with saline-lavaged controls, indices of inflammation (e.g. interleukin-8) were reduced in the surfactant-lavaged animals, and the function of surfactant lavaged from the lungs at postmortem was improved.[70]
Animal experimentation has also allowed investigation of the fluid volumes that should be used for lavage in MAS. Our group found that a total lavage volume of 30 mL/kg achieved an acceptable balance between recovery of meconium from the airspaces and retention of lavage fluid in the lung.[44] Total lavage volumes >30 mL/kg were not associated with significantly greater meconium recovery but did result in unacceptably high deposition of aqueous fluid in the lung (figure 3). We then examined whether this volume of 30 mL/kg should be administered in large or small aliquots, and found that aliquots of 15 mL/kg recovered considerably more meconium than aliquots of 3mL (43% vs 18%) and deposited less aqueous fluid (8.3 vs 12 mL/kg). Gas exchange and pulmonary mechanics were considerably better in the large aliquot group as a result. Our conclusion was that, during lavage, effective cleansing of the lung is best achieved by instillation of a relatively large amount of fluid into the lung at one time, and we have therefore targeted an aliquot volume of 15 mL/kg for our subsequent studies in human infants.
Meconium recovery and fluid deposition with increasing total lavage volume. (a) Mean and standard error for recovery of centrifugeable meconium solids and meconium pigment, graphed in relation to total lavage volume. Meconium recovery is expressed as a proportion of the amount of meconium instilled into the lung in the creation of the meconium aspiration syndrome model. Proportional meconium recovery rises with each 15 mL/kg increment in total lavage volume (p < 0.05, paired t-test), although beyond 30 mL/kg relatively little extra meconium is recovered. (b) Mean and standard error for fluid deposition (mL of residual lavage fluid per kg bodyweight) expressed relative to total lavage volume. Values for fluid deposition differ significantly with each 15 mL/kg increase in total lavage volume (p < 0.05, paired t-test), with no apparent tapering beyond 30 mL/kg. Error bars represent SD (reproduced from Dargaville et al.,[44] with permission; © 2003 American Thoracic Society).
6.2 Lung Lavage in Human Infants with MAS
In human infants, the available data on lavage therapy in MAS include case reports,[124–126] larger uncontrolled studies[108,127,128] and controlled studies in which lavage is compared with no lavage in historical controls,[109,129–132] concurrent controls[100,133,134] or, in two studies, infants allocated to the control arm of a randomised trial[135,136] (table III). These studies have used both saline[109,124,126–128,134,135] and dilute surfactant[100,125,129–133,135–137] as lavage fluids, with the combined evidence pointing to deleterious effects with saline lavage unless followed by bolus surfactant administration. The total lavage volume used in these investigations has ranged from 3 to 48 mL/kg, in aliquots of between 1mL and 15 mL/kg. Where used, the surfactant phospholipid concentration in the lavage fluid has ranged from 2.5 to 12 mg/mL, with most investigators using concentrations of around 5 mg/mL.[100,130–133,135,137] The lavage procedure has been performed using a variety of different instillation and suctioning techniques, with some investigators interrupting ventilation for one or both of these components of the lavage.
Considerable amounts of meconium appear to be removed by lavage in human infants, although only one study has examined this in a quantitative sense, finding on average 12.3% meconium solids (weight/volume) in the lavage return fluid.[130] Others have noted the return fluid to be meconium stained,[100,127,131,136] in some cases with identifiable meconium ‘clots’.[131] The lavage return fluid has also shown variable degrees of blood-staining in some studies,[100,130,131,133,136] reflecting the haemorrhagic alveolitis that is known to occur in MAS.[45] There has been no suggestion of new pulmonary haemorrhage as a result of lavage therapy. Volume of return fluid has been anywhere between 20% and 100% of the instilled volume, meaning that up to 24 mL/kg of aqueous fluid has been deposited into the lung during the lavage. This fluid would appear to clear rapidly from the alveolar space, both through the application of high ventilatory pressures and possibly through upregulation of epithelial sodium transport channels,[138] already known to be highly active in the neonatal lung.[139]
As would be expected given the nature of the procedure, lung lavage in ventilated human infants has been associated with transient hypoxaemia, with arterial oxygen saturation falling to fetal levels (60–70%) in several reports.[100,130,133,136] For the most part this hypoxaemia has not been accompanied by bradycardia or other cardiovascular disturbance, except where sedation was not adequate.[136] Time for recovery in oxygenation has generally been short, leading most investigators to conclude that it is possible to perform lung lavage safely in MAS, although other commentators have expressed concern that pulmonary hypertension will be irretrievably exacerbated.[140,141] Clearly, this question of safety must remain a focus of future studies of lung lavage in MAS.
Outcomes for lavaged infants in the studies reported to date have generally been favourable, with most investigators reporting better oxygenation after lavage than the pre-lavage baseline,[100,124–128,130,131,133,136,137] and also compared with oxygenation trends noted in historical or concurrent controls.[100,130,131,136] In some instances lung lavage appears to have averted the need for ECMO.[100,128]
The improvement in gas exchange after lung lavage in MAS appears to translate into somewhat earlier extubation, with pooled data from seven controlled studies reporting ventilatory outcome (77 lavaged infants, 79 controls) showing a weighted mean reduction of 2.4 ventilator days (95% CI 1.3, 3.4 days) in infants receiving lavage compared with controls.[100,130–133,136,137] Similarly, the overall rate of pneumothorax in six controlled studies reporting this ventilatory outcome (70 lavaged infants, 70 controls) was 8.5% in lavaged infants versus 30% in controls, with an odds ratio of 0.24 (95% CI 0.09, 0.61).[100,130,131,133,136,137]
Only two prospective, randomised controlled trials of lavage therapy in MAS have been reported to date. Ogawa[135] initiated a randomised controlled trial comparing surfactant lavage, saline lavage and no lavage in ventilated infants <12 hours of age with an FiO2 of at least 0.4. Total lavage volume was 10 mL/kg in five aliquots, using surfacten-TA 6 mg/mL or saline as the lavage fluid. This trial recruited 15 infants from 11 participating centres over 2 years, and the only existing report of the trial supplies data on 10 of the 15 enrollees (6 surfactant lavage, 4 saline lavage and 0 controls). Significant differences in ventilatory index and oxygenation were noted between the surfactant and saline lavage groups at 3 hours, reinforcing the limitations of saline as a lavage fluid. No comparisons with the non-lavaged control group were made in the report, and with the death of Professor Ogawa further data are unlikely to be forthcoming from this trial (Dr Tsutomo Kondo, personal communication, 2005).
Wiswell and co-workers[136] performed a phase I/II randomised controlled trial of surfactant lavage in conventionally ventilated infants with MAS who were at least 35 weeks gestation, <72 hours of age and had an OI between 8 and 25 inclusive. Surfactant lavage was performed using six aliquots of 8 mL/kg of sinapultide. The concentration of surfactant phospholipid was 2.5 mg/mL for the first four aliquots and 10 mg/mL for the last two. There were no clear differences between lavaged individuals and controls in terms of requirement for ECMO or other rescue therapies, air leak or duration of ventilation. There was a nonsignificant trend towards improved oxygenation in the lavaged infants (figure 4).
Improvement in oxygenation after surfactant lavage therapy in ventilated infants with meconium aspiration syndrome. Mean and standard error for oxygenation index over time in infants undergoing 6 × 8 mL/kg sinapultide (lucinactant) lavage, and controls receiving standard care (see table III for further details of the lavage treatment). A trend is noted towards improvement in oxygenation after surfactant lavage, which is not statistically significant (reproduced from Wiswell et al.,[136] with permission).
The results of the preliminary studies cited in this section have encouraged further randomised controlled trials of surfactant lavage therapy, two of which are currently underway. Wiswell and co-workers are engaged in a phase III trial of lavage using sinapultide,[142] using the same surfactant lavage protocol as in their phase I/II trial.[136] Our research group has commenced a multicentre international collaborative trial (the lessMAS [Lavage with Exogenous Surfactant Suspension in Meconium Aspiration Syndrome] trial),[143] which aims to recruit a total of 66 infants. The lavage details for this trial are a total lavage volume of 30 mL/kg in two aliquots of 15 mL/kg using dilute beractant at a concentration of 5 mg/mL. The primary outcome measure is duration of respiratory support. Further details of the trial and a video of the lavage procedure can be viewed at http://www.rch.org.au/neonatal_rch/lessmas.
The results of these ongoing studies will give more definitive guidance to neonatologists contemplating lavage therapy in ventilated infants with MAS. Until more data are forthcoming, our view is that lung lavage using dilute surfactant must be considered an unproven and experimental therapy, which should only be used in the context of a randomised controlled trial.
7. Conclusion
The multifaceted pathophysiology of MAS includes a considerable component of surfactant dysfunction. Bolus therapy with exogenous surfactant has found a place in the treatment of ventilated infants with MAS, although it is only likely to benefit these infants if given early and repeatedly. Lung lavage with dilute surfactant has emerged as a plausible alternative to bolus administration, although it currently remains in the realm of experimental therapy pending further investigation.
Note in Proof
A further randomised controlled trial of bolus surfactant therapy in MAS has been published since the preparation of this manuscript.[144]A short-term improvement in oxygenation was noted in surfactant-treated infants, but there was no significant difference between treated and control groups with respect to duration of ventilation, indicidence of pneumothorax or mortality.
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Cleary GM, Wiswell TE. Meconium-stained amniotic fluid and the meconium aspiration syndrome: an update. Pediatr Clin North Am 1998; 45: 511–29
Greenough A. Meconium aspiration syndrome: prevention and treatment. Early Hum Dev 1995; 41: 183–92
Roberten NRC. Aspiration syndromes. In: Greenough A, Roberten NRC, Milner AD, editors. Neonatal respiratory disorders. London: Arnold, 1996: 313–33
Wiswell TE, Bent RC. Meconium staining and the meconium aspiration syndrome: unresolved issues. Pediatr Clin North Am 1993; 40: 955–81
Bacsik RD. Meconium aspiration syndrome. Pediatr Clin North Am 1977; 24: 463–79
Kamlin O, Dargaville PA, for the Australia and New Zealand Neonatal Network. The epidemiology of meconium aspiration syndrome [abstract]. 6th Annual Scientific Conference of the Perinatal Society of Australia and New Zealand; 2002 Mar 9–13; Christchurch
Rossi EM, Philipson EH, Williams TG, et al. Meconium aspiration syndrome: intrapartum and neonatal attributes. Am J Obstet Gynecol 1989; 161: 1106–10
Sriram S, Wall SN, Khoshnood B, et al. Racial disparity in meconium-stained amniotic fluid and meconium aspiration syndrome in the United States, 1989–2000. Obstet Gynecol 2003; 102: 1262–8
Adhikari M, Gouws E. Meconium aspiration in South Africa. S Afr Med J 1995; 85: 891–3
Ellison V, Lui K, Dargaville PA. Aspiration of bile as a cause of respiratory distress in the newborn infant. J Pediatr 2004; 144: 389–90
Gordon E, South M, McDougall PN, et al. Blood aspiration syndrome as a cause of respiratory distress in the newborn infant. J Pediatr 2003; 142: 200–2
Yeh TF, Harris V, Srinivasan G, et al. Roentgenographic findings in infants with meconium aspiration syndrome. JAMA 1979; 242: 60–3
Yuksel B, Greenough A, Gamsu HR. Neonatal meconium aspiration syndrome and respiratory morbidity during infancy. Pediatr Pulmonol 1993; 16: 358–61
Rubin BK, Tomkiewicz RP, Patrinos ME, et al. The surface and transport properties of meconium and reconstituted meconium solutions. Pediatr Res 1996; 40: 834–8
Higgins ST, Wu AM, Sen N, et al. Meconium increases surfactant secretion in isolated rat alveolar type II cells. Pediatr Res 1996; 39: 443–7
Oelberg DG, Downey SA, Flynn MM. Bile salt-induced intracellular Ca++ accumulation in type II pneumocytes. Lung 1990; 168: 297–308
de Beaufort AJ, Pelikan DM, Elferink JG, et al. Effect of interleukin 8 in meconium on in-vitro neutrophil chemotaxis. Lancet 1998; 352: 102–5
Altshuler G, Hyde S. Meconium-induced vasocontraction: a potential cause of cerebral and other fetal hypoperfusion and of poor pregnancy outcome. J Child Neurol 1989; 4: 137–42
Holopainen R, Soukka H, Halkola L, et al. Meconium aspiration induces a concentration-dependent pulmonary hypertensive response in newborn piglets. Pediatr Pulmonol 1998; 25: 107–13
Yeh TF, Lilien LD, Barathi A, et al. Lung volume, dynamic lung compliance, and blood gases during the first 3 days of postnatal life in infants with meconium aspiration syndrome. Crit Care Med 1982; 10: 588–92
Goerke J, Clements JA. Alveolar surface tension and lung surfactant. In: Macklem PT, Mead J, editors. Handbook of physiology, section 3: the respiratory system. Bethesda (MD): American Physiological Society, 1986: 247–61
Jobe AH. Pathophysiology of respiratory distress syndrome and surfactant metabolism. In: Polin RA, Fox WW, editors. Fetal and neonatal physiology. 2nd ed. Philadelphia (PA): W.B. Saunders, 1998: 1299–314
Whitsett JA. Composition of pulmonary surfactant lipids and proteins. In: Polin RA, Fox WW, editors. Fetal and neonatal physiology. 2nd ed. Philadelphia (PA): W.B. Saunders, 1998: 1251–9
Wright JR, Clements JA. Metabolism and turnover of lung surfactant. Am Rev Respir Dis 1987; 136: 426–44
Jobe AH, Ikegami M, Jacobs HC, et al. Surfactant pool sizes and severity of respiratory distress syndrome in prematurely delivered lambs. Am Rev Respir Dis 1983; 127: 751–5
Hallman M, Merritt TA, Akino T, et al. Surfactant protein A, phosphatidylcholine, and surfactant inhibitors in epithelial lining fluid: correlation with surface activity, severity of respiratory distress syndrome, and outcome in small premature infants. Am Rev Respir Dis 1991; 144: 1376–84
Chida S, Phelps DS, Cordle C, et al. Surfactant-associated proteins in tracheal aspirates of infants with respiratory distress syndrome after surfactant therapy. Am Rev Respir Dis 1988; 137: 943–7
Ballard PL, Merrill JD, Godinez RI, et al. Surfactant protein profile of pulmonary surfactant in premature infants. Am J Respir Crit Care Med 2003; 168: 1123–8
Jobe AH. Pulmonary surfactant therapy. N Engl J Med 1993; 328: 861–8
Suresh GK, Soll RF. Current surfactant use in premature infants. Clin Perinatol 2001; 28: 671–94
Herting E, Rauprich P, Stichtenoth G, et al. Resistance of different surfactant preparations to inactivation by meconium. Pediatr Res 2001; 50: 44–9
Bernhard W, Mottaghian J, Gebert A, et al. Commercial versus native surfactants: surface activity, molecular components, and the effect of calcium. Am J Respir Crit Care Med 2000; 162: 1524–33
Seeger W, Grube C, Gunther A, et al. Surfactant inhibition by plasma proteins: differential sensitivity of various surfactant preparations. Eur Respir J 1993; 6: 971–7
Curosurf® (poractant alfra) intratracheal suspension: NDA 20–744 [package insert]. Parma: Chiesi Farmaceutici, S.p.A., 2000
bLES Biochemicals Inc. Product description: bLes (bovine lipid extract surfactant). London (ONT): bLES Biochemicals Inc., 1994 Jun
Cummings JJ, Holm BA, Hudak ML, et al. A controlled clinical comparison of four different surfactant preparations in surfactant-deficient preterm lambs. Am Rev Respir Dis 1992; 145: 999–1004
Infasurf® [package insert]. St Louis (MO): Forest Pharmaceuticals, Inc., 2003 Jun
Willson D. Calfactant. Expert Opin Pharmacother 2001; 2: 1479–93
Exosurf® (colfosceril palmitate, cetyl alcohol, tyloxapol) neonatal for intratracheal suspension [package insert]. Research Triangle Park (NC): Burroughs Wellcome Co., 1990 Aug
Babu KS, Woodcock DA, Smith SE, et al. Inhaled synthetic surfactant abolishes the early allergen-induced response in asthma. Eur Respir J 2003; 21: 1046–9
Cleary GM, Antunes MJ, Ciesielka DA, et al. Exudative lung injury is associated with decreased levels of surfactant proteins in a rat model of meconium aspiration. Pediatrics 1997; 100: 998–1003
Davey AM, Becker JD, Davis JM. Meconium aspiration syndrome: physiological and inflammatory changes in a newborn piglet model. Pediatr Pulmonol 1993; 16: 101–8
Wiswell TE, Peabody SS, Davis JM, et al. Surfactant therapy and high-frequency jet ventilation in the management of a piglet model of the meconium aspiration syndrome. Pediatr Res 1994; 36: 494–500
Dargaville PA, Mills JF, Headley BM, et al. Therapeutic lung lavage in the piglet model of meconium aspiration syndrome. Am J Respir Crit Care Med 2003; 168: 456–63
Dargaville PA, South M, McDougall PN. Comparison of two methods of diagnostic lung lavage in ventilated infants with lung disease. Am J Respir Crit Care Med 1999; 160: 771–7
Bui KC, Walther FJ, David-Cu R, et al. Phospholipid and surfactant protein A concentrations in tracheal aspirates from infants requiring extracorporeal membrane oxygenation. J Pediatr 1992; 121: 271–4
Lotze A, Stroud CY, Soldin SJ. Serial lecithin/sphingomyelin ratios and surfactant/albumin ratios in tracheal aspirates from term infants with respiratory failure receiving extracorporeal membrane oxygenation. Clin Chem 1995; 41: 1182–8
Dargaville PA, South M, McDougall PN. Surfactant and surfactant inhibitors in meconium aspiration syndrome. J Pediatr 2001; 138: 113–5
Lotze A, Whitsett JA, Kammerman LA, et al. Surfactant protein A concentrations in tracheal aspirate fluid from infants requiring extracorporeal membrane oxygenation. J Pediatr 1990; 116: 435–40
Moses D, Holm BA, Spitale P, et al. Inhibition of pulmonary surfactant function by meconium. Am J Obstet Gynecol 1991; 164: 477–81
Sun B, Curstedt T, Robertson B. Surfactant inhibition in experimental meconium aspiration. Acta Paediatr 1993; 82: 182–9
Bae CW, Takahashi A, Chida S, et al. Morphology and function of pulmonary surfactant inhibited by meconium. Pediatr Res 1998; 44: 187–91
Clark DA, Nieman GF, Thompson JE, et al. Surfactant displacement by meconium free fatty acids: an alternative explanation for atelectasis in meconium aspiration syndrome. J Pediatr 1987; 110: 765–70
Terasaka D, Clark DA, Singh BN, et al. Free fatty acids of human meconium. Biol Neonate 1986; 50: 16–20
Sun B, Herting E, Curstedt T, et al. Exogenous surfactant improves lung compliance and oxygenation in adult rats with meconium aspiration. J Appl Physiol 1994; 77: 1961–71
Tyler DC, Murphy J, Cheney FW. Mechanical and chemical damage to lung tissue caused by meconium aspiration. Pediatrics 1978; 62: 454–9
Berger TM, Allred EN, Van Marter LJ. Antecedents of clinically significant pulmonary hemorrhage among newborn infants. J Perinatol 2000; 20: 295–300
Pandit PB, Dunn MS, Colucci EA. Surfactant therapy in neonates with respiratory deterioration due to pulmonary hemorrhage. Pediatrics 1995; 95: 32–6
Fuchimukai T, Fujiwara T, Takahashi A, et al. Artificial pulmonary surfactant inhibited by proteins. J Appl Physiol 1987; 62: 429–37
Ikegami M, Rebello CM, Jobe AH. Surfactant inhibition by plasma: gestational age and surfactant treatment effects in preterm lambs. J Appl Physiol 1996; 81: 2517–22
Lachmann B, Eijking EP, So KL, et al. In vivo evaluation of the inhibitory capacity of human plasma on exogenous surfactant function. Intensive Care Med 1994; 20: 6–11
Colacicco G, Basu MK. Effect of serum albumin on dynamic force-area curve of dipalmitoyl lecithin. Respir Physiol 1978; 32: 265–79
Holm BA, Notter RH, Finkelstein JN. Surface property changes from interactions of albumin with natural lung surfactant and extracted lung lipids. Chem Phys Lipids 1985; 38: 287–98
Holm BA, Notter RH. Effects of hemoglobin and cell membrane lipids on pulmonary surfactant activity. J Appl Physiol 1987; 63: 1434–42
O’Brodovich HM, Weitz JI, Possmayer F. Effect of fibrinogen degradation products and lung ground substance on surfactant function. Biol Neonate 1990; 57: 325–33
Chen CT, Toung TJ, Rogers MC. Effect of intra-alveolar meconium on pulmonary surface tension properties. Crit Care Med 1985; 13: 233–6
Lu KW, William TH, Robertson B, et al. Polymer-surfactant treatment of meconium-induced acute lung injury. Am J Respir Crit Care Med 2000; 162: 623–8
Dargaville PA, Morley CJ. Overcoming surfactant inhibition with polymers. Acta Paediatr 2000; 89: 1397–400
Taeusch HW, Lu KW, Goerke J, et al. Nonionic polymers reverse inactivation of surfactant by meconium and other substances. Am J Respir Crit Care Med 1999; 159: 1391–5
Cochrane CG, Revak SD, Merritt TA, et al. Bronchoalveolar lavage with KL4-surfactant in models of meconium aspiration syndrome. Pediatr Res 1998; 44: 705–15
Bruni R, Fan BR, David-Cu R, et al. Inactivation of surfactant in rat lungs. Pediatr Res 1996; 39: 236–40
Seeger W, Gunther A, Thede C. Differential sensitivity to fibrinogen inhibition of SP-C- vs SP-B-based surfactants. Am J Physiol 1992; 262: L286–91
Almaas R, Robertson B, Linderholm B, et al. Reversal of meconium inhibition of pulmonary surfactant by ferric chloride, copper chloride, and acetic acid. Am J Respir Crit Care Med 2000; 162: 1789–94
Manalo E, Merritt TA, Kheiter A, et al. Comparative effects of some serum components and proteolytic products of fibrinogen on surface tension-lowering abilities of beractant and a synthetic peptide containing surfactant KL 4. Pediatr Res 1996; 39: 947–52
Mbagwu N, Bruni R, Hernandez-Juviel JM, et al. Sensitivity of synthetic surfactants to albumin inhibition in preterm rabbits. Mol Genet Metab 1999; 66: 40–8
Seeger W, Thede C, Gunther A, et al. Surface properties and sensitivity to protein-inhibition of a recombinant apoprotein C-based phospholipid mixture in vitro: comparison to natural surfactant. Biochim Biophys Acta 1991; 1081: 45–52
al-Mateen KB, Dailey K, Grimes MM, et al. Improved oxygenation with exogenous surfactant administration in experimental meconium aspiration syndrome. Pediatr Pulmonol 1994; 17: 75–80
Calkovska A, Sun B, Curstedt T, et al. Combined effects of high-frequency ventilation and surfactant treatment in experimental meconium aspiration syndrome. Acta Anaesthesiol Scand 1999; 43: 135–45
Hilgendorff A, Rawer D, Doerner M, et al. Synthetic and natural surfactant differentially modulate inflammation after meconium aspiration. Intensive Care Med 2003; 29: 2247–54
Hu X, Cao L, Lam LK, et al. Mitigation of meconium-induced lung injury by surfactant and inhaled nitric oxide is associated with suppression of nuclear transcription factor kappa B. Biol Neonate 2004; 87: 73–81
Lyra JC, Mascaretti RS, Precioso AR, et al. Different doses of exogenous surfactant for treatment of meconium aspiration syndrome in newborn rabbits. Rev Hosp Clin Fac Med Sao Paulo 2004; 59: 104–12
Rais-Bahrami K, Rivera O, Seale WR, et al. Effect of nitric oxide in meconium aspiration syndrome after treatment with surfactant. Crit Care Med 1997; 25: 1744–7
Robinson TW, Roberts AM. Effects of exogenous surfactant on gas exchange and compliance in rabbits after meconium aspiration. Pediatr Pulmonol 2002; 33: 117–23
Sevecova-Mokra D, Calkovska A, Drgova A, et al. Treatment of experimental meconium aspiration syndrome with surfactant lung lavage and conventional vs asymmetric high-frequency jet ventilation. Pediatr Pulmonol 2004; 38: 285–91
Sun B, Curstedt T, Robertson B. Exogenous surfactant improves ventilation efficiency and alveolar expansion in rats with meconium aspiration. Am J Respir Crit Care Med 1996; 154: 764–70
Sun B, Curstedt T, Song GW, et al. Surfactant improves lung function and morphology in newborn rabbits with meconium aspiration. Biol Neonate 1993; 63: 96–104
Hudson ML, Louder DS, Stribley RF. Surfactant distribution in the piglet model of meconium aspiration [abstract]. Pediatr Res 1996; 39: 217A
Chappell SE, Wolfson MR, Shaffer TH. A comparison of surfactant delivery with conventional mechanical ventilation and partial liquid ventilation in meconium aspiration injury. Respir Med 2001; 95: 612–7
Segerer H, van Gelder W, Angenent FW, et al. Pulmonary distribution and efficacy of exogenous surfactant in lung-lavaged rabbits are influenced by the instillation technique. Pediatr Res 1993; 34: 490–4
Segerer H, Scheid A, Wagner MH, et al. Rapid tracheal infusion of surfactant versus bolus instillation in rabbits: effects on oxygenation, blood pressure and surfactant distribution. Biol Neonate 1996; 69: 119–27
Auten RL, Notter RH, Kendig JW, et al. Surfactant treatment of full-term newborns with respiratory failure. Pediatrics 1991; 87: 101–7
Khammash H, Perlman M, Wojtulewicz J, et al. Surfactant therapy in full-term neonates with severe respiratory failure. Pediatrics 1993; 92: 135–9
Halliday HL, Speer CP, Robertson B. Treatment of severe meconium aspiration syndrome with porcine surfactant: Collaborative Surfactant Study Group. Eur J Pediatr 1996; 155: 1047–51
Blanke JG, Jorch G. Surfactant therapy in severe neonatal respiratory failure: multicenter study: II. Surfactant therapy in 10 newborn infants with meconium aspiration syndrome [in German]. Klin Pädiatr 1993; 205: 75–8
Lotze A, Knight GR, Martin GR, et al. Improved pulmonary outcome after exogenous surfactant therapy for respiratory failure in term infants requiring extracorporeal membrane oxygenation. J Pediatr 1993; 122: 261–8
Davis JM, Richter SE, Kendig JW, et al. High-frequency jet ventilation and surfactant treatment of newborns with severe respiratory failure. Pediatr Pulmonol 1992; 13: 108–12
Findlay RD, Taeusch HW, Walther FJ. Surfactant replacement therapy for meconium aspiration syndrome. Pediatrics 1996; 97: 48–52
Lotze A, Mitchell BR, Bulas DI, et al. Multicenter study of surfactant (beractant) use in the treatment of term infants with severe respiratory failure. Survanta in Term Infants Study Group. J Pediatr 1998; 132: 40–7
Soll RF, Dargaville P. Surfactant for meconium aspiration syndrome in full term infants. Cochrane Database Syst Rev 2000; (2): CD002054
Dargaville PA, Mills JF, Loughnan PM, et al. Large volume therapeutic pulmonary lavage in meconium aspiration syndrome [abstract]. Annual Scientific Meeting of the Royal Australian College of Physicians; 2002 May 6–8; Brisbane
Bhutani VK, Chima R, Sivieri EM. Innovative neonatal ventilation and meconium aspiration syndrome. Indian J Pediatr 2003; 70: 421–7
Wilson JM, Bower LK, Thompson JE, et al. ECMO in evolution: the impact of changing patient demographics and alternative therapies on ECMO. J Pediatr Surg 1996; 31: 1116–22
Hintz SR, Suttner DM, Sheehan AM, et al. Decreased use of neonatal extracorporeal membrane oxygenation (ECMO): how new treatment modalities have affected ECMO utilization. Pediatrics 2000; 106: 1339–43
Selecky PA, Wasserman K, Benfield JR, et al. The clinical and physiological effect of whole-lung lavage in pulmonary alveolar proteinosis: a ten-year experience. Ann Thorac Surg 1977; 24: 451–61
Wiswell TE, Smith RM, Katz LB, et al. Bronchopulmonary segmental lavage with Surfaxin (KL(4)-surfactant) for acute respiratory distress syndrome. Am J Respir Crit Care Med 1999; 160: 1188–95
Lober CW, Saltzman HA, Kylstra JA. Volume-controlled lung lavage in a woman with cystic fibrosis. Chest 1975; 68: 382–3
Ciravegna B, Sacco O, Moroni C, et al. Mineral oil lipoid pneumonia in a child with anoxic encephalopathy: treatment by whole lung lavage. Pediatr Pulmonol 1997; 23: 233–7
Burke-Strickland M, Edwards NB. Meconium aspiration in the newborn. Minn Med 1973; 56: 1031–5
Carson BS, Losey RW, Bowes WAJ, et al. Combined obstetric and pediatric approach to prevent meconium aspiration syndrome. Am J Obstet Gynecol 1976; 126: 712–5
Barrington KJ, Henderson CR, Cochrane CG, et al. Lung lavage with KL4 surfactant in a neonatal model of meconium aspiration: the importance of PEEP [abstract]. Pediatr Res 1998; 43: 166A
Foust R, Tran NN, Cox C, et al. Liquid assisted ventilation: an alternative ventilatory strategy for acute meconium aspiration injury. Pediatr Pulmonol 1996; 21: 316–22
Giuseppetti M, Wiswell TE. The use of saline lavage and surfactant therapy in the management of a piglet model of meconium aspiration syndrome [abstract]. Pediatr Res 1994; 35: 333A
Kuo CY, Hsueh C, Wang CR. Liquid ventilation for treatment of meconium aspiration syndrome in a piglet model. J Formos Med Assoc 1998; 97: 392–9
Lam BC, Yeung CY, Fu KH, et al. Surfactant tracheobronchial lavage for the management of a rabbit model of meconium aspiration syndrome. Biol Neonate 2000; 78: 129–38
Marraro G, Bonati M, Ferrari A, et al. Perfluorocarbon bronchoalveolar lavage and liquid ventilation versus saline bronchoalveolar lavage in adult guinea pig experimental model of meconium inhalation. Intensive Care Med 1998; 24: 501–8
Nakamura T, Matsuzawa S, Sugiura M, et al. A randomised control study of partial liquid ventilation after airway lavage with exogenous surfactant in a meconium aspiration syndrome animal model. Arch Dis Child Fetal Neonatal Ed 2000; 82: F160–2
Ohama Y, Itakura Y, Koyama N, et al. Effect of surfactant lavage in a rabbit model of meconium aspiration syndrome. Acta Paediatr Jpn 1994; 36: 236–8
Ohama Y, Ogawa Y. Treatment of meconium aspiration syndrome with surfactant lavage in an experimental rabbit model. Pediatr Pulmonol 1999; 28: 18–23
Paranka MS, Walsh WF, Stancombe BB. Surfactant lavage in a piglet model of meconium aspiration syndrome. Pediatr Res 1992; 31: 625–8
Schlösser RL, Veldman A, Fischer D, et al. Comparison of effects of perflubron and surfactant lung lavage on pulmonary gas exchange in a piglet model of meconium aspiration. Biol Neonate 2002; 81: 126–31
Sevecova D, Calkovska A, Drgova A, et al. Lung lavage using high-frequency jet ventilation in rabbits with meconium aspiration. Acta Paediatr 2003; 92: 314–9
Shaffer TH, Lowe CA, Bhutani VK, et al. Liquid ventilation: effects on pulmonary function in distressed meconium-stained lambs. Pediatr Res 1984; 18: 47–52
Tanveer A, Antunes MJ, Cleary GM, et al. Lung mechanics and inflammatory response in meconium injured rats following lung lavage with perfluorochemical or KL4 surfactant [abstract]. Pediatr Res 1998; 43: 299A
Ibara S, Ikenoue T, Murata Y, et al. Management of meconium aspiration syndrome by tracheobronchial lavage and replacement of Surfactant-TA. Acta Paediatr Jpn 1995; 37: 64–7
Kaneko M, Watanabe J, Ueno E. Surfactant lavage and replacement in meconium aspiration syndrome with pulmonary hemorrhage. J Perinat Med 2001; 29: 351–6
Mosca F, Colnaghi M, Castoldi F. Lung lavage with a saline volume similar to functional residual capacity followed by surfactant administration in newborns with severe meconium aspiration syndrome. Intensive Care Med 1996; 22: 1412–3
Su BH, Hu PS, Peng CT, et al. The effect of bronchial lavage and surfactant supplement on severe meconium aspiration syndrome. M Taiwan J Med 1998; 3: 191–7
Möller JC, Kohl M, Reiss I, et al. Saline lavage with substitution of bovine surfactant in term neonates with meconium aspiration syndrome (MAS) transferred for extracorporeal membrane oxygenation (ECMO): a pilot study. Crit Care (Lond) 1999; 3: 19–22
Kowalska K, Szymankiewicz M, Gadzinowski J. An effectiveness of surfactant lung lavage (SLL) in meconium aspiration syndrome (MAS) [in Polish]. Przegl Lek 2002; 59 Suppl. 1: 21–4
Lam BCC, Yeung CY. Surfactant lavage for meconium aspiration syndrome: a pilot study. Pediatrics 1999; 103: 1014–8
Salvia-Roiges MD, Carbonell-Estrany X, Figueras-Aloy J, et al. Efficacy of three treatment schedules in severe meconium aspiration syndrome. Acta Paediatr 2004; 93: 60–5
Szymankiewicz M, Gadzinowski J, Kowalska K. Pulmonary function after surfactant lung lavage followed by surfactant administration in infants with severe meconium aspiration syndrome. J Matern Fetal Neonatal Med 2004; 16: 125–30
Chang HY, Hsu CH, Kao HA, et al. Treatment of severe meconium aspiration syndrome with dilute surfactant lavage. J Formos Med Assoc 2003; 102: 326–30
Rosegger H, Engele H, Haas J. Tracheobronchial lavage: a supplementary measure in the initial management of meconium aspiration syndrome [in German]. Wien Klin Wochenschr 1987; 99: 843–7
Ogawa Y. Bronchial lavage with surfactant solution for the treatment of meconium aspiration syndrome. Hot Topics in Neonatology ’97 Conference Proceedings. Columbus (OH): Professional Services Dept, Ross Products, 1997: 259–64
Wiswell TE, Knight GR, Finer NN, et al. A multicenter, randomized, controlled trial comparing Surfaxin (Lucinactant) lavage with standard care for treatment of meconium aspiration syndrome. Pediatrics 2002; 109: 1081–7
Schlösser RL, Veldman A, Fischer D, et al. Lavage with exogenous surfactant in neonatal meconium aspiration syndrome [in German]. Z Geburtshilfe Neonatol 2002; 206: 15–8
Chesnutt MS, Nuckton TJ, Golden J, et al. Rapid alveolar epithelial fluid clearance following lung lavage in pulmonary alveolar proteinosis. Chest 2001; 120: 271–4
Bland RD. Loss of liquid from the lung lumen in labor: more than a simple “squeeze”. Am J Physiol Lung Cell Mol Physiol 2001; 280: L602–5
Kattwinkel J. Surfactant lavage for meconium aspiration: a word of caution. Pediatrics 2002; 109: 1167–8
Kinsella JP. Meconium aspiration syndrome: is surfactant lavage the answer? Am J Respir Crit Care Med 2003; 168: 413–4
Discovery Laboratories. Product pipeline: SRT for respiratory medicine [online]. Available from URL: http://www.discoverylabs.com/products-pipeline.htm [Accessed 2005 Sep 28]
Controlled trial of therapeutic lung lavage in meconium aspiration syndrome (the lessMAS trial) [online]. Available from URL: http://www.rch.org.au/neonatal_rch/lessmas [Accessed 2005 Sep 7]
Chinese Collaborative Study Group for Neonatal Respiratory Diseases. Treatment of severe meconium aspiration syndrome with porcine surfactant: a multicentre randomized controlled trial. Acta Paediatrica 2005; 94: 896–902
Acknowledgements
We thank Dr Bev Copnell and Professor Colin Morley for their assistance in preparing this manuscript.
The authors’ work on lavage therapy in meconium aspiration syndrome is supported by the Australian National Health and Medical Research Council (project grant number 284539). Associate Professor Peter Dargaville and Dr John Mills are Chief Investigators for the lessMAS trial, a multicentre international randomised controlled trial of surfactant lavage therapy in meconium aspiration syndrome. This trial is funded by the Australian National Health and Medical Research Council. Surfactant for the lessMAS trial is provided by Abbott Australasia.
No other conflicts of interest are declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dargaville, P.A., Mills, J.F. Surfactant Therapy for Meconium Aspiration Syndrome. Drugs 65, 2569–2591 (2005). https://doi.org/10.2165/00003495-200565180-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200565180-00003