Abstract
Objective
Meconium aspiration syndrome remains a relevant cause of neonatal respiratory failure and is associated with severe pulmonary changes including surfactant inactivation and pronounced inflammatory changes. The present study investigated the effect of two different surfactant preparations—recombinant surfactant protein C surfactant (rSP-C Surf) and natural bovine surfactant—on pulmonary gas exchange and inflammatory response.
Design and subjects
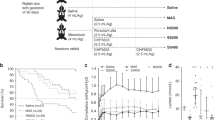
Twenty-three newborn piglets were intubated, mechanically ventilated, received 5 ml/kg 20% sterile meconium for induction of lung injury, and were randomized thereafter for controls (n=7), rSP-C Surf (n=8), or natural surfactant (n=8). Surfactants were given as an intratracheal bolus (75 mg/kg) and animals were further ventilated.
Measurements and results
Lung function variables, arterial blood gas samples and lung tissues were obtained. Histological evaluation was performed in right lung tissue using an established score. Cytokine mRNA expression (left lung tissue) was quantified using TaqMan real-time PCR (ΔΔCT method, normalized to controls). In addition to significant improvement in gas exchange and lung function, histological evaluation showed significantly lower sum scores in the rSP-C Surf group than in controls). Cytokine mRNA expression of IL-1β in whole lung tissue was significantly lower after administration of rSP-C Surf than in natural surfactant and controls whereas IL-10 mRNA expression was significantly induced in both surfactant groups.
Conclusions
Surfactant administration improved both gas exchange and pulmonary inflammatory cytokine transcription. Mechanisms underlying the differential inflammatory response in both surfactant preparations need to be further addressed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meconium aspiration syndrome (MAS) remains a relevant cause of respiratory failure in term neonates, and despite intensive perinatal treatment it is still associated with high neonatal mortality and morbidity [1]. MAS-induced pulmonary changes include typical histological characteristics [2], inflammatory cell infiltration with release of vasoactive substances [3], airway obstruction, and surfactant dysfunction and inactivation [4, 5]. Experimental models of MAS have therefore been established for studying the pathogenesis of respiratory failure in neonates. A number of studies have been performed addressing the pulmonary response to surfactant in MAS [6, 7]; however, results still remain controversial [8]. Type, dosage, and mode of surfactant administration are under investigation. Meconium constituents such as lysophosphatidylcholine, bilirubin, bile salts, and proinflammatory agents and the presence of hemoglobin in the lung are able to disrupt the surfactant monolayer formation and stabilization thus interfering with surface tension-lowering properties i.e., surfactant inactivation. Therefore the need for repetitive administration of natural surfactant to overcome its inactivation has been reported [9, 10], whereas recent studies revealed serious precautions against the lavage technique [11].
In addition to the improvement in MAS-induced airway obstruction and atelectasis following exogenous surfactant, its potential in influencing the pulmonary inflammatory response is discussed. As increased levels of tumor necrosis factor (TNF) α, interleukin (IL) 1β, 6, and 8, and low levels of IL-10 are known to be present in lung tissue after meconium aspiration [12, 13], and in vitro studies demonstrating an altered inflammatory response of polymorph nuclear leukocytes following surfactant incubation [14] seem to be of great potential.
Therefore the objective of the present study was to evaluate the effect of bolus administration of two different surfactant preparations-a natural bovine surfactant containing surfactant protein B (SP-B) and surfactant protein C (SP-C) and a synthetic surfactant containing recombinant SP-C (rSP-C Surf) on (a) variables of lung function and gas exchange, (b) lung histology, and (c) inflammatory cytokine transcription in a piglet model of MAS.
Materials and Methods
Animals
Twenty-three newborn piglets of either sex and a median age of 6 days (range 1–11) and a median weight of 2200 g (range 1900–2500) were studied. Animal experiments were approved by the State Ethics Committee for Animal Eexperiments of the state of Hesse, Germany, and performed according to the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health.
Protocol
Intravenous anesthesia was induced with ketamine (2 mg/kg body weight, b.w.; Inresa, Freiburg, Germany) and midazolam (0.2 mg/kg b.w., Roche, Grenzach-Wyhlen, Germany) after an intramuscular bolus of 50 mg/kg b.w. ketamine. Following relaxation with vercuronium (0.2 mg/kg b.w.; Inresa), the piglets were intubated with a cuffed endotracheal tube (3.0 mm outer diameter; Rüsch, Kernen-Rommelshausen, Germany). Continuous infusion with midazolam (1 mg/kg b.w. per hour), ketamine (2 mg/kg b.w. per hour), and vecuronium (0.6 mg/kg b.w. per hour) provided sedation, analgesia and muscular relaxation. Ringer’s solution was given intravenously at a rate of 5 ml/kg b.w. per hour. Animals were ventilated using a pressure controlled mode (Servo 900, Siemens, Germany), with a positive inspiratory pressure (PIP) of 15 cmH2O, a positive endexpiratory pressure (PEEP) of 2 cmH2O, and a respiratory rate of 30 cycles/min (rate of inspiration to expiration 30:70) at 100% oxygen (FIO21.0). Under these conditions normocapnia was observed. Body temperature was maintained in an art-specific range (38°–39°C) with radiant warmers and heating pads. An arterial catheter (20 G; Arrow, Erding, Germany) was placed into the right common carotid artery and a central venous line (4.0 F; Arrow) in the right femoral vein. Arterial blood gases (paO2, paCO2, and pH; ABL Radiometer 555, Diamond Diagnostics, Mass., USA), heart rate, blood pressure, central venous pressure, and electrocardiography (78354A Hewlett-Packard) were monitored before and during induction of lung injury, immediately before treatment and every 30 min thereafter. Tidal volumes were detected by pneumotachograph (CO2SMO plus 8100, Novametrix Medical Systems, USA) at the end of the endotracheal tube and ventilation efficiency index (VEI) was further calculated as: 3,800/(δP×δR×PaCO2) [15]. After a stabilization period of 30 min ventilation pressures were increased (PIP 25 cmH2O, PEEP 4 cmH2O) and remained unchanged during the residual ventilation period. Lung injury was induced in all animals by a bolus administration of 5 ml/kg b.w. of 20% meconium given in aliquots of 2–4 ml via the endotracheal tube under ongoing ventilation according to Soukka et al. [16]. Thirty minutes after induction of lung injury piglets were prospectively randomized to one of the following groups: group 1 (n=7) received intratracheal (i.t.) administration of 1.5 ml/kg b.w. physiological saline (Braun, Melsungen, Germany); group 2 (n=8) received 75 mg rSP-C Surf/kg b.w. intrathecally (Altana Pharma, Constance, Germany); and group 3 (n=8) received 75 mg bovine (SP-B and SP-C) surfactant/kg b.w. intrathecally with 75 mg corresponding 1.5 ml each. After another observation period of 330 min (360 min after induction of lung injury) piglets were killed using an overdose of potassium chloride and phenobarbitone. All animals survived the whole observation period with the exception of one control animal dying from severe respiratory failure. Immediately after death the chest was opened and a cannula was placed into the pulmonary artery, and the left atrium was opened and the lung was perfused in situ with Ringer’s lactate containing procaine (250 mg/l), eparine (20 U/ml), and calcium chloride (2.2 mmol/l) for 10 min. PIP was increased for 15 s to 25 cmH2O and thereafter reduced and left at 10 cmH2O. Thereafter tissue samples of the superior and inferior lobe of the left lung were extracted and immediately snap-frozen in liquid nitrogen for real time polymerase chain reaction (PCR) analyses. Afterwards right lungs were perfused with 300 ml of a formaldehyde (4.6%) and glutaraldehyde (0.5%) solution for approx. 10 min. Finally, the trachea was clamped at a PEEP of 10 cmH2O, and right lungs were removed and submersed in the above solution for histomorphological analyses.
Preparation of pooled meconium
Meconium was collected from healthy full-term neonates with history of neither perinatal complications nor maternal drug administration during pregnancy and delivery. Probes were tested for sterility, pooled, and further lyophilized and diluted with sterile saline to a 20% concentrated solution, equivalent to 65 mg/ml lyophilized meconium [16].
Therapeutic surfactants
Experiments were performed using a recombinant SP-C based surfactant (Altana) or a natural bovine surfactant. In the rSP-C Surf phenylalanine replaces cystein in positions 4 and 5 and isoleucin replaces methionin in position 32 within the recombinant human 34 amino acid SP-C sequence. The final surfactant contained 2% rSP-C (wt:wt) in phospholipids (dipalmitoylphosphatidylcholine and palmitoyloleoylphosphatidyl-glycerol at a 70:30 ratio (wt:wt), 50 mg/ml), plus 5% palmitic acid, which was added to facilitate the preparation of this surfactant product. The rSP-C Surf surfactant was delivered as lyophilized powder and was suspended in 0.9% sterile saline (Braun) to a concentration of 50 mg/ml phospholipids. The natural bovine surfactant, also provided as lyophilized powder, was resuspended in sterile saline 0.9% (Braun) to a final concentration of 50 mg/ml. Its composition has been described previously [17].
Lung histology
Tissue slides were obtained from dependent and nondependent parts of the right lung of each animal as described above and stained with hematoxylin-eosin to perform histological analyses. Lung histology was evaluated by a pathologist (M.E.), blinded to the animal’s group assignment, according to a previously described histological score [18]. Variables scored for histological evaluation were atelectasis, alveolar and interstitial inflammation, alveolar and interstitial hemorrhage, alveolar and interstitial edema, necrosis, and overdistension. The variables were scored using a five-point scale with no injury corresponding to 0 points and 4 points indicating maximum injury.
Measurements of cytokine mRNA expression by real-time PCR
Cytokine mRNA expression of IL-1β, IL-6, IL-8, IL-10, and transforming growth factor (TGF) β was measured in tissue of the left lung lobe, obtained as described above and stored at −80°C, using the real-time reverse-transcriptase PCR technique (TaqMan). To normalize gene expression mRNA of the housekeeping genes β-actin (A) and hypoxanthine-guanine-phosphoribosyl-transferase (H) was quantified. Cytokine mRNA expression was analyzed both for whole left lung tissue and in each left lung lobe separately.
For this purpose lung-tissue was homogenized in liquid nitrogen and total RNA was then extracted using the acid guanidinium-thiocyanate-phenolchloroform method (Roti Quick, Roth, Germany). The RNA concentration was measured spectrophotometrically and quality was estimated by agarose gel electrophoresis with ethidium bromide staining; 0.5 µg RNA was reverse transcribed in a volume of 100 µl at 42°C for 50 min after a denaturation step at 65°C with random hexamer primers and deoxyribonucleoside triphosphates (all chemicals from Gibco BRL, Calif., USA).
TaqMan real-time PCR
Real-time PCR has been confirmed to be a sensitive and precise tool for the quantification of nucleic acids and thus for gene expression studies [19]. Primers and probes for IL-1β, IL-6, IL-8, IL-10, TGF-β, hypoxanthine phosphoribosyltransferase (HPRT) and β-actin were designed using Primer Express software (Applied Biosystems) with reference to Hardt et al. [20] following a fixed set of recommendations to allow the application of standardized cycling conditions (2 min at 50°C, 10 min at 95°C, followed by 45 cycles for 15 s at 95°C and 1 min at 60°C). Control PCRs showed no signal for pure genomic DNA, confirming mRNA specificity. Primers were purchased from Roth and probes from Applied Biosystems. The reaction (total volume 25 µl) contained approximately 5 ng cDNA, primers and probes in a final concentration of 300 nM and 200 nM, respectively, as well as commercial reagents (Universal Master Mix, Applied Biosystems). Relative quantification of gene expression was performed using the ΔΔCt method, which results in a ratio of target gene expression to equally expressed housekeeping genes. The ratio found in a calibration sample that may be arbitrarily chosen serves as a reference point and is set to be 1. In this study control samples were subsumed for this purpose. HPRT and β-actin were chosen as housekeeping genes. To fulfill the requirements for the ΔΔCt method, PCR efficiency was determined by running cDNA dilution series (seven steps of 1:2 dilution performed in duplicate). PCR efficiency varied between 95.7% (IL-6) and 105.4% (IL-10).
Primer and TaqMan probes
-
Hypoxanthine-guanine-phosphoribosyl transferase:
-
Forward: 5′-TGGAAAGAATGTCTTGATTGTTGAAG-3′
-
Reverse: 5′-ATCTTTGGATTATGCTGCTTGACC-3′
-
TaqMan probe: 5′(VIC)-ACACTGGCAAAAVAATGCAAACCTTGCT-(TAMRA)3′
-
β-Actin:
-
Forward: 5′-TCATCACCATCGGCAACG-3′
-
Reverse: 5′-TTCCTGATGTCCACGTCGC-3′
-
TaqMan probe: 5′(VIC)-CCTTCCTGGGCATGGAGTCCTGC-(TAMRA)3′
-
Interleukin 1β:
-
Forward: 5′-GGTTTCTGAAGCAGCCATGG-3′
-
Reverse: 5′-GATTTGCAGCTGGATGCTCC-3′
-
TaqMan probe: 5′(FAM)-AAAGAGATGAAGTGCTGCACCCAAAACCTG-(TAMRA)3′
-
Interleukin 6:
-
Forward: 5′-GGGTAGGGAAGGCAGTAGCC-3′
-
Reverse: 5′-GAATCCCTCTCCACAAGCG-3′
-
TaqMan probe: 5′(FAM)-CTTCAGTGGAGTCGCCTTCTCCCTAA-(TAMRA)3′
-
Interleukin 8:
-
Forward: 5′-TTCTGCAGCTCTCTGTGAGGC-3′
-
Reverse: 5′-GGTGGAAAGGTGTGGAAGTC-3′
-
TaqMan probe: 5′(FAM)-TTCTGGCAAGAGTAAGTGCAGAACTTCGATG-(TAMRA)3′
-
Interleukin 10:
-
Forward: 5′-TTGGAGCTTGCTAAAGGCACT-3′
-
Reverse: 5′-CGGCGCTGTCATCAATTTCT-3′
-
TaqMan probe: 5′(FAM)-CACCTCCTCCACGGCCTTGCTCTT-(TAMRA)3′
-
Transforming growth factor β:
-
Forward: 5′-TACGCCAAGGAGGTCACCC-3′
-
Reverse: 5′-CAGCTCTGCCCGAGAGAGC-3′
-
TaqMan probe: 5′(FAM)-CTAATGGTGGAAAGCGGCAACCAAATGTA-(TAMRA)3′
Free fatty acid analysis of therapeutic surfactant preparations
Lipids in rSP-C Surf and natural bovine surfactant were extracted using methanol/chloroform according to the method of Bligh and Dyer [21]. Free fatty acids were converted to fatty acid methyl ester using diazomethane and then purified by means of thin layer chromatography (Silica 60 plates, Merck, Darmstadt, Germany), with toluol as developing solvent. Fatty acid methyl ester was identified using primuline [22] and subjected to gas chromatography as previously described [23, 24].
Statistical analysis and data presentation
Histological results are given as geometric mean ±standard deviation with 95% confidence intervals. Results of real-time PCR analyses are given as geometric mean and dispersion factor (lognormal standard deviation). Data were normalized to the housekeeping gene and further to the control values. Data analysis was performed using SPSS for Windows. The t test or the Mann-Whitney U test was used to compare two groups and analysis of variance or the Kruskal-Wallis test to compare of more than three groups, as appropriate. In the case of significance, Bonferroni’s and Dunn’s posthoc tests were applied, respectively. Significance was considered with a p value less than 0.05.
Results
Gas exchange and lung function
Prior to induction of acute respiratory failure by administration of meconium an adequate oxygenation was observed in all groups. After administration of meconium the arterial pO2, tidal volumes, and VEI were reduced with high significance in all animals (see Table 1; Fig. 1). After intratrached administration of either rSP-C Surf or natural bovine surfactant (75 mg/kg b.w.) arterial pO2, tidal volumes, and VEI significantly improved in surfactant treated animals as compared to controls. Initial effects were observed 30 min and maximum effects 330 min after bolus administration of rSP-C Surf and natural bovine surfactant (p<0.0001 vs. control; Table 1).
Histology
Data from 22 piglets were available for histological evaluation of tissue samples from the superior and inferior right lung lobes. Gross macroscopic examination of the lungs revealed severe dystelectasis without differences between left and right lungs. A differing degree of histological alterations was observed in each study group. Sum scores, including atelectasis, alveolar and interstitial inflammation, hemorrhage, edema, necrosis, and overdistension were significantly lower in the rSP-C Surf group (53±12; n=8) than controls (72±9; n=7; p<0.01; Fig. 2). There also was a trend towards lower sum scores in the natural bovine surfactant group (62±10; n=8) than in controls.
Lung histology sum scores for alveolar and interstitial inflammation, alveolar and interstitial hemorrhage, atelectasis, alveolar edema, interstitial edema, overdistension, and necrosis in the rSP-C Surf (75 mg/kg b.w.) and the natural surfactant group (nat SF; 75 mg/kg b.w.) compared to controls. Data given as boxplots, geometric mean, standard deviation, and 95% confidence interval. *p<0.05 vs. controls
Cytokine and growth factor expression
Lung tissue of 12 piglets (control n=3; rSP-C Surf n=5, natural bovine surfactant n=4) was available for real time PCR analyses; material of one piglet had to be excluded because of poor mRNA quality with degradation. Tissue samples of the superior and inferior left lung lobe showed significantly lower IL-1β mRNA expression in the rSP-C Surf group than in controls [p<0.02, normalized to HPRT (H) and β-actin (A)] and to natural bovine surfactant treated animals (p<0.05, H, A; Table 2; Fig. 3). IL-6, IL-8, and TGF-β mRNA expression tended to be higher in natural bovine surfactant treated animals than in rSP-C Surf and controls (Table 2). Cytokine mRNA expression of IL-10 was significantly higher in both surfactant groups than in controls in whole lung tissue (p<0.05, H, A; Table 2; Fig. 4).
Pulmonary IL-1β mRNA expression normalized to HPRT (H, left panel) or β-actin (A, right panel) in the superior (SL) and inferior (IL) lobe of the left lung in newborn piglets with experimentally induced MAS (5 ml/kg meconium) after administration of rSP-C Surf, natural bovine surfactant (nat SF), or physiological saline. Results are normalized to controls, logarithmic scale. **p<0.02 vs. controls, § p<0.05, §§ p<0.02 vs. natural bovine surfactant
Pulmonary IL-10 mRNA expression normalized to HPRT (H, left panel) or β-actin (A, right panel) in the superior (SL) and inferior (IL) lobe of the left lung in newborn piglets with experimentally induced MAS (5 ml/kg meconium) after administration of rSP-C Surf, natural bovine surfactant (nat SF), or physiological saline. Results are normalized to controls, logarithmic scale. *p<0.05, **p<0.02, ***p<0.01 vs. controls
Analyzing cytokine mRNA expression in both lung lobes separately, significantly lower levels of IL-1β and IL-6 mRNA were observed in the lower lung lobe from rSP-C Surf treated animals than in natural bovine surfactant (p<0.05, H, A) and controls (IL-1β, p<0.05, H; Table 2). Again in the natural bovine surfactant group a tendency to higher IL-6 and TGF-β mRNA expression levels was observed. IL-10 mRNA expression was significantly increased in the rSP-C Surf group in the upper and lower left lung lobe (p<0.05, A, vs. controls; Table 2), a trend to higher expression levels was observed for natural bovine surfactant.
Free fatty acid analysis
Chromatographic analyses revealed nearly 2% (w/w) arachidonic acid referring to total free fatty acid concentrations in the natural bovine surfactant samples. The fraction of free fatty acids in natural bovine surfactant was nearly 3% (wt:wt) compared to total phospholipids and significant amounts of ω-3 fatty acids eicosapentaenoic acid (1%) and docosahexaenoic acid (0.6%) were found in the analyzed natural bovine surfactant probes. The profiles of the tested natural bovine surfactant charges (n=3) were almost identical. In contrast the recombinant SP-C surfactant contained no arachidonic acid, and free fatty acids were composed mainly of palmitic acid (82%) and oleic acid (12%). Again, the profiles of different charges (n=3) were stable in the analyses.
Discussion
The present study investigated lung function and inflammatory pulmonary response in a well established piglet model of MAS [16] after bolus administration of two different surfactant preparations. Response to surfactant administration was determined by monitoring pulmonary gas exchange, tidal volumes, and pulmonary inflammatory reaction. Following the early phase of MAS with predominantly mechanically impaired gas exchange, expression of proinflammatory cytokines in meconium aspiration is the second step of the pathophysiological cascade leading to neonatal lung failure resembling acute respiratory distress syndrome (ARDS) [12, 13, 16]. Surfactant administration is a therapeutic option previously used in several studies on treatment strategies for ARDS-like lung failure in neonates [6, 10, 18, 25]. Therefore surfactant preparations with different protein contents and different administration techniques gained increasing interest [26, 27, 28, 29].
In the present study bolus administrations of two different surfactant preparations—rSP-C Surf and natural bovine surfactant—used in a piglet model of MAS showed an adequate response with improved gas exchange and tidal volumes. As recent studies revealed serious concerns against surfactant lavage techniques, bolus treatment was chosen for this experimental setting and confirmed for both surfactant preparations as an efficient and safe intervention in MAS. Apart from these “classical variables,” surfactant effects on the inflammatory response in MAS have not been studied systemically yet. As cytokines themselves play a crucial role in altering the surfactant system [30], interactions between these two pathophysiologically important opponents seem to be of considerable interest.
Because mRNA expression precedes protein formation, mRNA expression of early cytokines was quantified using TaqMan real-time PCR as a highly sensitive approach. It is a well established fact, lung maturation to show apicobasal pattern in the neonatal period. Furthermore initial distribution of meconium in MAS has been described to differ vertically involving more severely the lower lung areas [3, 31]. Therefore cytokine mRNA expression was analyzed both in whole lung tissue and for upper and lower lung lobe separately.
Administration of rSP-C Surf led to significantly lower proinflammatory cytokine mRNA expression in whole lung tissue than in natural bovine surfactant and controls. With the natural bovine surfactant mRNA expression of the proinflammatory cytokines even tended to be higher than in controls. As an indicator of direct anti-inflammatory properties of exogenous pulmonary surfactant preparations IL-10 mRNA expression was found to be induced after administration of both surfactant preparations. As IL-10 with its strong anti-inflammatory properties has been discussed as a potential therapeutic option for treatment of chronic inflammatory pulmonary disorders of the newborn [32, 33, 34], the present findings might implicate important clinical potentials.
The differential modulation of the inflammatory response was even more evident, when results were analyzed for upper and lower lung lobe separately, rSP-C Surf revealing most pronounced anti-inflammatory properties. Differing results in pro- and anti-inflammatory cytokines in the superior and inferior left lung lobe may be due to the above differences in maturation and regional distribution of meconium as well as exogenous surfactant. The trend towards a differential cytokine mRNA expression concerning Il-1β and IL-6, more evident in whole left lung tissue, may be due to time dependence in cytokine activation and to their differential role in the inflammatory cascade. Analyses of dynamics in cytokine expression could further explain the extent to which meconium and especially different surfactant preparations affect the complex system of inflammatory pulmonary response in the time course. Furthermore there is evidence in pulmonary inflammatory response a hierarchic regulation of cytokines to be apparent. Studies have shown IL-1 β to induce IL-8 expression in human bronchial epithelial cells [35].
Although anti-inflammatory properties of rSP-C Surf and natural bovine surfactant could be explained by a reduction in ventilator-associated lung injury, identical tidal volumes in both surfactant groups strongly contradict this explanation. Therefore differing qualitative and quantitative surfactant protein and phospholipid compositions must be considered as potential modifiers of the inflammatory response. Therefore effects of the recombinant surfactant might be due to its increased content of SP-C, whose dysfunction was shown to cause severe interstitial lung disease by dysregulation of intracellular surfactant processing [36]. Furthermore, SP-C was shown to interact with inflammation-stimulating agents such as lipopolysaccharides [37] and may thus have impact on pulmonary inflammatory processes. The above properties of SP-C and differing phospholipid compositions of surfactant preparations also may explain the results of other studies that have demonstrated different biophysical interactions [24, 29], gas exchange, and lung mechanics [28] after administration of various surfactant preparations. In contrast, recent reports found no difference in the inflammatory response after administration of four different surfactant preparations in a model of ventilator-associated lung injury [27].
Furthermore interactions between additional contents of the surfactant preparations and meconium itself or lung tissues must be considered. Phospholipase A2, present in meconium [38], has been discussed as being responsible for morphological alterations in lung injury [39] and additionally has been demonstrated to inactivate natural surfactant. These interactions might be different for various surfactant preparations, as it has been shown recently that resistance towards inactivation mediated by meconium of the rSP-C Surf is superior to animal lung’s derived surfactant preparations [29]. Furthermore, the rate of surfactant subtype conversion induced by meconium [40] may be altered by differing protein and phospholipid contents.
Another important property of phospholipase A2 is the generation of arachidonic acid, known to be a major precursor of eicosanoids, which mediate various inflammatory processes in both premature and adult lungs [41]. This precursor was found in the natural bovine surfactant preparation in contrast to the studied recombinant product as demonstrated chromatographically. This might explain previously reported activating properties of natural surfactant preparations [42].
Having established gas exchange and tidal volumes to be improved after bolus administration of both surfactant preparations, differences between the rSP-C and the natural bovine surfactant were demonstrated with respect to their immunomodulatory properties. Since there is evidence that persistently high levels of inflammatory cytokines are a pathophysiological mainstay for the development of chronic lung disease in newborns [33, 34], the evaluation of immunomodulatory surfactant properties seems to be of considerable interest. As the exact sequence of the reduced inflammatory response following surfactant in the present model of MAS is unclear, further studies are mandatory to address potential underlying mechanisms.
References
Rao S, Pavlova Z, Incerpi MH, Ramanathan R (2001) Meconium-stained amniotic fluid and neonatal morbidity in near-term and term deliveries with acute histologic chorioamnionitis and/or funisitis. J Perinatol 21:537–540
Wiswell TE, Peabody SS, Davis JM, Slayter MV, Bent RC, Merritt TA (1994) Surfactant therapy and high-frequency jet ventilation in the management of a piglet model of the meconium aspiration syndrome. Pediatr Res 36:494–500
Davey AM, Becker JD, Davis JM (1993) Meconium aspiration syndrome: physiological and inflammatory changes in a newborn piglet model. Pediatr Pulmonol 16:101–108
Wiswell TE, Bent RC (1993) Meconium staining and the meconium aspiration syndrome. Unresolved issues. Pediatr Clin North Am 40:955–981
Sun B, Curstedt T, Robertson B (1993) Surfactant inhibition in experimental meconium aspiration. Acta Paediatr 82:182–189
Findlay RD, Taeusch HW, Walther FJ (1996) Surfactant replacement therapy for meconium aspiration syndrome. Pediatrics 97:48–52
Gortner L, Pohlandt F, Bartmann P (1994) Bovine surfactant in full-term neonates with adult respiratory distress syndrome-like disorders. Pediatrics 93:538
Wiswell TE (2001) Advances in the treatment of the meconium aspiration syndrome. Acta Paediatr Suppl 90:28–30
Sun B, Curstedt T, Song GW, Robertson B (1993) Surfactant improves lung function and morphology in newborn rabbits with meconium aspiration. Biol Neonate 63:96–104
Möller JC, Kohl M, Reiss I, Diederich W, M. NE, Göpel W, Gortner L (1999) Saline lavage with substitution of bovine surfactant in term neonates with meconium aspiration syndrome (MAS) transferred for extracorporeal membrane oxygenation (ECMO): a pilot study. Crit Care 3:19–22
Kattwinkel J (2002) Surfactant lavage for meconium aspiration: a word of caution. Pediatrics 109:1167–1168
Zagariya A, Bhat R, Uhal B, Navale S, Freidine M, Vidyasagar D (2000) Cell death and lung cell histology in meconium aspirated newborn rabbit lung. Eur J Pediatr 159:819–826
Beaufort AJ de, Pelikan DM, Elferink JG, Berger HM (1998) Effect of interleukin 8 in meconium on in-vitro neutrophil chemotaxis. Lancet 352:102–105
Tegtmeyer FK, Gortner L, Ludwig A, Brandt E (1996) In vitro modulation of induced neutrophil activation by different surfactant preparations. Eur Respir J 9:752–757
Davis AJ, Jobe AH, Hafner D, Ikegami M (1998) Lung function in premature lambs and rabbits treated with a recombinant SP-C surfactant. Am J Respir Crit Care Med 157:553–559
Soukka H, Rautanen M, Halkola L, Kero P, Kaapa P (1997) Meconium aspiration induces ARDS-like pulmonary response in lungs of ten-week-old pigs. Pediatr Pulmonol 23:205–211
Gortner L, Pohlandt F, Disse B, Weller E (1990) Effects of bovine surfactant in premature lambs after intratracheal application. Eur J Pediatr 149:280–283
Lotze A, Mitchell BR, Bulas DI, Zola EM, Shalwitz RA, Gunkel JH (1998) Multicenter study of surfactant (beractant) use in the treatment of term infants with severe respiratory failure. Survanta in Term Infants Study Group. J Pediatr 132:40–47
Heid CA, Stevens J, Livak KJ, Williams PM (1996) Real time quantitative PCR. Genome Res 6:986–994
Hardt K von der, Schoof E, Kandler MA, Dotsch J, Rascher W (2002) Aerosolized perfluorocarbon suppresses early pulmonary inflammatory response in a surfactant-depleted piglet model. Pediatr Res 51:177–182
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Wright RS (1971) A reagent for the non-destructive location of steroids and some other lipophilic materials on silica gel thin-layer chromatograms. J Chromatogr 59:220–221
Mayer K, Schmidt R, Muhly-Reinholz M, Bogeholz T, Gokorsch S, Grimminger F, Seeger W (2002) In vitro mimicry of essential fatty acid deficiency in human endothelial cells by TNFalpha impact of omega-3 versus omega-6 fatty acids. J Lipid Res 43:944–951
Schmidt R, Meier U, Yabut-Perez M, Walmrath D, Grimminger F, Seeger W, Gunther A (2001) Alteration of fatty acid profiles in different pulmonary surfactant phospholipids in acute respiratory distress syndrome and severe pneumonia. Am J Respir Crit Care Med 163:95–100
Khammash H, Perlman M, Wojtulewicz J, Dunn M (1993) Surfactant therapy in full-term neonates with severe respiratory failure. Pediatrics 92:135–139
Lam BC, Yeung CY, Fu KH, Wong KY, Chan FL, Tsoi NS (2000) Surfactant tracheobronchial lavage for the management of a rabbit model of meconium aspiration syndrome. Biol Neonate 78:129–138
Ikegami M, Jobe AH (2002) Injury responses to different surfactants in ventilated premature lamb lungs. Pediatr Res 51:689–695
Cummings JJ, Holm BA, Hudak ML, Hudak BB, Ferguson WH, Egan EA (1992) A controlled clinical comparison of four different surfactant preparations in surfactant-deficient preterm lambs. Am Rev Respir Dis 145:999–1004
Herting E, Rauprich P, Stichtenoth G, Walter G, Johansson J, Robertson B (2001) Resistance of different surfactant preparations to inactivation by meconium. Pediatr Res 50:44–49
Vayrynen O, Glumoff V, Hallman M (2002) Regulation of surfactant proteins by LPS and proinflammatory cytokines in fetal and newborn lung. Am J Physiol Lung Cell Mol Physiol 282:L803–L810
Cleary GM, Wiswell TE (1998) Meconium-stained amniotic fluid and the meconium aspiration syndrome. An update. Pediatr Clin North Am 45:511–529
Li YH, Brauner A, Jonsson B, Van der Ploeg I, Soder O, Holst M, Jensen JS, Lagercrantz H, Tullus K (2001) Inhibition of macrophage proinflammatory cytokine expression by steroids and recombinant IL-10. Biol Neonate 80:124–132
Jones CA, Cayabyab RG, Kwong KY, Stotts C, Wong B, Hamdan H, Minoo P, deLemos RA (1996) Undetectable interleukin (IL)-10 and persistent IL-8 expression early in hyaline membrane disease: a possible developmental basis for the predisposition to chronic lung inflammation in preterm newborns. Pediatr Res 39:966–975
De Dooy JJ, Mahieu LM, Van Bever HP (2001) The role of inflammation in the development of chronic lung disease in neonates. Eur J Pediatr 160:457–463
Shimotake TK, Zhou L, Hershenson MB, Schreiber MD (2003) Interleukin (IL)-1b Induces IL-8 Expression in Human Bronchial Epithelial Cells Via a Protein Kinase C (PKC)-d Dependent Pathway. Pediatr Res 53:402A
Nogee LM, Dunbar AEr, Wert SE, Askin F, Hamvas A, Whitsett JA (2002) A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med 344:573–579
Augusto LA, Synguelakis M, Johansson J, Pedron T, Girard R, Chaby R (2003) Interaction o pulmonary surfacant protein C with CD14 and lipopolysaccharide. Infect Immun 71:61–67
Schrama AJ, de Beaufort AJ, Sukul YR, Jansen SM, Poorthuis BJ, Berger HM (2001) Phospholipase A2 is present in meconium and inhibits the activity of pulmonary surfactant: an in vitro study. Acta Paediatr 90:412–416
Holopainen R, Aho H, Laine J, Peuravuori H, Soukka H, Kaapa P (1999) Human meconium has high phospholipase A2 activity and induces cellular injury and apoptosis in piglet lungs. Pediatr Res 46:626–632
Kakinuma R, Shimizu H, Ogawa Y (2002) Effect of meconium on the rate of in vitro subtype conversion of swine pulmonary surfactant. Eur J Pediatr 161:31–36
Davidson D, Drafta D, Wilkens BA (1995) Elevated urinary leukotriene E4 in chronic lung disease of extreme prematurity. Am J Respir Crit Care Med 151:841–845
Moya FR, Hoffman DR, Zhao B, Johnston JM (1993) Platelet-activating factor in surfactant preparations. Lancet 341:858–860
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00134-005-2655-8
Recombinant surfactant has been kindly provided by Altana Pharma, Constance, Germany. These results were presented in part at the meetings of the Society for Pediatric Research at Baltimore, Md., in 2002 and at Seattle, Wash., USA, in 2003.
Rights and permissions
About this article
Cite this article
Hilgendorff, A., Rawer, D., Doerner, M. et al. Synthetic and natural surfactant differentially modulate inflammation after meconium aspiration. Intensive Care Med 29, 2247–2254 (2003). https://doi.org/10.1007/s00134-003-1984-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-003-1984-8