Summary
Synopsis
Cefotaxime is well established as an effective and well tolerated antibacterial drug for 3 times daily parenteral treatment of a variety of moderate to severe infections in hospitalised patients. Its frequency of administration has recently been reassessed with a 12-hourly regimen.
Comparative studies in hospitalised patients with nosocomial or community-acquired lower respiratory tract infections, demonstrate the similar clinical and bacteriological efficacy of twice daily cefotaxime 1 or 2g and the same daily dose of ceftriaxone, usually administered once daily. Cefotaxime 2g twice daily was also similar in efficacy to ceftriaxone 2g once daily. Retrospective and post-marketing studies also reveal the similar efficacy of cefotaxime administered twice and 3 times daily, and pharmacoeconomic studies suggest that total direct costs of treatment with cefotaxime compared is similar to that with other third generation cephalosporins in currently used dosage regimens.
When administered twice daily, cefotaxime is, thus, an effective antibacterial agent for the treatment of hospitalised patients outside the intensive care unit with a variety of mild to moderate non-CNS infections caused by susceptible organisms. When appropriately administered twice daily there is potential to lower the cost of antibacterial treatment without compromising efficacy.
Antibacterial Activity
Recent studies involving large numbers of clinical isolates of Enterobacteriaceae from intensive care and haematology and/or oncology units in Europe and medical centres in the US confirm earlier data indicating that the antibacterial activity of cefotaxime is generally similar to that of ceftazidime and ceftriaxone, and superior to that of cefuroxime, against Enterobacteriaceae. Tested strains of both ampicillin-susceptible and -resistant Haemophilus influenzae are susceptible to low concentrations (≤0.07 mg/L) of cefotaxime, and 90% of Moraxella catarrhalis and Neiserria gonorrhoeae strains (both penicillin-susceptible and -resistant) were inhibited by cefotaxime ≤0.5 mg/L.
Cefotaxime is more active than ceftazidime against methicillin-susceptible Staphylococcus aureus [minimum inhibitory concentration (MIC) ≈ 4 mg/L], S. epidermidis and Streptococcus pneumoniae, and in most studies the minimum inhibitory cefotaxime concentration for 90% (MIC90) of S. pneumoniae was ≤0.25 mg/L. The susceptibility of viridans streptococci to cefotaxime varies, but MIC90 values were generally between 0.12 and 2 mg/L. The MIC90 values of cefotaxime for Bacteroides fragilis ranged from 1.56 to 32 mg/L.
The active metabolite of cefotaxime, desacetyl-cefotaxime, exhibits antibacterial activity that is generally less than that of the parent drug. There is evidence that desacetyl-cefotaxime may prolong the microbiological action of cefotaxime, particularly when both substances have inherent activity against an organism, and the synergistic interaction between cefotaxime and desacetyl-cefotaxime against some organisms is well documented. A combination of cefotaxime and desacetyl-cefotaxime exhibited synergy or partial synergy against the majority of tested susceptible Enterobacteriaceae and the majority of B. fragilis. These findings may explain in part the enhanced activity of cefotaxime in vivo compared with that expected from in vitro microbiological results.
Synergy between cefotaxime and aminoglycosides has been shown for some Enterobacteriaceae. Cefotaxime and ampicillin have shown synergism against Listeria monocytogenes and cefotaxime and amoxicillin against Enterococcus faecalis. Similarly, combinations of cefotaxime with fosfomycin showed synergy against S. pneumoniae in vitro, a finding which was confirmed in vivo using the fibrin clot model. Recent studies have documented the in vitro synergistic effect between cefotaxime and vancomycin or teicoplanin against resistant strains of S. pneumoniae. A combination of cefotaxime and ofloxacin was synergistic against the great majority of Enterobacteriaceae and Pseudomonas aeruginosa and all tested S. pneumoniae.
Epidemiological studies have generally indicated that the susceptibility to cefotaxime of Gram-negative aerobic bacteria, particularly Enterobacteriaceae, has remained relatively stable over a period of 3 to 5 years.
Pharmacokinetic Properties
Single-dose intravenous administration of cefotaxime 1 and 2g results in mean maximum plasma concentrations (Cmax) of 81 to 102 and 174 to 214 mg/L, respectively. Cmax values after intramuscular administration of cefotaxime are about one-quarter to one-third of those after intravenous administration of the same dose. The plasma concentration of cefotaxime exceeds that of desacetyl-cefotaxime for about 2 hours after single-dose intravenous administration; plasma concentrations 8 hours after a 30-minute infusion of cefotaxime 2g were 0.51 and 1.09 mg/L for cefotaxime and desacetyl-cefotaxime, respectively. Compared with healthy volunteers, plasma concentrations are somewhat higher in elderly patients with bacteraemia. After 12 hours, plasma desacetyl-cefotaxime concentrations were higher than those of cefotaxime.
Earlier studies have shown, and more recent studies confirmed, that cefotaxime concentrations shown to be inhibitory to susceptible bacteria in vitro are achieved in most body tissues following administration of usual therapeutic dosages. The mean plasma elimination half-life (t½β) of 0.82 to 1.43 hours for cefotaxime and 2.02 to 2.13 hours for desacetyl-cefotaxime in healthy volunteers is prolonged in preterm and low birth-weight neonates, in elderly patients [mean creatinine clearance of 42 ml/min (2.52 L/h)], in patients with renal impairment (creatinine clearance <10 ml/min) and in patients with cirrhosis and ascites or chronic parenchymal liver disease and jaundice.
The t½β of individual third generation cephalosporins has often been used as a principal determinant for selection of dose interval, and in some instances, of a particular drug within this class. However, determinants of effective antimicrobial therapy include many other pharmacokinetic, pharmacodynamic and clinical properties. The antibacterial efficacy of β-lactam antibiotics is time-rather than concentration-dependent, and because of the importance of MIC values, surrogate relationships that relate pharmacokinetic parameters to MIC have been studied. Included among these are the time that the plasma concentration of the drug exceeds the MIC (time > MIC), Cmax to MIC ratio and area under the inhibitory concentration-time curve (AUIC24). After treatment with cefotaxime there was a correlation between the time > MIC and the numbers of bacteria remaining in the lungs of mice with K. pneumoniae infection. Also, threshold values for AUIC24 defined in animal studies are achieved for a variety of bacteria following twice daily administration of cefotaxime to healthy volunteers. Thus, it appears feasible to administer cefotaxime 1 or 2g 12-hourly as monotherapy to adults for treatment of infection caused by bacteria with an MIC of ≤1 mg/L.
Therapeutic Efficacy
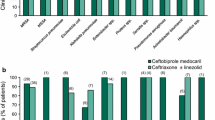
The efficacy of cefotaxime intravenous or intramuscular twice daily has been studied in hospitalised patients with a variety of infections. Studies comparing cefotaxime with other antimicrobial drugs randomly allocated patients to treatment groups, but typically, microbiological documentation was available in only a minority of patients. Few studies provided details of follow-up procedures or efficacy data at specified periods after the end of treatment. Most often the efficacy of cefotaxime was compared with that of ceftriaxone. In patients with nosocomial pneumonia, the efficacy of cefotaxime 2g twice daily was similar to that of ceftriaxone 2g twice daily, and cefotaxime 2g 3 times daily initially, followed by twice daily administration, was more clinically effective than routinely used antibacterial regimens.
When administered intravenously or intramuscularly in the treatment of patients with community-acquired pneumonia, bronchopneumonia or acute exacerbation of chronic bronchitis, the efficacy of cefotaxime 1g twice daily was similar to that of ceftriaxone 1g twice or once daily administered by the same route. At the higher dosage of 2g twice daily intravenously, cefotaxime had similar clinical efficacy to ceftriaxone 2g once daily administered by the same route and a short course of cefotaxime 2g twice daily followed by cefixime 400mg once daily orally. The bacteriological efficacy of cefotaxime and ceftriaxone was also generally similar.
Patients in an intensive care unit who developed serious lower respiratory tract infections responded similarly to treatment with cefotaxime 2g twice or 3 times daily.
Cefotaxime 2g twice daily had similar clinical and bacteriological efficacy to the same dosage regimen of aztreonam in patients with complicated and/or nosocomial urinary tract infection. In another study, the rate of eradication or presumed eradication of pathogens in patients with serious urinary tract infections treated with cefotaxime 2g twice daily was not significantly different compared with that with ceftriaxone 2g once daily.
Twice daily intravenous administration of cefotaxime 2g has also been shown to be at least as effective as ceftriaxone in the treatment of skin and soft tissue infections.
A postmarketing surveillance study in hospitalised patients (n=1636) revealed that clinical and bacteriological response rates were similar following treatment with cefotaxime 2g twice or 3 times daily. Similarly, retrospective analysis of results of treatment of bacteraemia, septicaemia, urogenital, intra-abdominal, CNS or lower respiratory tract infections with cefotaxime showed a clinical response rate in 97 and 96% of patients treated with cefotaxime 1 and 2g twice daily, respectively. Retrospective clinical evaluation of the same regimens in elderly Japanese patients with pneumonia indicated that clinical efficacy was not influenced by underlying hepatic, renal, haematological or neurological disease, but was decreased in patients with initially poor health status and in those with concomitant lung neoplasm.
Tolerability
The majority of adverse events associated with cefotaxime are mild and transient. There is no reliable indication of the relative incidence of adverse events during twice daily versus more frequent administration of cefotaxime. However, the tolerability profile appears similar with twice daily and more frequent regimens, with mild gastrointestinal complaints being the most common events, followed by pruritus, rash and phlebitis at the injection site. The incidence of adverse events in patients treated with a variety of regimens of cefotaxime was between 5 and 8%, with discontinuation necessary in only 1 to 2%.
In comparative studies, the incidence of adverse events was reported to be similar with cefotaxime and ceftriaxone, or in some instances greater with ceftriaxone, which caused a higher incidence of digestive adverse events.
Pharmacoeconomic Considerations
The relative costs of treatment with the third generation cephalosporins have been determined from known acquisition costs alone, from acquisition, preparation and administration costs, and from total direct costs including acquisition, preparation, monitoring and estimated costs arising from complications. Whether there are real cost differences between cefotaxime and ceftriaxone with respect to laboratory monitoring and adverse events is debatable. Comparative cost information from direct measurement of difference in disease course or outcome is not yet available. When assessed by each of these methods, the averaged cost of treatment with cefotaxime (mostly 3 times daily) has mostly been lower than that with ceftriaxone once or twice daily. Substitution of cefotaxime for ceftriaxone has also been associated with cost savings, although contrary findings have also been reported. There appears to be further potential for cost containment if cefotaxime is administered twice daily.
Dosage and Administration
When administered twice daily (12-hourly), the recommended dose of cefotaxime is usually 1 to 2g, intravenously or intramuscularly. Twice daily administration should be considered in the treatment of monomicrobial infections caused by susceptible organisms, in patients without neutropenia and not in an intensive care unit. Twice daily administration may also be considered in the treatment of moderately serious infection caused by susceptible organisms in elderly patients, who would be expected to eliminate the drug more slowly than younger patients.
It is not yet clear how long administration should be continued after patients become afebrile or have other evidence of bacterial elimination. Prudence and anecdotal experience suggest that administration for an additional 48 to 72 hours is appropriate.
Similar content being viewed by others
References
NationalCommittee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests. v. 14 No. 16. Villanova, Pennsylvania: National Committee for Clinical Laboratory Standards, 1994
Carmine AA, Brogden RN, Heel.RC, et al. Cefotaxime: a review of its antibacterial activity, pharmacological properties and therapeutic use. Drugs 1983; 25: 223–89
Todd PA, Brogden RN. Cefotaxime. An update of its pharmacology and therapeutic use. Drugs 1990 Oct; 40: 608–51
Amyes SGB, Baird DR, Crook DW, et al. A multicentre study of the in-vitro activity of cefotaxime, cefuroxime, ceftazidime, ofloxacin and ciprofloxacin against blood and urinary pathogens. J Antimicrob Chemother 1994 Nov; 34: 639–48
Ansorg R, Primavesi CA, von-Recklinghausen G. Inhibitory and bactericidal activity of cefpirome and cefotaxime against blood culture isolates. Chemotherapy 1990; 36: 24–8
Choi K-H, Kim D-J, Shim J-S, et al. In-vitro and in-vivo activity of DWC-751, a new cephalosporin. J Antimicrob Chemother 1994 Jun; 33: 1233–7
Fukasawa M, Sumita Y, Tada E, et al. In vitro antibacterial activity of meropenem [in Japanese]. Chemotherapy (Tokyo) 1992 Apr; 40 Suppl. 1: 74–89
Inoue E, Mitsuhashi S, Inoue M. In vitro antibacterial activity of cefclidin and interaction with beta-lactamases [in Japanese]. Chemotherapy (Tokyo) 1992 Sep; 40 Suppl. 4: 1–13
Jones RN, Pfaller MA, Allen SD, et al. Antimicrobial activity of cefpirome. An update compared to five third-generation cephalosporins against nearly 6000 recent clinical isolates from five medical centers. Diagn Microbiol Infect Dis 1991 Jul–Aug; 14: 361–4
Liu Y-C, Huang W-K, Cheng D-L. Antibacterial activity of cefepime in vitro. Chemotherapy (Basel) 1994 Nov–Dec; 40: 384–90
Maejima T, Inoue M, Mitsuhashi S. In vitro antibacterial activity of KP-736, a new cephem antibiotic. Antimicrob Agents Chemother 1991 Jan; 35: 104–10
Martínez-Beltrán J, Cantón R, Liñares J, et al. Multicentre comparative study on the antibacterial activity of FK-037, a new parenteral cephalosporin. Eur J Clin Microbiol Infect Dis 1995 Mar; 14: 244–52
Masuyoshi S, Mitsuhashi S, Inoue I, et al. In vitro and in vivo antibacterial activity and beta-lactamase stability of cefepime, a new parenteral cephalosporin [in Japanese]. Chemotherapy (Tokyo) 1991 Jun; 39 Suppl. 2: 1–14
Nakayama I, Yamaji E, Hirata H, et al. In vitro antibacterial activity of FK037, a new parenteral cephalosporin [in Japanese]. Chemotherapy (Tokyo) 1994 Oct; 42 Suppl. 3: 428–32
Sumita Y, Mitsuhashi S, Inoue M. In vitro antibacterial activity of meropenem, a new carbapenem antibiotic [in Japanese]. Chemotherapy (Tokyo) 1992 Apr; 40 Suppl. 1: 1–15
Verbist L. Epidemiology and sensitivity of 8625 ICU and hematology/oncology bacterial isolates in Europe. Scand J Infect Dis 1993; Suppl. 91: 14–24
Watanabe N-A, Hiruma R, Katsu K. In vitro evaluation of E1077, a new cephalosporin with a broad antibacterial spectrum. Antimicrob Agents Chemother 1992 Mar; 36: 589–97
Bauernfeind A, Schweighart S, Eberlein E, et al. In vitro activity and stability against novel beta-lactamases of investigational beta-lactams (cefepime, cefpirome, flomoxef, SCE 2787 and piperacillin plus tazobactam) in comparison with established compounds (cefotaxime, latamoxef and piperacillin). Infection 1991; 19 Suppl. 5: S264–75
Shah PM, Knothe H. The in vitro activity of flomoxef compared to four other cephalosporins and imipenem. Infection 1991; 19 Suppl. 5: S279–83
Toyosawa T, Miyazaki S, Tsuji A. In vitro and in vivo antibacterial activities of E1077, a novel parenteral cephalosporin. Antimicrob Agents Chemother 1993 Jan; 37: 60–6
Gu JW, Neu HC. In vitro activity of Ro 23-9424, a dual-action cephalosporin, compared with activities of other antibiotics. Antimicrob Agents Chemother 1990 Feb; 34: 189–95
Jones RN, Erwin ME, Barrett MS, et al. Antimicrobial activity of E-1040, a novel thiadiazolyl cephalosporin compared with other parenteral cephems. Diagn Microbiol Infect Dis 1991 Jul–Aug; 14: 301–9
Murphy SP, Erwin ME, Jones RN. Cefquinome (HR 111V): in vitro evaluation of a broad-spectrum cephalosporin indicated for infections in animals. Diagn Microbiol Infect Dis 1994 Sep; 20: 49–55
Neu HC, Gu J-W, Fang W. In vitro activity and beta-lactamase stability of LJC 10, 627. Antimicrob Agents Chemother 1992 Jul; 36: 1418–23
Kayser FH. In vitro activity of cefpodoxime in comparison with other oral beta-lactam antibiotics. Infection 1994 Sep–Oct; 22: 370–5
Stefani S, Pellegrino MB, D’Amico G, et al. In vitro activity of a new broad-spectrum, beta-lactamase-stable oral cephalosporin, cefixime, in comparison with other drugs, against Haemophilus influenzae, Haemophilus parainfluenzae, Moraxella catarrhalis and Streptococcal pneumoniae. Chemotherapy (Basel) 1992 Jan–Feb; 38: 36–45
Alós JI, Gómez-Garcés JL, Cogollos R, et al. Susceptibilities of ampicillin-resistant strains of Salmonella other than S. typhi to 10 antimicrobial agents. Antimicrob Agents Chemother 1992 Aug; 36: 1794–6
Lepage P, Bogaerts J, Van GC, et al. Multiresistant Salmonella typhimurium systemic infection in Rwanda. Clinical features and treatment with cefotaxime. J Antimicrob Chemother 1990 Sep; 26 Suppl. A: 53–7
Chantot JF, Klich M, Teutsch G, et al. Antibacterial activity of RU44790, a new N-tetrazolyl monocyclic beta-lactam. Antimicrob Agents Chemother 1992 Aug; 36: 1756–63
Wise R, Andrews JM. In vitro activities of two glycylcyclines. Antimicrob Agents Chemother 1994 May; 38: 1096–102
Blondeau JM, Yaschuk Y. In vitro activities of ciprofloxacin, cefotaxime, ceftriaxone, chloramphenicol and rifampicin against fully susceptible and moderately penicillin-resistant Neisseria meningitidis. Antimicrob Agents Chemother 1995; 39(11): 2577–9
Nakashio S, Susa C, Iwasawa H, et al. In vitro antimicrobial activity of SY5555, a new oral penem [in Japanese]. Chemotherapy (Tokyo) 1994 Apr; 42 Suppl. 1: 656–63
Nakashio S, Iwasawa H, Kanemitsu K, et al. Antimicrobial activity of piperacillin with tazobactam, a new beta-lactamase inhibitor, in vitro against recent clinical isolates [in Japanese]. Chemotherapy (Tokyo) 1994 Oct; 42 Suppl. 2: 385–95
Cormican MG, Jones RN. Antimicrobial activity of cefotaxime tested against infrequently isolated pathogenic species (unusual pathogens). Diagn Microbiol Infect Dis 1995 May–Jun; 22: 43–8
Klugman KP, Saunders J, Khoosal M. In-vitro activity of cefepime against bacterial pathogens from hospitalized patients. J Antimicrob Chemother 1993 Jul; 32: 164–6
Fujimoto T, Watanabe M, Inoue M. In-vitro antibacterial activity of DQ-2556 and its stability to various beta-lactamases. J Antimicrob Chemother 1990 Sep; 26: 329–41
Florez C, Perez MJ, Aretio R, et al. Susceptibility of Streptococcus pneumoniae to five antibiotics. J Antimicrob Chemother 1992 Nov; 30: 727–8
Scheid WM. Is there a place for carbapenems in the therapy of meningitis? Curr Opin Infect Dis 1994 Oct; 7 Suppl. 1: 33–7
Haas DW, Stratton CW, Griffin JP, et al. Diminished activity of ceftizoxime in comparison to cefotaxime and ceftriaxone against Streptococcus pneumoniae. Clin Infect Dis 1995 Mar; 20: 671–6
Jetté LP, Ringuette L, Dascal A, et al. Pneumococcal resistance to antimicrobial agents in the province of Québec, Canada. J Clin Microbiol 1994 Oct; 32: 2572–5
Spangler SK, Jacobs MR, Appelbaum PC. Susceptibilities of 177 penicillin-susceptible and -resistant pneumococci to FK-037, cefpirome, cefepime, ceftriaxone, cefotaxime, ceftazidime, imipenem, biapenem, meropenem, and vancomycin. Antimicrob Agents Chemother 1994 Apr; 38: 898–900
Lozniewski A, Dap G, Conroy MC, et al. Antibiotic susceptibility of Streptococcus pneumoniae strains isolated from respiratory tract infections in Nancy from 1990 to 1991. Med Mal Infect 1994 Jun–Jul; 24: 780–4
Barry AL, Brown SD, Fuchs PC. In vitro activity of ceftizoxime, ceftazidime, cefuroxime, ceftriaxone, cefotaxime and penicillin against Streptococcus pneumoniae as determined by three quantitative methods. Eur J Clin Microbiol Infect Dis 1996; 15: 344–6
Barry AL, Brown SD, Novick WJ. In virto activities of cefotaxime, ceftriaxone, ceftazidime, cefpirome and penicillin against Streptococcus pneumoniae isolates. Antimicrob Agents Chemother 1995; 39 (Oct): 2193–6
Waites KB, Swiatlo E, Gray BM. Comparative activities of parenteral cephalosporins against penicillin-resistant Streptococcus pneumoniae isolated from pediatric patients. Curr Ther Res 1996 July; 57(7): 489–96
Potgieter E, Carmichael M, Koornhof HJ, et al. In vitro antimicrobial susceptibility of viridans streptococci isolated from blood cultures. Eur J Clin Microbiol Infect Dis 1992 Jun; 11: 543–9
Aldridge KE, Gelfand M, Relier LB, et al. A five-year multi-center study of the susceptibility of the Bacteroides fragilis group isolates to cephalosporins, cephamins, penicillins, clindamycin, and metronidazole in the United States. Diagn Microbiol Infect Dis 1994 Apr; 18: 235–41
Croco JL, Erwin ME, Jennings JM, et al. Evaluation of the Etest for antimicrobial spectrum and potency determinations of anaerobes associated with bacterial vaginosis and peritonitis. Diagn Microbiol Infect Dis 1994 Dec; 20: 213–9
Kato N, Sawa K, Muto Y, et al. Antibacterial activity of a new parenteral cephem, cefclidin, against anaerobic bacteria and Gardnerella vaginalis. Chemotherapy (Tokyo) 1992 Sep; 40 Suppl. 4: 31–8
Mikamo H, Izumi K, Ito K, et al. In vitro antibacterial activity of FK037, a new parenteral broad-spectrum cephalosporin, against recent clinical isolates in the fields of obstetrics and gynecology. Chemotherapy (Basel) 1994 May–Jun; 40: 161–6
Quintiliani R, Nightingale CH, Tilton R. Comparative pharmacokinetics of cefotaxime and ceftizoxime and the role of desacetylcefotaxime in the antibacterial activity of cefotaxime. Diagn Microbiol Infect Dis 1984; 2: 63S-70S
Jones RN. Cefotaxime and desacetylcefotaxime antimicrobial interactions: the clinical relevance of enhanced activity, a review. Diagn Microbiol Infect Dis 1995 May–Jun; 22: 19–34
Nanetti A, La PM. In-vitro comparison of the activity of cefotaxime and desacetylated cefotaxime and of their combination. J Chemother 1990 Jun; 2: 159–263
Molinari G, Saverino D, Paglia P, et al. Synergistic antibacterial interaction of cefotaxime and desacetylcefotaxime. J Chemother 1991 Feb; 3: 6–12
Canawati HN. A reassessment of the activity of the 3rd-generation cephalosporins against anaerobes and Staphylococcusaureus. Am J Surg 1992 Oct; 164 Suppl.: 24–7
Stratton CW, Kernodle DS, Eades SC. Evaluation of cefotaxime alone and in combination with desacetylcefotaxime against strains of 1Staphylococcus aureus that produce variants of staphylococcal β-lactamase. Diagn Microbiol Infect Dis 1989; 12: 57–65
Reller LB. Interaction of cefotaxime and desacetylcefotaxime against pathogenic bacteria: assessment with the serum bactericidal test. Diagn Microbiol Infect Dis 1984; 2 Suppl. 2: 55–61
Stratton CW, Aldridge KE, Gelfand MS. In vitro killing of penicillin-susceptible, -intermediate, and -resistant strains of Streptococcus pneumoniae by cefotaxime, ceftriaxone and ceftizoxime: a comparison of bactericidal and inhibitory activity with achievable CSF levels. Diagn Microbiol Infect Dis 1995 May–Jun; 22: 35–42
Lemmen S, Kropec A, Engels I, et al. Serum bactericidal activity after administration of four cephalosporins in healthy volunteers. Eur J Clin Microbiol Infect Dis 1993 Nov; 12: 856–60
Deeter RG, Weinstein MP, Swanson KA, et al. Crossover assessment of serum bactericidal activity and pharmacokinetics of five broad-spectrum cephalosporins in the elderly. Anti-microb Agents Chemother 1990 Jun; 34: 1007–13
Chin N-X, Neu HC. Cefotaxime and desacteylcefotamime: an example of advantageous antimicrobial metabolism. Diagn Microbiol Infect Dis 1984; 2 suppl.: 215–315
Schrinner E, Limbert M, Seeger K, et al. The in vitro antimicrobial activity of desacetylcefotaxime compared to other related β-lactams. Diagn Microbiol Infect Dis 1984; 2 Suppl.: 13–20
Amicosante G, Perilli M, Felici A, et al. CTX and its desacetyl derivative (DES-CTX): interaction with some representative beta-lactamases and their related pattern of resistance to newer selected clinical isolates. Drugs Exp Clin Res 1990; 16(11): 549–56
Bauernfeind A, Grimm H, Schweighart S. A new plasmidic cefotaximase in a clinical isolate of Escherichia coli. Infection 1990 Sep–Oct; 18: 294–8
Franceschini N, Galleni M, Frere JM, et al. A class-A betalactamase from Pseudomonas-stutzeri that is highly active against monobactams and cefotaxime. Biochem J 1993 Jun 15; 292: 697–700
Hupková M, Blahová J, Babálová M, et al. Transferable resistance to cefotaxime in nosocomial Klebsiella pneumoniae and Escherichia coli strains due to their production of extended-spectrum beta-lactamase in Slovakia. J Chemother 1995 Feb; 7: 16–20
Payne DJ, Amyes SGB. Stability of cefdinir (Cl-983, FK482) to extended-spectrum plasmid-mediated beta-lactamases. J Med Microbiol 1993 Feb; 38: 114–7
Podbielski A, Schonling J, Melzer B, et al. Molecular characterization of a new plasmid-encoded SHV-type betalactamase (SHV-2 variant) conferring high-level cefotaxime resistance upon Klebsiella pneumoniae. J Gen Microbiol 1991 Mar; 137 (Pt 3): 569–78
Portier H, Kazmierczak A, Lucht F, et al. Cefotaxime in combination with other antibiotics for the treatment of severe methicillin-resistant staphylococci infections. Infection 1985; 13 Suppl. 1: 123–8
Murray PR. Activity of cefotaxime-aminoglycoside combinations against aminoglycoside-resistant pseudomonads. Anti-microb Agents Chemother 1980; 17: 474–6
Hoogkamp-Korstanje JAA. Activity of cefotaxime and ceftriaxone alone and in combination with penicillin, ampicillin and piperacillin against neonatal meningitis pathogens. J Antimicrob Chemother 1985; 16: 327–34
Mainardi JL, Goldstein FW, Acar JF, et al. Synergistic effect of amoxicillin and cefotaxime against Enterococcus faecalis. Antimicrob Agents Chemother 1995; 39(9): 1984–7
Barakett V, Lesage D, Delisle F, et al. Synergy of cefotaxime and fosfomycin against penicillin-resistant pneumococci. J Antimicrob Chemother 1993 Jan; 31: 105–9
Bajaksouzian S, Visalli MA, Jacobs MR, et al. Antipneumococcal activities of cefpirome and cefotaxime, alone and in combination with vancomycin and teicoplanin, determined by checkerboard and time-kill methods. Antimicrob Agents Chemother 1996; 40(9): 1973–6
Kerr JR. In vitro activities of two drug combinations of ceftazidime, cefotaxime, cefuroxime, ciprofloxacin, chloramphenicol, imipenem and temocillin against clinical isolates of Pseudomonas cepacia from patients with cystic fibrosis. Int J Antimicrob Agents 1993 Dec; 3: 205–9
Jones RN, Johnson DM, Barrett MS, et al. Antimicrobial activity of isepamicin (SCH21420, 1-N-HAPA gentamicin B) combinations with cefotaxime, ceftazidime, ceftriaxone, ciprofloxacin, imipenem, mezlocillin and piperacillin tested against gentamicin-resistant and susceptible Gram-negative bacilli and enterococci. J Chemother 1991 Oct; 3: 289–94
Bauernfeind A, Grimm H, Klietmann W, et al. The influence of sulbactam on the in vitro activity of mezlocillin, pipericillin and cefotaxime. Int J Antimicrob Agents 1996; 6: S15–26
Schmalreck AF, Wildfeuer A. In vitro activity against clinically important Gram-positive and Gram-negative bacteria of sulbactam, alone and in combination with ampicillin, cefotaxime, mezocillin, and piperacillin. Arzneimittel Forschung 1990 Oct; 40: 1145–55
Cormican MG, Erwin ME, Jones RN. Bactericidal activity of cefotaxime, desacetylcefotaxime, rifampin, and various combinations tested at cerebrospinal fluid levels against penicillin-resistant Streptococcus pneumoniae. Diagn Microbiol Infect Dis 1995 May–Jun; 22: 119–24
Jenkins SG, Lewis JW. Synergistic interaction between ofloxacin and cefotaxime against common clinical pathogens. Infection 1995; 23(3): 154–61
Friedland IR, McCracken GH. Management of infections caused by antibiotic resistant Streptococcus pneumoniae. N Engl J Med 1994; 331(6): 377–82
Baquero F, Loza E, Morosini MI, et al. Beta-lactam-resistant Streptococcus pneumoniae: new bacterial pathogens? Eur Resp Rev 1994 Sep; 4 Rev. 22: 316–20
Jones RN. The antimicrobial activity of cefotaxime: comparative multinational hospital isolate surveys covering 15 years. Infection 1994; 22 Suppl. 3: 152–60
Rodríguez-Noriega E, Morfin-Otero R, Atilano-Duran G, et al. Bacterial resistance to antimicrobial agents in Mexico. Drug Invest 1992; 4 Suppl. 2: 2–8
Wiedemann B, Dietz H. Cefotaxime - unchanged antibacterial activity over years? Diagn Microbiol Infect Dis 1995 May–Jun; 22: 5–12
Parry MF. Aztreonam susceptibility testing. A retrospective analysis. Am J Med 1990 Mar 23; 88: 7S-11S (discussion 38S-42S)
Sirot DL, Goldstein FW, Soussy CJ, et al. Resistance to cefotaxime and seven other beta-lactams in members of the family Enterobacteriaceae: a 3-year survey in France. Antimicrob Agents Chemother 1992 Aug; 36: 1677–81
Fdez AA, Alonso R, Colom K, et al. Multicenter study of cefotaxime resistance -1993 [in Spanish]. Rev Esp Quimioter 1994 Mar; 7: 57–61
Alonso R, Fdez-Aránguiz A, Colom K, et al. Multicenter study of the cefotaxime resistance and detection-characterization of extended broad spectrum beta-lactamases [in Spanish]. Rev Esp Quimioter 1992 Dec; 5: 301–6
Colom K, Fdz-aranguiz A, Alonso R, et al. Five-year survey of cefotaxime resistance in Spain. Microbial Drug Resistance 1995; 1(4): 327–30
Fuchs PC, Barry AL, Brown SD, et al. Survey of antimicrobial activity of four commonly used third generation cephalosporins tested against recent bacterial isolates from ten American medical centers, and assessment of disk diffusion test performance. Diagn Microbiol Infect Dis 1996; 24: 213–9
Bryan CS, John JF, Pai MS, et al. Gentamicin versus cefotaxime for therapy of neonatal sepsis. Am J Dis Child 1985; 139: 1086–9
Füssle R, Biscoping J, Behr R, et al. Development of resistance by Enterobacter cloacae during therapy of pulmonary infections in intensive care patients. Clin Investig 1994 Dec; 72: 1015–9
Chow JW, Fine MJ, Shlaes DM, et al. Enterobacter bacteremia: clinical features and emergence of resistance during therapy. Ann Intern Med 1991; 115: 585–90
Jones RN. The current and future impact of antimicrobial resistance among nosocomial pathogens. Diagn Microbiol Infect Dis 1992; 15: 3–10
Craig WA. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad spectrum cephalosporins. Diagn Microbiol Infect Dis 1995 May–Jun; 22): 89–96
Chavanet P, Beloeil H, Pechinot A, et al. In vivo activity and pharmacokinetics of cefotaxime or ceftriaxone in combination with fosfomycin in fibrin clots infected with highly penicillin-resistant Streptococcus pneumoniae. Antimicrob Agents Chemother 1995; 39(8): 1736–43
Ho I, Aswapokee P, Fu KP, et al. Pharmacokinetic parameters of cefotaxime following intravenous and intramuscular administration of single and multiple doses. In: Nelson, Grassi, editors. Current Chemotherapy and Infectious Diseases. Washington, DC: American Society for Microbiology, 1980: 116–8
Lüthy R, Blaser J, Bonetti A, et al. Human pharmacokinetics of ceftazidime in comparison to moxalactam and cefotaxime. J Antimicrob Chemother 1981; 8 Suppl. B: 273–6
Lüthy R, Blaseer J, Bonetti A, et al. Comparative multi-dose pharmacokinetics of cefotaxime, moxalactam and ceftazidime. Antimicrob Agents Chemother 1981; 20: 567–75
McKendrick MW, Geddes AM, Wise R. Clinical experience with cefotaxime. In: Nelson, Grassi, editors. Current Chemotherapy and Infectious Disease. Washington, DC: American Society for Microbiology, 1980: 123–5
Fu KP, Aswapokee P, Ho I, et al. Pharmacokinetics of cefotaxime. Antimicrob Agents Chemother 1979; 16: 592–7
Neu HC, Aswapokee P, Fu KP, et al. Cefotaxime kinetics after intravenous and intramuscular injection of single and multiple doses. Clin Pharmacol Ther 1980; 27: 677–85
Ings RMJ, Fillastre J-P, Godin M, et al. The pharmacokinetics of cefotaxime and its metabolites in subjects with normal and impaired renal function. Rev Infect Dis 1982; 4 Sept.: 379–91
Ohkawa M, Kuroda K. A double-blind clinical trial of cefotaxime and cefazolin in complicated urinary tract infections. J Antimicrob Chemother 1980; 6 Suppl. A: 231–3
Bouchet JL, Aparicio M, Vincon G, et al. Pharmacokinetic considerations for treatment of bacterial peritonitis during continuous ambulatory peritoneal dialysis. Contrib Nephrol 1991; 89: 96–107
Albin HC, Demotes-Mainard FM, Bouchet JL, et al. Pharmacokinetics of intravenous and intraperitoneal cefotaxime in chronic ambulatory peritoneal dialysis. Clin Pharmacol Ther 1985; 38: 285–9
Hasegawa H, Imada A, Horiuchi A, et al. Pharmacokinetics of cefotaxime in patinets undergoing haemodialysis and continuous ambulatory peritoneal dialysis. J Antimicrob Chemother 1984; 14 Suppl. B: 135–42
Heim KL, Halstenson CE, Comty CM, et al. Disposition of cefotaxime and desacetylcefotaxime during continuous ambullatory peritoneal dialysis. Antimicrob Agents Chemother 1986; 30: 15–9
Vallee F, LeBel M. Comparative study of pharmacokinetics and serum bactericidal activity of ceftizoxime and cefotaxime. Antimicrob Agents Chemother 1991 Oct; 35: 2057–64
Jonsson M, Walder M. Pharmacokinetics of intravenous antibiotics in acutely ill elderly patients. Eur J Clin Microbiol Infect Dis 1986; 5: 629–33
Goodpasture HC, Gerlach EH, Jones RN, et al. Optimal cefotaxime dosing for Gram-negative bacteremia: effective trough serum bactericidal titer and drug concentrations 8 and 12 hr after 1 or 2G infusions. Diagn Microbiol Infect Dis 1989; 12: 101–5
Matsumoto T, Yoshida T, Ohura T, et al. Pharmacokinetic and clinical studies on cefotaxime in plastic and reconstructive surgery. Jpn J Antibiot 1987; 40: 311–24
Shyu WC, Quintiliani R, Nightingale CH, et al. Effect of protein binding on drug penetration into blister fluid. Antimicrob Agents Chemother 1988; 32: 128–30
Ueda T, Sakai K, Fujimoto M. Studies of cefotaxime serum concentrations during surgery under general anaesthesia and its passage into the wound fluid after surgery for breast cancer. Infection 1985; 13 Suppl. 1: 43–5
Runyon BA, Akriviadis EA, Sattler FR, et al. Ascitic fluid and serum cefotaxime levels in patients treated for spontaneous bacterial peritonitis [abstract]. Hepatology 1988; 8: 1246
Just H-M, Bassler M, Frank V, et al. Pentration of cefotaxime into heart valves, subcutaneous and muscle tissue of patients undergoing open-heart surgery. J Antimicrob Chemother 1984; 14: 431–4
Robbs JV, Kharsany A. Serum and tissue concentrations of intravenous cefotaxime during aortic surgery. J Antimicrob Chemother 1984; 14 Suppl. B: 113–6
Shio K, Matsuda S, Kusumoto C, et al. Concentrations of cefotaxime in serum and myocardial tissue. Jpn J Antibiot 1984; 37: 1035–9
Hägele D, Frühwirth O, Strehl E, et al. Konzentrationsbestimmung eines antibiotikums (Cefotaxime) in der vaginalhaut nach präopertaiver intravenoser Gabe. Geburtschilfe und Frauenheilkunde 1983; 43: 217–9
Adam D, Naber KG. Concentrations of ceftriaxone in prostate adenoma tissue. Chemotherapy (Basel) 1984; 30: 1–6
Sjolin J, Erikksson N, Arneborn P, et al. Penetration of cefotaxime and desacetylcefotaxime into brain abscesses in humans. Antimicrob Agents Chemother 1991 Dec; 35: 2606–10
Wittmann DH. Chemotherapeutic principles of difficult to treat infections in surgery: II. Bone and joint infections. Infection 1980; 8: 330–3
Endo F, Matsui N, Watanabe T, et al. Penetration of cefotaxime into the human bone marrow blood. Jpn J Antibiot 1986; 39: 1273–8
Iwamori H, Adachi N. Studies on transfer of cefotaxime into bone marrow blood. Jpn J Antibiot 1986; 39: 733–8
Sakurai M, Shibuya S, Miyagishima J, et al. Concentration of cefotaxime and its metabolite in the human bone marrow blood. Jpn J Antibiot 1986; 39: 739–45
Loos U, Essers L. Bronchopulmonary disposition of cefotaxime [abstract]. Eur Respir J 1990 Sep; 3 Suppl. 10: 88
Fick RB, Alexander MR, Prince RA, et al. Penetration of cefotaxime into respiratory secretions. Antimicrob Agents Chemother 1987; 31: 815–7
Matsuoka T, Ota H, Takeda J. Basic and clinical studies of cefotaxime. Jpn J Antibiot 1986; 39: 726–32
Petrikkos G, Androulakis M, Goumas P, et al. A comparative study of cefoxitin, cefotaxime, maxalactam and aztreonam kinetics in saliva. Chemioterapia 1987; 6: 355–8
Scaglione F, Raichi M, Fraschini F. Serum protein binding and extravascular diffusion of methoxyimino cephalosporins. Time courses of free and total concentrations of cefotaxime and ceftriaxone in serum and pleural exudate. J Antimicrob Chemother 1990 Sep; 26 Suppl. A: 1–10
Hary L, Andrejak M, Leleu S, et al. The pharmacokinetics of ceftriaxone and cefotaxime in cirrhotic patients with ascites. Eur J Clin Pharmacol 1989; 36: 613–6
Vincent P, Colombel JF, Husson HO, et al. Pharmacocinétique du céfotaxime chez malades cirrhotiques avec ou sans ascite. Presse Med 1988; 17: 2331–4
Runyon BA, Akriviadis EA, Sattler FR. Ascitic fluid and serum cefotaxime and desacetyl cefotaxime levels in patients treated for bacterial peritonitis. Dig Dis Sci 1991 Dec; 36: 1782–6
Peterson J, Stewart RDM, Catto GRD, et al. Pharmacokinetics of intraperitoneal cefotaxime treatment of peritonitis in patients on ambulatory peritoneal dialysis. Nephron 1985; 40: 79–82
Bald M, Rascher W, Bonzel KE, et al. Pharmacokinetics of intraperitoneal cefotaxime in children with peritonitis undergoing continuous ambulatory peritoneal dialysis. Perit Dial Int 1990; 10(4): 311–3
Asmar BI, Thirumoorthi MC, Buckley JA, et al. Cefotaxime diffusion into cerebrospinal fluid of children with meningitis. Antimicrob Agents Chemother 1985; 28: 138–40
Bègué P, Floret D, Mallet E, et al. Pharmacokinetics and clinical evaluation of cefotaxime in children suffering with purulent meningitis. J Antimicrob Chemother 1984; 14 Suppl. B: 161–5
Beyssac E, Cardot J-M, Colnet G, et al. Pharmacokinetics of cefotaxime and its desacetyl metabolite in plasma and cerebrospinal fluid. Eur J Drug Metab Pharmacokinet 1987; 12: 91–102
Brückner O, Collmann H, Borner K. Cefotaxime levels in ventricular cerebrospinal fluid, determined by bioassay and by high performance liquid chromatography. Chemotherapy (Basel) 1983; 29: 237–43
Feldstein TJ, Uden DL, Larson TA. Cefotaxime for treatment of bacterial meningitis in infants and children. Pediatr Infect Dis J 1987; 6: 471–5
Humbert G, Leroy A, Nair S, et al. Concentrations of cefotaxime and the desacetyl metabolite in serum and CSF of patients with meningitis. J Antimicrob Chemother 1984; 13: 487–94
Peretti P, Sueri L, Tosi M, et al. Cefotaxime in the cerebrospinal fliud and serum of patinets with purulent meningitis. J Antimicrob Chemother 1984; 14 Suppl. B: 117–23
Trang JM, Jacobs RF, Kearns GL, et al. Cefotaxime and desacetylcefotaxime pharmacokinetics in infants and children with meningitis. Antimicrob Agents Chemother 1985; 28: 791–5
Nau R, Prange HW, Muth P, et al. Passage of cefotaxime and ceftriaxone into cerebrospinal fluid of patients with uninflamed meninges. Antimicrob Agents Chemother 1993 Jul; 37: 1518–24
Hashira S, Koike Y, Fujii R. Fundamental and clinical investigations of cefotaxime in neonates. Jpn J Antibiot 1982; 35: 1737–48
von Hattingberg HM, Marget W, Belohradsky BH, et al. Pharmacokinetics of cefotaxime in neonates and children: clinical aspects. J Antimicrob Chemother 1980; 6 Suppl. A: 113–8
Heimann G, Eickschen M, Seeger K. Pharmakokinetik von Cefotaxime im Kindesalter. Infection 1980; 8 Suppl. 4: 454–9
Kafetzis DA, Brater DC, Kapiki AN, et al. Treatment of severe neonatal infections with cefotaxime. Efficacy and pharmacokinetics. J Pediatr 1982; 100: 483–9
Kobayashi Y, Haruta T, Okura K, et al. Evaluation of cefotaxime in treatment of infections in newborns. Jpn J Antibiot 1982; 35: 1801–15
McCracken GH, Threlkeld NE, Thomas ML. Pharmacokinetics of cefotaxime in newborn infants. Antimicrob Agents Chemother 1982; 21: 683–4
Aujard Y, Brion F, Jacqz-Aigrain E, et al. Pharmacokinetics of cefotaxime and desacetylcefotaxime in the newborn. Diagn Microbiol Infect Dis 1989; 12: 87–91
Kearns GL, Young RA. Pharmacokinetics of cefotaxime and desascetylcefotaxime in the young. Diagn Microbiol Infect Dis 1995 May–Jun; 22: 97–104
Wise R, Wright N, Wills PJ. Pharmacology of cefotaxime and its desacetyl metabolite in renal and hepatic disease. Antimicrob Agents Chemother 1981; 19: 526–31
Vos MC, Vincent HH, Yzerman EPF, et al. Drug clearance by continuous haemodiafiltration: results with the AN-69 capillary haemofilter and recommended dose adjustment for seven antibiotics. Drug Invest 1994 Jun; 7: 315–22
Höffken G, Lode H, Koeppe P, et al. Pharmacokinetics of cefotaxime and desacetylcefotaxime in cirrhosis of the liver. Chemotherapy (Basel) 1984; 30: 7–17
Ko RJ, Sattler FR, Nichols S, et al. Pharmacokinetics of cefotaxime and desacetylcefotaxime in patients with liver disease. Antimicrob Agents Chemother 1991 Jul; 35: 1376–80
Kuse E, Vogt P, Rosenkranz B. Pharmacokinetics of cefotaxime in patients after liver transplantation. Infection 1990 Sep–Oct; 18: 268–72
Burckart GJ, Ptachcinski RJ, Jones DH, et al. Impaired clearance of ceftizoxime and cefotaxime after orthotopic liver transplantation. Antimicrob Agents Chemother 1987; 31: 323–4
Kearns GL, Young RA, Jacobs RF. Cefotaxime dosage in infants and children. Pharmacokinetic and clinical rationale for an extended dosage interval. Clin Pharmacokinet 1992 Apr; 22: 284–97
Turnidge JD. Pharmacodynamic (kinetic) considerations in the treatment of moderately severe infections with cefotaxime. Diagn Microbiol Infect Dis 1995 May–Jun; 22: 57–70
Nix DE, Schentag JJ. Role of pharmacokinetics and pharmacodynamics in the design of dosage shedules for q12h cefotaxime alone, and in combination with other antibiotics. Diagn Microbiol Infect Dis 1995 May–Jun; 22: 71–6
Fremaux A, Sissia G, Geslin P. In vitro post antibiotic effect of cefuroxime, and three other beta-lactams against penicillin susceptible and resistant Streptococcus pneumoniae [abstract]. 34th Interscience Conference on Antimicrobial Agents and Chemotherapy, American Society for Microbiology, 1994 Oct 4–7, Orlando, Florida.
Nicolau DP, Patel KB, Quintiliani R, et al. Cephalosporin-metronidazole combinations in the management of intra-abdominal infections. Diagn Microbiol Infect Dis 1995 May–Jun; 22: 189–94
Sullivan MC, Nightingale CH, Quintiliani R, et al. Comparison of the pharmacodynamic activity of cefotaxime plus metronidazole with cefoxitin and ampicillin plus sulbactam. Pharmacotherapy 1995; 15(4): 479–86
Wittmann DH. The role of cefotaxime in the treatment of surgical infections. Diagn Microbiol Infect Dis 1995; 22 (May–June): 173–82
Doern GV. The in vitro activity of cefotaxime versus bacteria involved in selected infections of hospitalised patients outside of the intensive care unit. Diagn Microbiol Infect Dis 1995 May–Jun; 22: 13–8
Jones RN. Summation. Diagn Microbiol Infect Dis 1995; 22: 243–7
Brunch HP, Kujath P. Cefotaxime twice daily for the treatment of post-operative pneumonia. 19th International Congress of Chemotherapy, 1995 July 16–21,Montreal.
Holzheimer RG, Jendrissek A, Kaufmann T, et al. Sucessful cefotaxime treatment with a daily dose of 2 X 2g in nosocomial pneumonia of surgical patients [Abstract 1215]. Can J Infect Dis 1995; 6 (July) Suppl. C: 330C
Keller C. Prospective evaluation of twice daily cefotaxime in the treatment of hospitalised patients with severe infections. Diagn Microbiol Infect Dis 1995 May–Jun; 22: 159–62
Bruch H-P, Kujath P, and the German Cefotaxime Study Group. Study of cefotaxime twice daily for the therapy of postoperative pneumonia. Diagn Microbiol Infect Dis 1995 May–Jun; 22: 203–8
Dansey RD, Jacka PJ, Strachan SA, et al. Comparison of cefotaxime with ceftriaxone given intramuscularly 12-hourly for community-acquired pneumonia. Diagn Microbiol Infect Dis 1992 Jan; 15: 81–4
Dolmann AL, Bataglia A, De Luca M, et al. Cefotaxime (IG, administered twice daily) and ceftriaxone (IG, administered once daily) in the treatment of community-acquired pneumonia in hospitalised patients [Abstract 3106]. Can J Infect Dis 1995; 6 (July) Suppl. C: 408C
Fernandez-Guerrero M, Gudiol F, Rodriguez-Torres A, et al. Nosocomial pneumonia: comparative multicentre trial between monotherapy with cefotaxime and treatment with antibiotic combinations. Infection 1991; 19 Suppl 6: S320–5
Gris P, Deman R, Van Schil L, et al. A randomised, multinational study comparing cefotaxime and ceftriaxone in lower respiratory tract infections in hospitalised patients [Abstract 3118]. Can J Infect Dis 1995; 6 (July) Suppl. C: 410C
Maesen FP, Davies BI, van-den-Bergh JJ, et al. Cefodizime and cefotaxime in acute exacerbations of chronic bronchitis: a randomized double-blind prospective study in 180 patients. J Antimicrob Chemother 1990 Mar; 25: 413–22
Philips MJ, Hart G, Frith PA, et al. Intravenous third-generation cephalosporins are effective and safe in the treatment of non intensive care unit-acquired lower respiratory tract infections. 19th International Congress of Chemotherapy, 1995 July 16–21, Montreal.
Shah PM, Stille W. Cefotaxime versus ceftriaxone for the treatment of nosocomial pneumonia: Results of a multicenter study. Diagn Microbiol Infect Dis 1995 May–Jun; 22: 171–2
Simmons BP, Gelfand MS, Grogan J, et al. Cefotaxime twice daily versus ceftriaxone once daily: a randomised controlled study in patients with serious infections. Diagn Microbiol Infect Dis 1995 May–Jun; 22: 155–8
Vogel F. Treatment of lower respiratory tract infections including pneumonia. Comparative study with i.vl cefotaxime/oral cefixime versus parenteral cefotaxime [in German]. Fortschr Med 1994 Oct 10; 112: 395–8
Cade JF, Presneill J, Keighley C. Efficacy of a low dose of cefotaxime in serious chest infections. Chest 1992 May; 101: 1393–8
Naber KG, Dette GA, Kees F, et al. Pharmacokinetics, in vitro activity, therapeutic efficacy and clinical safety of aztreonam vs. cefotaxime in the treatment of complicated urinary tract infections. J Antimicrob Chemother 1986; 17: 517–27
Sangaret AM, German P, Djanhan Y, et al. Cefotaxime in caesarian sections in patients with chorioamniotitis. J Antimicrob Chemother 1984; 14 Suppl. B: 285–9
Bakkaloglu A, Saatci U, Soylemezoglu O, et al. Comparison of ceftriaxone versus cefotaxime for childhood upper urinary tract infections. J Chemother 1996; 8(1): 59–62
Young LS. Review and reassessment of dosing schedules for cefotaxime in selected medical indications. Diagn Microbiol Infect Dis 1995 May–Jun; 22: 147–54
Bergquist SO, Eriksson L, Eriksson S, et al. Retrospective analysis of the efficacy of cefotaxime sodium dosed twice daily: The Swedish experience. Diagn Microbiol Infect Dis 1995 May–Jun; 22: 163–6
Inamatsu T. Treatment of pneumonia in the elderly with cefotaxime monotherapy: clinical evaluation. Eur Resp Rev 1994 Sep; 4 Rev. 22: 329–32
Cadranel JF, Bismuth E, Attali P, et al. Cefotaxime-related hypoprothrombinaemia in a patient with acute panccreatitis: a case with a possible clue to the pathogenesis. Am J Gastroenterol 1989; 84: 686–8
Vertolli U, Vinci C, Naso A. Hypoprothrombinemia and cephalosporins in uremia. Part II. Nephron 1995 Jan; 69: 111
Danan G, Erlinger S. Hypoprothrombinemia and cefotaxime [letter]. Am J Gastroenterol 1990 Jun; 85: 763–4
Cadranel JF, Attali P, Bismuth E, et al. Hypoprothrombinemia and cefotaxime. [letter]. Am J Gastroenterol 1990 Jun; 85: 763–4
Salama A, Goettsche B, Scheiffer T, et al. Immune complexmediated intravascular hemolysis due to IgM cephalosporin dependent antibody. Transfusion 1987; 27: 460–3
Shulman IA, Arndt PA, McGehee W, et al. Cefotaxime-induced immune hemolytic anemia due to antibodies reacting in vitro by more than one mechanism. Transfusion 1990 Mar–Apr; 30: 263–6
Funada H, Ishizaki T, Kuroda T, et al. Cefotaxime-associated diarrhoea and Clostridium difficile. Jpn J Antibiot 1984; 37: 555–7
Nolan N, Tighe B, Cooney C, et al. Cefotaxime and pseudomembranous colitis. Lancet 1985; 2: 888
Impallomeni M, Galletly NP, Wort SJ, et al. Increased risk of diarrhoea caused by Clostridium difficile in elderly patients receiving cefotaxime. BMJ 1995 November 18; 311: 1345–6
Lesna M, Parkham DM. Mortality due to C. difficile colitis in elderly people has been underestimated [Letter]. BMJ 1996 March 23; 312:778
Rothschild E, Rauss A, Danan G. Risk of diarrhoea due to Clostridium difficile during cefotaxime treatment [Letter]. BMJ 1996 March 23; 312: 778
Lode H. Cefotaxime for the 1990’s. Diagn Microbiol Infect Dis 1995
Taugourdeau MC, Star PEG. Taking a positive approach in cost management: STAR, a cost-containment computerized system. Eur Resp Rev 1994 Sep; 4 Rev. 22: 336–42
Lee J, Carlson JA, Chamberlain MA. A team approach to hospital formulary replacement. Diagn Microbiol Infect Dis 1995 May–Jun; 22: 239–42
Rawlings RD. Therapeutic exchange of cefotaxime for ceftriaxone: evaluation, implementation, and subsequent cost savings at a 300-bed community hospital. Diagn Microbiol Infect Dis 1995 May–Jun; 22: 235–8
Lee CKK, Glen DJ. Cefotaxime and ceftriaxone use evaluation in pediatrics: considerations of cost effectiveness. Diagn Microbiol Infect Dis 1995 May–Jun; 22: 231–4
Roark MK, Reed WE. Econotherapeutics. Diagn Microbiol Infect Dis 1995 May–Jun; 22: 209–18
Vander Linde JB, Stein GE, Malburg DE. A drug use evaluation and cost analysis of the thrid generation cephalosporins. PT 1995 Apr: 198–211
Gladen HE. Evaluating the cost-effectiveness of treatment with third-generation cephalosporins. Diagn Microbiol Infect Dis 1992 Jan; 15: 99–105
Burke JP, Pestotnik SL, Classen DC, et al. A retrospective analysis of twice daily cefotaxime compared to conventional therapy for the treatment of infections in a USA hospital. Diagn Microbiol Infect Dis 1995 May–Jun; 22: 167–70
Smyth ETM, Barr JG, O’Neill CA, et al. An assessment of the hidden and total antibiotic costs of four parenteral cephalosporins. PharmacoEconomics 1995 December; 8(6): 541–50
Plosker GL, Benfield P. Cefotaxime: a pharmacoeconomic evaluation of its use in serious infections. PharmacoEconomics In press; 11 (1)
Gutensohn A, Bunz D, Frighetto L, et al. Outcome of a ceftriaxone/cefotaxime interchange programme in a major teaching hospital. Chemotherapy (Basel) 1991 Jun; 37 Suppl. 3: 15–21
Ramirez JA. Switch therapy in community-acquired pneumonia. Diagn Microbiol Infect Dis 1995 May–Jun; 22: 219–24
Morales JO, Snead H. Efficacy and safety of intravenous cefotaxime for treating pneumonia in outpatients. Am J Med 1994 Aug 15; 97 Suppl. 2A: 28–33
Williams DN. Reducing costs and hospital stay for pneumonia with home intravenous cefotaxime treatment: results with a computerized ambulatory drug delivery system. Am J Med 1994 Aug 15; 97 Suppl. 2A: 50–5
Williams DN, Bosch D, Boots J. Safety, efficacy, and cost savings in an outpatient intravenous antibiotic program. Clin Ther 1993 Jan–Feb; 15: 169–79
Szof C, Walker PC. Incompatibility of cefotaxime sodium and vancomycin sulfate during Y-site administration. Am J Hosp Pharm 1993 Oct; 50: 2054–7
Lewis JD, El-Gendy A. Cephalosporin-pentamidine isethionate incompatibilities. American Journal of Health-System Pharmacy 1996 June 15; 53: 1461–2
Dajani AS. Cefotaxime use in pediatric infections. Diagn Microbiol Infect Dis 1995 May–Jun; 22: 105–10
Leggiadro RJ, Barrett FF, Chesney PJ, et al. Invasive pneumococci with high level penicillin and cephalosporin reistance at a mid-south children’s hospital. Pediatr Infect Dis J 1994 Apr; 13: 320–2
Schliamser SE, Cars O, Norrby, SR. Neurotoxicity of betalactam antibiotics: predisposing factors and pathogenesis. J Antimicrob Chemother 1991 Apr; 27: 405–25
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: W.R. Bowie, Division of Infectious Diseases, University of British Columbia, Vancouver, British Columbia, Canada; R.G. Finch, Department of Microbial Diseases, The City Hospital, Nottingham, England; D.H.E. Gladen, Valley Medical Center, Fresno, California, USA; R.N. Jones, Department of Pathology, University of Iowa College of Medicine, Iowa City, Iowa, USA; U, Kumazawa, Faculty of Medicine, Kyushu University, Fukuoka, Japan; S.W.B Newsom, Departments of Geriatrics and Bacteriology, Papworth Hospital, Cambridge, England; A. Rimola, Liver Unit, Hospital Clinic i Provincial, Villarroel, Barcelona, Spain.
Rights and permissions
About this article
Cite this article
Brogden, R.N., Spencer, C.M. Cefotaxime. Drugs 53, 483–510 (1997). https://doi.org/10.2165/00003495-199753030-00009
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199753030-00009