Abstract
Ceftobiprole, the active metabolite of the prodrug ceftobiprole medocaril (Zevtera®), is a new generation broad-spectrum intravenous cephalosporin with activity against methicillin-resistant Staphylococcus aureus. Ceftobiprole exhibits potent in vitro activity against a number of Gram-positive and Gram-negative pathogens associated with hospital-acquired pneumonia (HAP) and community-acquired pneumonia (CAP). It is the first cephalosporin monotherapy approved in the EU for the treatment of both HAP (excluding ventilator associated-pneumonia [VAP]) and CAP. In phase III trials, ceftobiprole medocaril was noninferior, in terms of clinical cure rates at the test-of-cure visit, to ceftazidime plus linezolid in patients with HAP and to ceftriaxone ± linezolid in patients with CAP severe enough to require hospitalization. In patients with HAP, noninferiority of ceftobiprole medocaril to ceftazidime plus linezolid was not demonstrated in a subset of patients with VAP. In patients with CAP, ceftobiprole medocaril was effective in those at risk for poor outcomes (pneumonia severity index ≥91, Pneumonia Patient Outcomes Research Team score IV–V or bacteraemic pneumonia). In the phase III trials, ceftobiprole medocaril was generally well tolerated, with ≈10 % of patients discontinuing the treatment because of adverse events. The most common treatment-related adverse events occurring in ceftobiprole recipients in the trials in patients with HAP or CAP included nausea, diarrhoea, infusion site reactions, vomiting, hepatic enzyme elevations and hyponatraemia. Therefore, ceftobiprole medocaril monotherapy offers a simplified option for the initial empirical treatment of patients with HAP (excluding VAP) and in those with CAP requiring hospitalization.
Similar content being viewed by others
Intravenously administered new generation broad-spectrum cephalosporin, with activity against methicillin-resistant Staphylococcus aureus (MRSA) |
First anti-MRSA cephalosporin monotherapy to be approved in the EU for both HAP (excluding ventilator-associated pneumonia) and CAP |
Noninferior to ceftazidime plus linezolid in patients with HAP (excluding patients with ventilator-associated pneumonia) and ceftriaxone ± linezolid in those with CAP |
Generally well tolerated |
Offers simplified monotherapy option relative to combination therapies for initial empirical treatment |
1 Introduction
Hospital-acquired pneumonia (HAP) [nosocomial pneumonia], which includes ventilator-associated pneumonia (VAP), is one of the most common hospital-acquired infections, accounting for one-quarter of all infections in intensive care units (ICUs) [1–3]. In a European multicentre observational study, of 827 patients with HAP admitted to ICUs, 27.1, 56.2 and 16.7 % of patients had HAP (non-VAP), VAP and very early onset VAP, respectively [1]. Community-acquired pneumonia (CAP) is a common infectious disease worldwide. For example, in the UK, the annual incidence of CAP is 5–11 cases per 1,000 adult patients and 22–42 % of these patients are admitted to hospital, with 1.2–10 % of the hospitalized patients being managed in an ICU [4]. The incidence of CAP dramatically increases with increasing age [4, 5]. Both HAP [2, 3] and CAP [4, 5] are associated with significant morbidity, mortality and treatment cost.
The most common bacteria causing HAP are Enterobacteriaceae (such as Klebsiella spp., Enterobacter spp., Serratia spp.), Staphylococcus aureus, Pseudomonas aeruginosa and Acinetobacter baumannii [1, 6], and the most common bacteria causing CAP are Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis [5]. Polymicrobial infection was reported in approximately one-third of patients with HAP in a survey of European ICUs [1] and in 11 % of patients with CAP admitted to the ICU in a Spanish observational study [7]. Pathogens that are resistant to antibacterials, particularly methicillin-resistant S. aureus (MRSA) and multi-drug-resistant S. pneumoniae (MDRSP), are associated with poor outcomes and higher treatment cost [3, 5].
According to the European guidelines, initial empirical treatment options for HAP [6] are penicillins/β-lactamase inhibitor, cephalosporins (cefuroxime, cefotaxime, ceftriaxone, ceftazidime), carbapenems (imipenem, meropenem) and fluoroquinolones (levofloxacin, moxifloxacin, ciprofloxacin); the options for CAP [8] are penicillins/β-lactamase inhibitor ± a macrolide, cephalosporins ± a macrolide, or fluoroquinolones. Selection of antibacterials should be based on whether the onset of HAP is early or late [6] and risk factors for specific pathogens, such as P. aeruginosa, Acinetobacter spp. and MRSA [6, 8]. If MRSA is suspected or identified, vancomycin, linezolid or teicoplanin ± rifampin should be used [6, 8]. There is no single agent that provides coverage against both MRSA and multidrug-resistant pneumococci. Thus there is a need for such agents.
Ceftobiprole medocaril (Zevtera®), an intravenously administered water soluble prodrug of ceftobiprole, is a new generation broad-spectrum cephalosporin with anti-MRSA activity. Currently, it is the first anti-MRSA cephalosporin to receive approval (in the EU) for both hospital (excluding VAP)- and community-acquired pneumonia [9, 10]. This review focuses on the pharmacological properties of ceftobiprole, and its clinical efficacy and tolerability in adult patients with hospital- and community-acquired pneumonia, as approved in the EU. The efficacy of ceftobiprole in complicated skin and soft tissue infections (cSSTI) has been reviewed elsewhere [11].
2 Antibacterial Activity
2.1 Mechanism of Action
Ceftobiprole medocaril is a member of the pyrrolidinone-3-ylidenemethyl cephem series of cephalosporins (Fig. 1) [12, 13]. As with β-lactam antibacterial agents in general, ceftobiprole exerts its antibacterial activity by binding to important penicillin-binding proteins (PBPs) and inhibiting their transpeptidase activity [13], which is essential for the synthesis of the peptidoglycan layer of bacterial cell walls. Ceftobiprole binds to multiple PBPs in clinically relevant pathogens, which provides its broad activity spectrum [13–17].
The anti-MRSA activity of ceftobiprole is attributed to its rapid and tight binding to the mutant PBP2a form (encoded by the mecA gene) that confers methicillin-resistance [13]. Ceftobiprole showed high affinity for PBP2a from various MRSA strains, with half maximal inhibitory concentrations (IC50) ranging from 0.31 to 0.9 mg/L [15, 17]. In the MRSA OC 3726 strain, ceftobiprole IC50 value for PBP2a was 0.9 mg/L, compared with >50 mg/L for ceftriaxone or ceftazidime [15]. Ceftobiprole retains its activity against strains that express divergent mecA gene homologues (mecC or mecALGA251) [9].
In S. pneumoniae strains, alterations in PBP-1a, -2x and -2 are known to confer resistance to β-lactam antibacterials, and ceftobiprole shows different degrees of affinity for these PBPs [15]. In a penicillin-resistant strain of S. pneumoniae OC 8819, compared with ceftriaxone, ceftobiprole exhibited a lower affinity for PBP1a (IC50 0.1 vs. 0.02 mg/L) and a greater affinity for PBP2x (1 vs. 8 mg/L), with both agents showing a similar low affinity for PBP2b (>8 mg/L) [15]. In a penicillin-susceptible strain of S. pneumoniae OC 8865, ceftobiprole IC50 values for PBP-1a, -2x and -2b were 0.03, 0.01 and 0.06 mg/L, respectively, compared with 0.01, 0.03 and >1 mg/L for ceftriaxone [15]. The binding profile of ceftobiprole to wild-type or mutated PBP2b appeared more like penicillin than cephalosporins, such as ceftriaxone [14].
Ceftobiprole binds to the low-affinity PBP5 that confers penicillin resistance in enterococci [16]. In a laboratory-derived penicillin-resistant strain of Enterococcus faecium, IC50 values for unmutated PBP5 were 1.4 mg/L for ceftobiprole compared with 8 mg/L for benzylpenicillin and >200 mg/L for other cephalosporins (cefepime and ceftazidime) [16].
Ceftobiprole had strong affinity for important PBPs in Gram-negative bacteria [15]. For example, ceftobiprole IC50 values for PBP2 and PBP3 were 0.2 mg/L for both in the Escherichia coli MC4100 strain, and 3 and 0.1 mg/L in the P. aeruginosa PAO1 strain. In both strains, the affinity of ceftobiprole for PBP3 was generally similar to that of other cephalosporins (ceftriaxone and/or ceftazidime, and cefepime). However, the affinity of ceftobiprole for PBP2 was 20-fold higher in the E. coli strain and >10-fold higher in the P. aeruginosa strain, compared with ceftazidime. In the latter strain, the affinity of ceftobiprole for PBP2 was also 2.7-fold higher than that of cefepime. Ceftobiprole did not show high affinity for PBP5 in E. coli MC4100, or PBP-5 or -6 in P. aeruginosa PAO1 (IC50 >8 and >32 mg/L, respectively) [15].
2.2 In Vitro Activity
A review published in 2008 [11] summarized the in vitro activity of ceftobiprole against clinically relevant pathogens collected from around the world. This section primarily focuses on the in vitro activity of ceftobiprole against clinical isolates of Gram-positive and Gram-negative bacteria collected from Europe between 2005 and 2011 [18–33], including data from the SENTRY antimicrobial surveillance programme [18, 31] and the Ceftobiprole Local Antibiotic Susceptibility Surveillance (CLASS) study conducted in Europe and the Middle East [21, 26, 30]. Some data are available as abstracts [19–25, 30] or posters [32, 33] only. The main focus of this section is the pathogens associated with HAP or CAP that are mentioned in the UK summary of product characteristics (SPC) [representative SPC for the decentralized procedure in the EU] for ceftobiprole [9].
The in vitro activity of the active metabolite, ceftobiprole, was assessed using the minimum inhibitory concentration (MIC) required to inhibit the growth of 90 % (MIC90) of the target bacterial isolates. MICs were determined by the Clinical and Laboratory Standards Institute (CLSI) broth microdilution method in all studies, with the exception of the CLASS study which used the Etest method. As ceftobiprole is approved only in the EU, the European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoints are the most relevant for interpreting the susceptibility. According to the UK SPC [9], the EUCAST breakpoints indicating susceptibility to ceftobiprole are ≤2 mg/L for S. aureus, including MRSA, ≤0.5 mg/L for S. pneumoniae and ≤0.25 mg/L for Enterobacteriaceae. Based on the pharmacokinetic/pharmacodynamic target for Gram-negative organisms, a non-species specific susceptibility breakpoint of ≤4 mg/L is indicated for ceftobiprole. There is insufficient evidence to establish a susceptibility breakpoint for P. aeruginosa [9].
2.2.1 Gram-Positive Aerobic Bacteria
The in vitro activity of ceftobiprole and comparator agents against selected Gram-positive bacteria is summarized in Table 1. All susceptibility rates reported are based on the EUCAST breakpoints.
Ceftobiprole showed good activity against S. aureus, including MRSA and methicillin-susceptible S. aureus (MSSA) [MIC90 2 and 0.5 mg/L, respectively] and coagulase-negative staphylococci (CoNS), including methicillin-resistant CoNS (MR-CoNS) and methicillin-susceptible CoNS (MS-CoNS) [Table 1]. In the SENTRY programme, 4,147 of 15,426 (26.9 %) S. aureus clinical isolates collected from Europe, Turkey and Israel during 2005–2010 were MRSA, and 98.3 % of these strains were susceptible to ceftobiprole [31].
Ceftobiprole showed activity against S. aureus strains that were not susceptible to linezolid, daptomycin or vancomycin (MIC90 2 mg/L against strains not susceptible to linezolid [n = 21] or daptomycin [n = 32], vancomycin-intermediate S. aureus [VISA; n = 12] and heterogeneous VISA [hVISA; n = 32], and 1 mg/L against vancomycin-resistant S. aureus [VRSA; n = 10]); all strains were 100 % susceptible to ceftobiprole [34].
Ceftobiprole MIC90 values ranged from 1 to 4 mg/L in S. aureus strains with different staphylococcal cassette chromosome (SCC)mec and/or multilocus sequence types [28, 34]. MIC90 values were 4, 2, 2 and 1 mg/L against laboratory strains with SCCmec types I, II, III, and IV, respectively; ceftobiprole susceptibility was 71.4 % for type I and 100 % for all other types [34]. Among hospital- or community-acquired MRSA (n = 100 and 16) and hVISA (n = 12) clinical isolates collected from Italian medical centers during 2007–2008, MIC90 values were as follows: 4 mg/L against hospital-acquired MRSA with sequence types (ST)-SCCmec types ST247-IA, ST247-I/IA, ST239-IIIA or ST228-I, and hVISA with ST8/247/239/228; 2 mg/L against community-acquired MRSA with ST5/8/30/80-IV or ST88-V; and 1 mg/L against hospital-acquired MRSA with ST8-I or ST22-IV [28].
Ceftobiprole displayed potent in vitro activity against S. pneumoniae and β-haemolytic or viridans group streptococci (Table 1). Among S. pneumoniae clinical isolates, ceftobiprole showed similar potent in vitro activity against penicillin-resistant and penicillin-susceptible strains (susceptibility 100 % for both; Table 1).
Among Enterococcus spp., ceftobiprole is active against E. faecalis, but not against E. faecium (Table 1). Overall, the in vitro activity of ceftobiprole against the major groups of Gram-positive bacterial pathogens appears to be similar to that of linezolid, teicoplanin and vancomycin (Table 1).
2.2.2 Gram-Negative Aerobic Bacteria
The activity of ceftobiprole and comparator agents against selected Gram-negative aerobic bacteria is summarized in Table 2. Susceptibility rates are based on the EUCAST breakpoints where available or CLSI breakpoints otherwise.
Ceftobiprole showed very good in vitro activity against Gram-negative fastidious respiratory pathogens such as H. influenzae and M. catarrhalis (Table 2). Ceftobiprole retains activity against H. influenzae strains that produce β-lactamase (MIC90 0.06 mg/L for both β-lactamase-positive and -negative strains; n = 156 and 867, respectively) [24]. In 71 H. influenzae isolates collected from French teaching hospitals, ceftobiprole showed a similar activity against ampicillin-susceptible and -nonsusceptible isolates (MIC90 0.12 mg/L for both) [35].
In SENTRY [31], 83.4 % of 17,480 Enterobacteriaceae isolates collected from Europe, Turkey and Israel during 2005–2010 were susceptible to ceftobiprole based on the EUCAST breakpoint of ≤0.25 mg/L, and 12.7% of isolates had a MIC value of ≥8 mg/L. Among 3,594 Enterobacteriaceae isolates of European origin, ceftobiprole showed potent in vitro activity against ceftazidime-susceptible strains but not against ceftazidime-nonsusceptible strains (MIC90 0.12 and >32 mg/L, respectively); in ceftazidime-susceptible strains, cefepime and ceftriaxone had MIC90 values of 0.12 and 0.5 mg/L, respectively [29].
As with cefepime, ceftazidime and ceftriaxone, ceftobiprole shows in vitro activity against E. coli, Klebsiella pneumoniae and Proteus mirabilis strains that do not produce extended spectrum β-lactamase (ESBL) [ESBL-negative], but is generally inactive against ESBL-producing (ESBL-positive) strains of these organisms (Table 2). Ceftobiprole and cefepime are generally active against Citrobacter and Enterobacter spp., although these agents were inactive against Citrobacter and Enterobacter cloacae strains that overproduce AmpC β-lactamase (i.e. derepressed AmpC mutants); in contrast, ceftazidime and ceftriaxone are inactive against these organisms, irrespective of AmpC mutant status (Table 2). Like cefepime and ceftazidime, ceftobiprole is active against Serratia spp., including Serratia marcescens, with good activity for all three agents seen only against ceftazidime-susceptible strains (Table 2).
Among nonfermentative Gram-negative bacteria, the in vitro activity of ceftobiprole against P. aeruginosa was somewhat similar to that of cefepime and ceftazidime, and ceftobiprole had limited activity against Acinetobacter spp. (Table 2).
2.2.3 Anaerobic Bacteria
Among anaerobic bacteria, ceftobiprole is generally active against Clostridium spp. and Fusobacterium spp. (MIC90 ≤8 mg/L) but inactive against Bacteroides spp., Prevotella spp. and Veillonella spp. (MIC90 >128 mg/L) [11].
2.2.4 Bacteria Causing Atypical Pneumonia
In vitro data indicate that the following pathogens are not susceptible to ceftobiprole: Chlamydophila pneumoniae, Burkholderia cepacia complex, Mycoplasma pneumoniae, Mycobacterium spp., Norcardia spp. and Stenotrophomonas maltophilia [9].
2.2.5 Bactericidal and Post-Antibiotic Effect
The ratio of minimum bactericidal concentration (MBC) to MIC (MBC90/MIC90 or MBC/MIC) for ceftobiprole against MRSA ranged from 1 to 4 [28, 36–39]. Ceftobiprole was bactericidal (≥3 log10 CFU/mL reduction from the initial inoculum) at 0.5–4 × MIC against all or most of MRSA clinical isolates tested, including community- and hospital-acquired isolates [28, 36–38]. The tested strains included hVISA [28], VISA and VRSA [38], and MR-CoNS [36]. Ceftobiprole was bacteriostatic against some multi-drug resistant strains, including hVISA [28]. Against a VISA isolate, bactericidal activity was noted at MIC, but not at higher concentrations [36]. In one in vitro study, a paradoxical bactericidal effect (“Eagle effect”) was observed in 90 % of the tested strains, where after an initial killing at 1 or 2 × MIC of ceftobiprole, growth of the pathogen population increased at higher concentrations, followed by further killing; the reason for this effect was not clear [28].
In an in vitro pharmacodynamic/pharmacokinetic model, human-simulated regimen of ceftobiprole (500 mg every 8 h) was bactericidal against MSSA, community- and healthcare-associated MRSA, VISA and VRSA strains, whereas a human-simulated regimen of vancomycin (1 g every 12 h) was bacteriostatic against MSSA, MRSA and VISA and showed no activity against VRSA [40].
Ceftobiprole was effective in preventing the intracellular growth of MRSA in THP-1 macrophages and keratinocytes, whereas cefoxitin, ceftriaxone, cephalexin, cefuroxime were less effective or ineffective in these cells [41]. The increased intracellular activity of ceftobiprole appears, at least in part, to be because of its relatively greater ability to bind with PBP2a at acidic pH conditions, compared with the other cephalosporins [41].
Ceftobiprole was bactericidal against 10 of 12 S. pneumoniae clinical isolates, including strains that were resistant to penicillin, macrolides and/or fluoroquinolone (at 2 × MIC) [42], 2 of 2 β-lactamase-positive and 2 of 2 vancomycin-resistant E. faecalis isolates (at 2–8 × MIC) [43], 10 of 10 H. influenzae isolates (4 β-lactamase-positive, 2 β-lactamase-negative, 2 β-lactamase-positive amoxicillin clavulanate-resistant and 2 β-lactamase-negative ampicillin-resistant strains) [at 2 × MIC] and 1 of 2 β-lactamase-positive M. catarrhalis strains (at 4 × MIC) [44]. Against P. aeruginosa, ceftobiprole 4 mg/L (0.5–2 × MIC for six tested strains) decreased viable bacterial counts by 1.5–2 log10 CFU/mL at 6 h, but a subsequent increase in the count relative to the initial inoculum was observed at 24 h for all six strains [45]. Based on MIC90 and MBC90 values, the bactericidal activity of ceftobiprole was similar to that of cefepime and ceftriaxone against H. influenzae, K. pneumoniae and E. cloacae (ESBL-negative) and that of cefepime against P. aeruginosa [46].
In vitro, ceftobiprole showed a modest post-antibiotic effect against Gram-positive organisms: 1.4–3.1, 0–1.8 and 0–0.9 h against pneumococci, staphylococci and enterococci, respectively [47]. In a neutropenic mouse model of thigh infection, escalating doses of ceftobiprole produced post-antibiotic effects of 3.8–4.8 h against a MRSA strain and 0–0.8 h against a penicillin-resistant S. pneumoniae (PRSP) strain [48].
2.2.6 Synergy Studies
Several studies have investigated the synergy between ceftobiprole and other antibacterial agents [45, 49–54], with some of these studies available only as abstracts [51–53]. Ceftobiprole showed synergistic activity in combination with vancomycin against MRSA and glycopeptide-intermediate S. aureus, both in vitro [51] and in vivo [49], and against VISA in vivo [50]. The combination was indifferent against MSSA in vitro [51]. Ceftobiprole has also demonstrated in vitro synergistic activity in combination with daptomycin (against daptomycin-nonsusceptible MRSA isolates with various resistance phenotypes [53] and against 4 of 6 daptomycin-susceptible and -resistant vancomycin-resistant enterococci [52]), plazomicin (against 17 of 47 MRSA strains with various resistance phenotypes) and amikacin or levofloxacin (against P. aeruginosa [45]). The synergistic effect of adding ceftobiprole to any antibacterial agent has not been assessed in clinical studies.
2.3 In Vivo Activity
In murine pneumonia [55–58] or thigh infection [48] models (see also Sect. 2.5), ceftobiprole has demonstrated bactericidal effects against S. aureus (including MRSA) [48, 55, 56], S. pneumoniae (including MDRSP) [48, 58], E. coli and P. aeruginosa [48], K. pneumoniae and E. cloacae [48, 57], and H. influenzae [57].
Subcutaneous ceftobiprole treatment was associated with clearance of S. pneumoniae (penicillin-, and/or ceftriaxone- and cefotaxime-resistant strains) from lungs and blood in a murine acute pneumonia model [58]. At 39 h post challenge, ceftobiprole-treated mice had four orders of magnitude lower lung titers than that of untreated control mice and at the end of the treatment, pneumococci was not detected in lung or blood in mice treated with ceftobiprole [58].
Subcutaneous ceftobiprole was as effective as intramuscular cefepime or intraperitoneal ceftriaxone in reducing lung titers of H. influenzae and ESBL-negative E. cloacae and K. pneumoniae in a murine pneumonia model (p < 0.05 vs. untreated mice for all three drugs and no significant difference between the drugs); none of the three cephalosporins were effective against ESBL-positive K. pneumoniae [57].
Subcutaneous ceftobiprole did not promote the growth of Clostridium difficile or production of C. difficile toxin in mice cecal content samples; whereas, subcutaneous ceftazidime, ceftriaxone, cefoxitin or cefotaxime all significantly (p ≤ 0.01 vs. saline control) promoted the growth of C. difficile, with the toxin detected in ≥75 % of the samples [59].
2.4 Resistance Development
Single- and multi-step resistance selection studies suggest that ceftobiprole has low potential for selection of resistance among Gram-positive and -negative bacteria [13, 36, 42, 44]. In a serial passage study performed with high inocula of an MRSA strain (S. aureus 745), exposure to ceftobiprole increased MIC only by twofold and the resistance was not sustained after re-isolation [13]. In another study [36], resistance selection with ceftobiprole was performed with ten staphylococcal strains, including MSSA, MRSA, VRSA, VISA, MS-CoNS and MR-CoNS. After 50 serial passages at subinhibitory concentrations, ceftobiprole selected seven clones with a MIC increase of fourfold and three clones with a MIC increase of ≤2-fold; a single clone displayed the maximum increase in MIC of 8 mg/L [36]. Similar results were reported in serial passage studies performed with ten strains of S. pneumoniae (MIC increased twofold with five strains) [42], eight strains of H. influenzae (MIC increased ≈4-fold with two strains and ≈2-fold with one strain) and two strains of M. catarrhalis (no change in MIC) [44]. Single-passage studies performed with staphylococcal strains [36], S. pneumoniae [42], H. influenzae and M. catarrhalis [44] show that resistant mutation frequencies were low with ceftobiprole.
A lack of emergence of resistance after ceftobiprole treatment was also demonstrated in isolates of S. pneumoniae [58], H. influenzae, E. cloacae and ESBL-negative K. pneumoniae [57] collected from murine models of pneumonia. While no increase in MIC was noted for S. pneumoniae [58], the difference between pre- and post-treatment MIC values were within one dilution step for other pathogens [57].
As with other cephalosporins, the potential mechanisms of resistance to ceftobiprole include inactivation of the drug by bacterial β-lactamases [60], mutations in the mecA gene which encodes PBP2a [61] and overexpression of the mexXY efflux system [62].
An enzyme kinetics study conducted with purified bacterial β-lactamases showed that ceftobiprole was stable to hydrolysis by staphylococcal PC1 penicillinase, and Ambler Class A (TEM-1) and Class C (AmpC) β-lactamases produced by Gram-negative bacteria [60]. Ceftobiprole was labile to hydrolysis by the Class A enzymes (serine carbapenemases [KPC-2, SME-3], broad-spectrum β-lactamases [SHV-1] and ESBLs [CTX-M-15, K1, TEM-26]) produced by Enterobacteriaceae; however, the hydrolytic stability did not always correspond with MIC values (≤0.5 mg/L for strains that produce SME-3, TEM-26 and SHV-1). Ceftobiprole was also labile to hydrolysis by the Class B metallo-β-lactamases (IMP-1, VIM-2) and Class D β-lactamases (OXA-10) produced by P. aeruginosa. Ceftobiprole, cefepime and ceftazidime were hydrolyzed slowly by AmpC β-lactamases produced by Enterobacteriaceae, with slight differences seen in activities of the enzymes from different species/strains. In general, ceftobiprole and cefepime had lower MIC values than ceftazidime against Enterobacteriaceae strains that produce AmpC β-lactamases [60].
Ceftobiprole is inactive against P. aeruginosa and Acinetobacter strains that overexpress chromosomal AmpC β-lactamases (i.e. AmpC-derepressed mutants) [9]. The predominant mechanism of resistance in single-step P. aeruginosa mutants selected by ceftobiprole appeared to be increased transcription of the mexXY RNA, not upregulation of AmpC [62].
2.5 Pharmacokinetic/Pharmacodynamic Considerations
As with other cephalosporins, the pharmacodynamic index that best correlates with the antimicrobial efficacy of ceftobiprole is the proportion of dosing interval that the serum concentration of free drug exceeds the MIC (%fT>MIC) [63].
Ceftobiprole exposure targets required for bacterial killing in human studies were derived from animal studies in neutropenic, leucopenic or immunocompetent murine models of staphylococcal pneumonia [55, 56], acute pneumococcal pneumonia [58], pneumonia induced with Gram-negative pathogens [57] and thigh infection [48, 64]. For S. aureus, including community- and hospital-acquired MRSA strains (MIC 0.25–2 mg/L), fT>MIC values were: 8.8–25.4 % for bacterial stasis; 13.5–19.8 % for 1 log10 CFU/mL reduction from baseline; 23–39.1 % for 2 log10 CFU/mL reduction from baseline [48, 55, 56, 64]. The corresponding values for S. pneumoniae (MIC 0.03–1 mg/L), including penicillin-resistant strains, were: 8.4–22.2, 13.8–18.4 and 18.4–31.8 % [48, 64]. In the acute pneumonia model induced with four S. pneumoniae strains (MIC 0.008–1 mg/L), T>MIC values (presumed to be the % of dosing interval the total drug concentration was above the MIC) ranged from 9 to 18 % for ceftobiprole [CFU reduction target not stated] [58]. For Gram-negative pathogens, fT>MIC values for bacterial stasis and 2 log10 CFU/mL reduction from baseline were: 41.9 and 57.8 % for E. coli (MIC 0.06 mg/L); 41.2 and 59.2 % for K. pneumoniae (MIC 0.06 mg/L); 35.6–44.6 and 40.9–100 % for E. cloacae (MIC 0.5–2.0 mg/L); 46.7 and 98.8 % for P. aeruginosa (MIC 2.0 mg/L) [48]. For ESBL-negative E. cloacae (MIC ≤0.125 mg/L) and ESBL-negative K. pneumoniae (MIC 0.5 mg/L), fT>MIC values for 1 log10 CFU/mL reduction from baseline were 44.3 and 35.2 %, respectively [12, 57].
A pharmacokinetic/pharmacodynamic study hypothesized that a rational ceftobiprole dose choice against MRSA in humans can be derived based on the proportion of dosing interval that the epithelial lining fluid (ELF) concentration of total drug exceeds the MIC (%T>MIC ELF) [55]. In a murine model of staphylococcal pneumonia, ceftobiprole %T>MIC ELF values for 1 log10 and 2 log10 CFU/g reduction in S. aureus in lung tissue were 12.9 and 24 %, respectively. These data were then extrapolated to humans using concentration–time profile of ceftobiprole in human ELF. Based on these calculations, following 500 mg every 8 h infused over 2 h, probabilities of achieving %T>MIC ELF target in humans were modelled to be 85.6 % for 1 log10 CFU/mL reduction and 79.7 % for 2 log10 CFU/mL reduction in S. aureus. These target rates were obtained by taking ceftobiprole MIC distribution for MRSA into account [55]. It must be noted that a correlation between ELF concentration and clinical efficacy is not empirically established for any antibacterial agent.
The probability of attaining fT>MIC targets with various dosing regimens of ceftobiprole in humans was assessed using population pharmacokinetic modelling and Monte Carlo simulations [65, 66]. An initial set of simulations was performed using data from a multiple ascending dose study (n = 12) [66]. With ceftobiprole 500 mg every 8 h infused over 30 min, the probability of attaining 40, 50 and 60 % fT>MIC targets was 100, 99 and 79 %, respectively, for MIC values up to 4 mg/L [66]. Thus a 500 mg every 8 h regimen was considered optimal to provide coverage against Gram-positive bacteria with MICs of ≤4 mg/L [63].
A second set of simulations were performed using data from 150 subjects who participated in phase I/II studies, including a renal impairment study [65]. With ceftobiprole 500 mg every 8 h infused over 2 h, the probability of attaining a 50 % fT>MIC target was >80 % for a MIC of 4 mg/L in subjects with normal renal function (creatinine clearance [CLCR] 80–120 ml/min). The probabilities of attaining a 50 % fT>MIC target (near maximal killing effect) were 98.8 and 99.9 % for MRSA and MSSA, respectively (MIC90 1 and 0.5 mg/L). The probabilities of attaining a 60 % fT>MIC target (near maximal killing effect) for AmpC-producing and AmpC-nonproducing bacilli, and P. aeruginosa were 87.8, 94.1 and 62.0 %, respectively (AmpC-producers and P. aeruginosa had MIC90 values of 16 and 32 mg/L and >90 % of AmpC-nonproducing isolates had a MIC of ≤0.25 mg/L) [65].
Based on these two sets of Monte Carlo simulations, a ceftobiprole regimen of 500 mg every 8 h infused over 2 h was selected for phase III trials in patients with Gram-positive and Gram-negative pathogens [63]. A validation study [67] showed that the probability of target attainment based on the initial Monte Carlo simulation [66] adequately predicted the actual exposure to ceftobiprole in a phase III study (see Sect. 4.1 for trial design details) in patients with HAP, including severely ill patients. An additional analysis [68] of data from this trial showed that %fT>MIC was the most significant (p < 0.0001) predictor of microbiological eradication at the end of treatment evaluation and one of the significant (p = 0.0062) independent predictors of clinical outcome at the test-of-cure (TOC) evaluation (based on the highest MIC of any pathogen cultured at baseline or end of treatment, using multiple logistic regression analyses). According to regression analyses, a significant (p = 0.0029) correlation was seen between %fT>MIC and clinical cure when %fT>MIC was ≥51.1 %; a significant (p < 0.0001) correlation was also observed for microbiological eradication when %fT>MIC was ≥62.2 % [68]. Similar results are reported in patients with CAP in a phase III trial (see Sect. 4.2 for trial design details), where ceftobiprole or ceftriaxone %fT>MIC strongly correlated with microbiological eradication [69].
3 Pharmacokinetic Properties
3.1 General Profile
The pharmacokinetics of intravenous ceftobiprole medocaril have been reviewed in detail previously [63]. This section provides a brief overview of ceftobiprole medocaril pharmacokinetics, focusing mainly on the approved regimen (500 mg every 8 h infused over 2 h) for patients with hospital- or community-acquired pneumonia.
After intravenous administration, the prodrug ceftobiprole medocaril is rapidly and almost completely converted to the active drug, ceftobiprole (Fig. 1). The plasma concentrations of ceftobiprole reached the peak at the end of infusion, followed by a biphasic decline, reflecting an initial rapid distribution into other body compartments and a gradual terminal elimination [70, 71]. The pharmacokinetics of ceftobiprole are linear over a range of 125–1,000 mg after single- [71] or multiple- [70] dose administration and are time-independent [9, 63]. In subjects with normal renal function, steady-state concentrations of ceftobiprole were attained on the first day of dosing with ceftobiprole medocaril every 8 h, with no appreciable accumulation [9, 63].
On day 5, following multiple intravenous infusion of 500 mg every 8 h infused over 2 h in healthy volunteers, the mean maximum plasma concentration of ceftobiprole was 33.0 µg/mL and the area under the plasma concentration–time curve (AUC) from 0 to 8 h (AUC8) was 102 µg·h/mL [9, 63]. The volume of distribution at steady state was 15.5 L [9, 63]. The mean AUC of ceftobiprole in the ELF was 25.5 % of that of plasma in healthy volunteers receiving 500 mg every 8 h infused over 2 h [55]. The extent of ceftobiprole penetration into the ELF of patients with pneumonia is unknown.
A small fraction of ceftobiprole is metabolized to the microbiologically inactive open-ring metabolite [9]. Systemic exposure of the open-ring metabolite accounts for ≈4 % of the parent drug exposure in subjects with normal renal function. Because of minimal metabolism, the potential for other drugs to interact with ceftobiprole is minimal. In vitro studies indicated that ceftobiprole is not an inhibitor of or substrate to the p-glycoprotein transporter system and it does not inhibit breast cancer resistance protein, multidrug resistance protein 1, multidrug resistance-associated protein 2, organic anion transporters 1 (OAT1) and OAT3, or organic cation transporters 1 (OCT1) and OCT2. Ceftobiprole is potentially a weak substrate of the renal tubule cells uptake transporters, OAT1 and OCT2. Ceftobiprole is an inhibitor of the hepatocyte uptake transporters, organic anion transporting polypeptides (OATP) 1B1 and OATP1B3 [9].
In vitro, ceftobiprole is not an inhibitor of or substrate to the cytochrome P450 system [63]. Binding of ceftobiprole to plasma proteins is minimal (16 %) and is independent of the drug and protein concentrations [9, 63].
Ceftobiprole is primarily eliminated through renal excretion, with an elimination half-life (t1/2) 3.3 h, a total systemic clearance of 4.98 L/h and a renal clearance 4.28 L/h [9, 63]. Following intravenous administration of ceftobiprole medocaril, 87.8 % of the administered dose was recovered in the urine, with the active metabolite (ceftobiprole) accounting for 80–90 % of the recovered dose [63, 70]. Ceftobiprole is eliminated predominantly by glomerular filtration [9, 70]. Ceftobiprole does not undergo tubular secretion and only a fraction is reabsorbed; consequently, renal drug-drug interactions are not expected with ceftobiprole [9].
3.2 Special Patient Populations
The pharmacokinetics of ceftobiprole medocaril are affected by renal impairment [9, 63, 72] and, therefore, adjustment of dosage or infusion duration is necessary for subjects with renal impairment (see Sect. 6).
A renal impairment study in 20 male subjects with normal renal function, or mild, moderate or severe renal impairment (CLCR >80, 51–80, 30–50 and <30 ml/min, respectively) showed that systemic exposure to ceftobiprole increased with decreasing renal function [63, 72]. Ceftobiprole AUC from time zero to the last measurable concentration (AUClast) increased by 29 % in subjects with mild renal impairment, and 2.5- and 3.3-fold in those with moderate or severe renal impairment, compared with those who had normal renal function. Subjects with moderate or severe renal impairment had decreased total systemic clearance (62 and 75 %) and renal clearance (78 and 91 %) of ceftobiprole, compared with those with normal renal function. The elimination half-life was the longest in subjects with severe renal impairment (11 vs. 3.5 h in normal subjects) [63, 72].
In patients with end stage renal disease (ESRD) requiring dialysis, AUCs of ceftobiprole (and the open-ring metabolite) are substantially increased, compared with healthy subjects [9]. In patients with a CLCR of >150 mL/min, systemic clearance of ceftobiprole was 40 % higher and the volume of distribution is 30 % greater than in subjects with normal renal function. In patients with ESRD who were on hemodialysis, ceftobiprole is hemodialysable with an extraction ratio of 0.7 [9].
The pharmacokinetics of ceftobiprole are not expected to be affected by hepatic impairment, as it undergoes minimal hepatic metabolism [9]. Dosage adjustment is not necessary based on gender, race or body weight, or in elderly patients with normal renal function [9].
4 Therapeutic Efficacy
The clinical efficacy of intravenous ceftobiprole medocaril for the treatment of adult patients with HAP [73] or CAP [74] has been evaluated in two separate double-blind, multinational, phase III noninferiority trials, both fully published. Additional data from these trials are available in the UK public assessment report (PAR) [representative PAR for the decentralized procedure in the EU] [12].
Eligibility criteria and trial design details for both trials are summarized in Table 3. Efficacy of ceftobiprole medocaril was assessed in intent-to-treat (ITT), clinically evaluable (CE), microbiological ITT or microbiologically evaluable (ME) populations (see Tables 3, 4 for definitions and number of patients).
4.1 Hospital-Acquired Pneumonia
In the HAP trial, patients were stratified according to infection type (non-VAP or VAP) and within each strata, further stratified according to baseline Acute Physiology and Chronic Health Evaluation (APACHE) II scores (8–19 or 20–25); patients with VAP were further stratified by ventilation duration (<5 or ≥5 days) [73]. Eligible patients were randomized to ceftobiprole medocaril or ceftazidime plus linezolid for 7–14 days (see Table 4 for treatment regimens). Additional open-label fluoroquinolone or an aminoglycoside was allowed in patients at risk for pseudomonal infections [73]. While patients who received non-study systemic antibiotics for pneumonia were considered to have clinical failure, those who received such treatment for indications other than pneumonia were excluded from the CE population [73].
The primary endpoint was the clinical cure rate at the TOC visit in the ITT and CE populations of patients with HAP (non-VAP plus VAP), as defined in Table 3 [73]. The key secondary endpoint was microbiological eradication rate at the TOC visit in the microbiological ITT and ME populations [73].
Baseline demographic and clinical characteristics of the ITT population (n = 781; 571 patients had non-VAP and 210 had VAP) were generally similar between the two treatment groups [73]. There were more men in the ceftobiprole medocaril group than in the ceftazidime plus linezolid group (71 vs. 62 %). At baseline, 46 % of the ITT population were aged ≥65 years (mean age 61 years [12]), and a large proportion of patients were severely ill (41 and 13 % of patients had an APACHE II score of ≥15 and ≥20, respectively). Furthermore, 73 % of patients had systemic inflammatory response syndrome (SIRS) and 11 % of patients had bacteremia. A valid baseline pathogen was found in 69 % of patients, with 37 and 48 % of patients having a valid Gram-positive and Gram-negative pathogen, respectively. Approximately 11, 13 and 24 % of the ITT population had MRSA, pseudomonas and polymicrobial infections, respectively. In the ME population, pathogens isolated at baseline were S. aureus (42.5 %) [39 % MRSA], Enterobacteriaceae (37.7 %), P. aeruginosa (18.4 %), A. baumannii (9.3 %), S. pneumoniae (7.8 %) and Haemophilus (5.4 %) [73].
In patients with HAP (non-VAP plus VAP), ceftobiprole medocaril was noninferior to ceftazidime plus linezolid in terms of clinical cure rates at the TOC visit in the ITT and CE populations (primary endpoint; Table 4) [73].
Microbiological eradication rates in patients with HAP (non-VAP plus VAP) are summarized in Table 4 [73]. In the ITT population, 30-day all-cause mortality in the ceftobiprole medocaril and ceftazidime plus linezolid groups was 19.4 versus 18.5 % (difference +1.0; 95 % CI −4.5 to 6.5) and the corresponding 30-day pneumonia-specific mortality was 6.6 versus 6.2 % (difference +0.5; 95 % CI −2.9 to 3.9) [12].
4.1.1 In Patients with Non-VAP
In patients with non-VAP, ceftobiprole medocaril was noninferior to ceftazidime plus linezolid in terms of clinical cure rate at TOC in the ITT (59.6 vs. 58.8 %; difference +0.8; 95 % CI −7.3 to 8.8; n = 287 and 284) and CE (77.8 vs. 76.2 %; difference +1.6; 95 % CI −6.9 to 10.0; n = 198 and 185) populations [73].
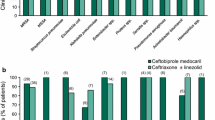
Clinical cure rates at the TOC visit in the ME population of patients with non-VAP, analyzed by the most commonly isolated pathogens at baseline are shown in Fig. 2. The rates were generally similar between patients who had Gram-positive and Gram-negative pathogens at baseline. For most Enterobacteriaceae species and P. aeruginosa, clinical cure rates were similar between the two treatment groups, although the rates were lower with ceftobiprole medocaril than with ceftazidime plus linezolid for patients who had A. baumanii or Haemophilus spp. at baseline (Fig. 2).
Clinical cure rates at the test-of-cure visit by the most common baseline pathogens in the microbiologically evaluable population of patients with hospital-acquired pneumonia (excluding ventilator-associated pneumonia) [73]. The numbers above the bars are the numbers of patients with that particular baseline pathogen. BPR ceftobiprole medocaril, CAZ ceftazidime, LZD linezolid, MRSA methicillin-resistant Staphylococcus aureus, MSSA methicillin-susceptible S. aureus, spp. species
In the CE population, clinical cure rates were generally similar between the treatment groups in subgroup analyses by baseline demographic and clinical characteristics (age, sex, geographical region, APACHE II score, care facility [ICU vs. non-ICU], pre-study antibiotics and antipseudomonal antibiotics) [73].
In the CE population, 38 ceftobiprole medocaril recipients and 37 ceftazidime plus linezolid recipients required mechanical ventilation during treatment, or developed pneumonia within 48 h after ventilation started, and thus did not fall within the definition of VAP (see Table 3 for definition of VAP) [73]. Among these patients, 55.3 % of ceftobiprole medocaril recipients and 40.5 % of ceftazidime plus linezolid recipients achieved clinical cure at TOC (difference +14.7; 95 % CI −7.6 to 37.1) [73].
More ceftobiprole medocaril than ceftazidime plus linezolid recipients (86.9 vs. 78.4 %; difference +8.5; 95 % CI 0.9–16.1) in the CE population showed an early (i.e. after 4 days’ treatment) clinical improvement, as assessed by the investigator based on the resolution of clinical signs and symptoms, with the largest difference seen for patients with MRSA at baseline (94.7 vs. 52.6 %; difference +42.1, 95 % CI 17.5–66.7) [73].
In patients with non-VAP, microbiological eradication rates at TOC in ceftobiprole medocaril and ceftazidime plus linezolid groups were 48.6 versus 53.6 % (difference −5.0; 95 % CI −15.3 to 5.3) in the microbiological ITT population (n = 179 and 181), and 62.9 versus 67.5 % (difference −4.6; 95 % CI −16.7 to 7.6) in the ME population (n = 116 and 120) [73].
In the ITT population of patients with non-VAP, 30-day all-cause mortality (16.7 vs. 18.0 %; difference −1.2; 95 % CI −7.4 to 5.0) and 30-day pneumonia-specific mortality (5.9 vs. 5.6 %; difference 0.3; 95 % −3.5 to 4.1) were similar between ceftobiprole medocaril and ceftazidime plus linezolid recipients [73].
4.1.2 In Patients with VAP
In patients with VAP, noninferiority of ceftobiprole medocaril to ceftazidime plus linezolid was not demonstrated in terms of clinical cure rate at TOC. In the ITT population, clinical cure rates were 23.1 versus 36.8 % (difference −13.7; 95 % CI −26.0 to −1.5; n = 104 and 106) and the corresponding rates in the CE population were 37.7 versus 55.9 % (difference −18.2; 95 % CI −36.4 to 0; n = 53 and 59) [73]. Similar results were obtained for the microbiological outcome; in the microbiological ITT population, microbiological eradication rate in the ceftobiprole medocaril and ceftazidime plus linezolid groups were 20.0 versus 34.9 % (difference −14.9; 95 % CI −27.9 to −1.9; n = 90 and 86) and the corresponding rates in the ME population were 30.4 versus 50.0 % (difference −19.6; 95 % CI −38.8 to −0.4; n = 46 and 50) [73].
In the ITT population of patients with VAP, 30-day all-cause mortality was 26.9 versus 19.8 % in the ceftobiprole medocaril and ceftazidime plus linezolid groups (difference +7.1; 95 % CI −4.3 to 18.5) and the corresponding 30-day pneumonia-specific mortality was 8.7 versus 7.5 % (difference +1.1; 95 % CI −6.3 to 8.5) [73].
4.2 Community-Acquired Pneumonia
In the CAP trial, patients were stratified according to Pneumonia Severity Index (PSI) score (<91 or ≥91) and need for antistaphylococcal treatment, and were then randomized to ceftobiprole medocaril or ceftriaxone ± linezolid for 7–14 days (see Table 4 for treatment regimens) [74]. After day 3, at the investigators discretion, patients were eligible to switch to oral cefuroxime 500 mg every 12 h if they met protocol-defined criteria (significant improvement in clinical symptoms and signs; reduction in body temperature for ≥24 h without using antipyretics; white blood cell count and bands [%] within the reference range; no clinically significant deterioration on chest radiograph; negative blood cultures; and, stable vital signs) [74].
The primary endpoint was the clinical cure rate at the TOC visit in the ITT and CE population, as defined in Table 3 [74]. Secondary efficacy endpoints, in the hierarchical order they were tested, were microbiological eradication rate in the microbiological ITT and ME populations at TOC visit, clinical cure rate by baseline PSI score in the ITT and CE populations and 30-day pneumonia-specific mortality in the ITT and CE populations [74].
There were no significant between-group differences in demographic and baseline characteristics of the ITT population (n = 638) [74]. The mean age was 54.5 years and ≈43 % of patients were female. With respect to CAP risk/severity characteristics at baseline, 37 and 18 % of patients were aged ≥65 and ≥75 years, 22 % of patients had a PSI score of ≥91, 54 % of patients had SIRS and ≈4 % of patients had bacteraemia. A typical bacterial pathogen was isolated at baseline in 28.8 % of the ITT population, with S. pneumoniae (n = 68) and H. influenzae (n = 26) being the most common [74].
The mean duration of ceftobiprole medocaril treatment in the CE population (data not reported for the ITT population) was 7.2 days in patients who received intravenous therapy only (n = 103) and 4.8 days in those who switched to oral cefuroxime (n = 128), and the corresponding duration of ceftriaxone ± linezolid treatment was 7.8 and 5.1 days (n = 101 and 137, respectively) [74]. In the ceftriaxone group, 34 patients received linezolid for a mean of 5.8 days [74].
In patients with CAP, ceftobiprole medocaril was noninferior to ceftriaxone ± linezolid in terms of clinical cure rates achieved at TOC visit in the ITT and CE population (primary endpoint; Table 4) [74].
The severity of pneumonia at baseline had no effect on clinical cure rates at the TOC visit in ceftobiprole medocaril and ceftriaxone ± linezolid groups, with no significant differences seen between patients with baseline PSI scores of <91 (85.6 vs. 88.3 %) or ≥91 (90.2 vs. 84.5 %), according to a subgroup analysis in the CE population [74]. Similar results were observed in the ITT population (data not reported) [74].
Additional subgroup analyses showed that the between-group treatment differences in clinical cure rates in the CE population were not significantly different within subgroups of age (<65 vs. ≥65 years; <75 vs. ≥75 years), Pneumonia Patient Outcomes Research Team (PORT) score (I–V), bacteraemia (present vs. absent) and SIRS (present vs. absent) [74]. Clinical cure rates in the ceftobiprole medocaril versus ceftriaxone ± linezolid groups at the TOC visit in the CE population in patients at risk for poor outcomes were: age ≥75 years 92.3 versus 86.0 %; PORT score IV 89.6 versus 84.6 %; PORT score V 100 versus 83.3 %; CAP complicated by bacteraemia 85.7 versus 85.7 %; and, presence of SIRS 84.6 versus 86.7 % [74].
In the ME population, clinical cure rates at TOC analyzed by the most commonly isolated pathogens at baseline are generally similar between the treatment groups (Fig. 3). Two patients in the ceftobiprole medocaril group and three patients in the ceftriaxone ± linezolid group had MDRSP at baseline and all achieved clinical cure at TOC visit. In patients with S. pneumoniae and PSI ≥91 at baseline, clinical cure rate was 100 % with ceftobiprole medocaril (n = 10) and 83 % with ceftriaxone ± linezolid (n = 6) [74].
Clinical cure rates at the test-of-cure visit by the most common baseline pathogens in the microbiologically evaluable population of patients with community-acquired pneumonia [74]. The numbers above the bars are the numbers of patients with that particular baseline pathogen. There were no patients with MRSA, H. parahaemolyticus or K. oxytoca at baseline in the comparator group. θ indicates 0 % clinical cure rate. BPR ceftobiprole medocaril, CRO ceftriaxone, LZD linezolid, MRSA methicillin-resistant Staphylococcus aureus, MSSA methicillin-susceptible S. aureus
Ceftobiprole medocaril was noninferior to ceftriaxone ± linezolid in terms of microbiological eradication rates achieved at TOC visit in the microbiological ITT and ME population (Table 4) [74]. Subgroup analyses showed that the rates were significantly (p = 0.025) different between patients who switched versus those did not switch to oral cefuroxime [74]. Among those who switched, microbiological eradication rates were significantly (based on 95 % CI) lower with ceftobiprole medocaril than with ceftriaxone ± linezolid (89 vs. 100 %; 95 % CI for the treatment difference −20.8 to −0.8; n = 37 and 41, respectively) [74]. However, among patients who did not switch, the corresponding rates were 87.1 versus 80.0 % (n = 31 and 35, respectively) [12]. No significant difference in microbiological eradication rates was observed for comparisons within other strata and subgroups (data not reported) [74].
According to a post hoc analysis of the CAP trial data, among patients with PORT risk class ≥IV, more ceftobiprole medocaril than ceftriaxone ± linezolid recipients achieved an early clinical response at day 3 (78 vs. 61 %; difference 17.4; 95 % CI 2.3–32.5; ITT population), assessed using the criteria suggested by the Foundation for the National Institutes of Health based on four clinical symptoms: cough, dyspnea, pleuritic chest pain and sputum production [75].
During the first 30 days of treatment, pneumonia-specific mortality was low in both ceftobiprole medocaril and ceftriaxone ± linezolid groups (1 vs. 3 patients in the ITT population and 0 vs. 2 patients in the CE population) [74].
At the late follow-up visit (28–35 days post therapy), clinical cure rates were sustained in 99 % of the CE population in both treatment groups, with no microbiological relapse in the ME population in either group [74].
5 Tolerability
Tolerability data for intravenous ceftobiprole medocaril discussed in this section are from the HAP [73] and CAP [74] trials discussed in Sect. 4, with some data available on file [76]. Additional data from these trials are reported in the UK PAR [12]. In addition, combined ceftobiprole medocaril safety data for 1,668 patients who participated in clinical studies for HAP, CAP or cSSTIs are available in the UK SPC [9].
Ceftobiprole medocaril was generally well tolerated in patients with HAP or CAP in phase III trials [12, 73, 74]. Although the majority of patients in ceftobiprole medocaril and comparator groups reported at least one treatment-emergent adverse event (77.5 vs. 77.7 % in the HAP trial and 70.0 vs. 64.6 % in the CAP trial), relatively few patients discontinued treatment because of these adverse events (14.0 vs. 10.4 % in the HAP trial and 5.8 vs. 3.7 % in the CAP trials) [12, 74].
The most common (incidence ≥5 %) adverse events occurring in ceftobiprole medocaril recipients in the HAP trial were diarrhoea (11 vs. 15 % in the ceftazidime plus linezolid recipients), hypokalemia (10 vs. 8 %), hyponatraemia (10 vs. 6 %), pyrexia (9 vs. 8 %), vomiting (7 vs. 3 %) and anemia (5 vs. 5 %) [76]. The most common (incidence ≥5 %) adverse events occurring in ceftobiprole medocaril recipients in the CAP trial were nausea (10 vs. 4 % in ceftriaxone ± linezolid recipients), vomiting (9 vs. 3 %), diarrhoea (7 vs. 9 %) and headache (7 vs. 7 %) [76].
The most common treatment-related adverse events are summarized in Table 5. In the HAP trial, the incidence of treatment-related diarrhoea was numerically lower with ceftobiprole medocaril than with ceftazidime plus linezolid [73]. In the CAP trial, the incidence of treatment-related adverse events were significantly greater with ceftobiprole medocaril than with ceftriaxone ± linezolid, mainly because of significant between-group differences in the incidence of nausea and vomiting (Table 5) [74]. In the combined analysis of patients with HAP, CAP or cSSTIs [9], the most common (incidence ≥3 %) adverse events reported in ceftobiprole medocaril recipients were nausea, vomiting, diarrhoea, infusion site reactions, hypersensitivity (including urticaria, pruritic rash and drug hypersensitivity) and dysgeusia.
The incidences of serious adverse events (SAE) in ceftobiprole medocaril versus comparator groups were 36.3 versus 31.9 % in the HAP trial and 11.3 versus 11.5 % in the CAP trial [12]. However, these events were considered treatment-related only in a small proportion of patients (3.9 vs. 3.1 % in the HAP trial [73]; 1.0 vs. 1.2 % in the CAP trial [12]). Treatment-related SAEs occurring in ceftobiprole medocaril recipients in the HAP trial were hyponatraemia (4 patients) and coma (2 patients), with the following occurring in one patient each: cardiac arrest, nausea, vomiting, no therapeutic response, pyrexia, hypersensitivity, bronchopneumonia, C. difficile colitis, lung abscess, QT prolongation, increased hepatic enzymes, abnormal laboratory test, hypocalcaemia, convulsion, pulmonary oedema, respiratory distress, respiratory failure and shock [12]. In the CAP trial, treatment-related serious anemia, anaphylactic shock and viral infection occurred in one ceftobiprole medocaril recipient each [12]. Of note, these data show that treatment-related serious C. difficile colitis is rare with ceftobiprole medocaril.
Ceftobiprole medocaril 500 mg every 8 h for 7 days had no significant ecological impact on the normal human intestinal flora, with no C. difficile strains or toxins detected in faecal samples, in healthy volunteers [77].
6 Dosage and Administration
In the EU, in adults (≥18 years of age) with HAP (excluding VAP) or CAP, the recommended dosage of ceftobiprole medocaril is 500 mg administered every 8 h as a 2-h intravenous infusion [9]. In patients with CAP, after completing ≥3 days’ ceftobiprole medocaril therapy and depending on the patient’s clinical response, switching to an appropriate oral antibiotic therapy may be considered [9].
In patients with supranormal CLCR (>150 mL/min), an infusion duration of 4 h is recommended [9]. Dosage adjustment is not necessary in patients with mild renal impairment (CLCR 50–80 mL/min). However, in patients with CLCR <50 mL/min, dosage should be reduced as follows: 500 mg every 12 h infused over 2 h in patients with moderate renal impairment (CLCR 30–<50 mL/min), 250 mg every 12 h infused over 2 h in those with severe renal impairment (CLCR <30 mL/min) and 250 mg every 24 h in those with ESRD with or without intermittent dialysis. The UK SPC states that ceftobiprole medocaril should be used with caution in patients with severe renal impairment, as the dosage recommendation is based on limited clinical data [9].
Ceftobiprole medocaril is contraindicated in patients with hypersensitivity to ceftobiprole or other cephalosporin class of antibacterial, and in those with an immediate and severe hypersensitivity (e.g. anaphylactic reaction) to any other type of β-lactam antibacterial agent (e.g. penicillins or carbapenems) [9].
Local prescribing information should be consulted for details on special warnings, precautions and potential drug interactions related to the use of ceftobiprole medocaril.
7 Current Status of Ceftobiprole Medocaril in Patients with Hospital- or Community-Acquired Pneumonia
Intravenous ceftobiprole medocaril has been approved in the EU for the treatment of adults with HAP (excluding VAP) or CAP infections, using the decentralized procedure, with the UK as the Reference Member State and Austria, Belgium, Denmark, Finland, France, Germany, Italy, Luxembourg, Norway, Spain and Sweden as the Concerned Member States [9, 10]. It is also in pre-registration stage in Switzerland for these indications [78]. Current European treatment guidelines for HAP [6] and CAP [4, 8] were published prior to the approval of ceftobiprole medocaril and thus, ceftobiprole medocaril is not considered in these guidelines.
Ceftobiprole medocaril shows broad-spectrum in vitro activity against many Gram-positive and Gram-negative pathogens that cause HAP and CAP, including S. aureus, MRSA, PRSP, H. influenzae (including β-lactamase-producing strains), M. catarrhalis, E. coli and K. pneumoniae (see Sect. 2.2). Of note, ceftobiprole medocaril has demonstrated in vitro activity against S. aureus strains that were resistant to vancomycin and those that are not susceptible to linezolid, the well-known MRSA agents (see Sect. 2.2.1). In vitro susceptibility rate for P. aeruginosa was ≈65 % with ceftobiprole medocaril (based on non-species specific EUCAST breakpoint) and >75 % with cefepime or ceftazidime (Table 2). As with other cephalosporins, ceftobiprole medocaril shows limited activity against Acinetobacter spp., and is susceptible to hydrolysis by enzymes (e.g. ESBLs) produced by Enterobacteriaceae (see Sect. 2.2.2). The UK SPC cautions that the prevalence of ESBL-producing Enterobacteriaceae should be considered when initiating treatment with ceftobiprole medocaril [9]. Ceftobiprole medocaril has low potential for selection of resistance among Gram-positive and Gram-negative bacteria (see Sect. 2.4).
Intravenous ceftobiprole medocaril was shown to be noninferior to ceftazidime plus linezolid for the treatment of HAP and ceftriaxone ± linezolid for the treatment of CAP (in hospitalized patients) in two separate phase III registrational trials (see Sect. 4). In both trials, at the TOC visit, clinical cure rates were similar between the ceftobiprole medocaril and comparator groups in the ITT and CE populations (primary endpoint). In patients with HAP (excluding VAP) or CAP, ceftobiprole medocaril has shown clinical efficacy against those who had S. aureus, including MRSA, S. pneumoniae, commonly prevalent Enterobacteriaceae and P. aeruginosa at baseline (Figs. 2, 3). Of note, in patients with non-VAP, clinical cure rates were similar between ceftobiprole medocaril and ceftazidime plus linezolid recipients who had P. aeruginosa at baseline (Fig. 2).
In the HAP trial, noninferiority of ceftobiprole medocaril in terms of clinical cure (or microbiological eradication) was not demonstrated in a small subset of patients with VAP. Consequently, ceftobiprole medocaril is not approved for patients with VAP. Interestingly, in mechanically ventilated patients with non-VAP, clinical outcomes were in favor of ceftobiprole medocaril (see Sect. 4.1.1), suggesting that mechanical ventilation by itself is not associated with poor outcomes. A post hoc multivariate logistic regression analysis did not reveal any individual or combination of patient factors (such as baseline pathogens, sex, age, comorbidities and vasopressor use) that could explain the differential outcome in patients with VAP [73]. Furthermore, there was no differences in ceftobiprole medocaril pharmacokinetics between non-VAP and VAP groups [68]. It is thought that the small sample size and the substantial heterogeneity in baseline characteristics in the VAP subgroup may have contributed to the differential outcomes [73]. Further investigation assessing the efficacy of ceftobiprole medocaril in patients with VAP may be warranted.
The pivotal trial in patients with CAP had some strengths and limitations. It is the first trial to evaluate the efficacy of ceftobiprole medocaril in patients with CAP requiring hospitalization and intravenous antibacterial treatment, and it included patients with various disease severity/risk characteristics [74]. According to the UK PAR [12], the comparators and the noninferiority margin are considered acceptable. However, limitations of the trial included potential bias against including extremely ill patients and inclusion of a large number of patients with a PSI score of <91 (77 % of the CE population) or a PORT score of I or II (≈48 % of the CE population). Nevertheless, subgroup analyses showed that patients with a PSI score of ≥91 or PORT score III–V benefited from ceftobiprole medocaril treatment (see Sect. 4.2).
Ceftobiprole medocaril is generally well tolerated in patients with HAP or CAP in phase III trials, with ≈10 % of patients discontinuing the treatment because of adverse events (see Sect. 5). The most common treatment-related adverse events occurring in ceftobiprole medocaril recipients included nausea, diarrhoea, infusion site reactions, vomiting, hepatic enzyme elevations and hyponatraemia. Ceftobiprole medocaril has no significant impact on the normal human intestinal flora, and C. difficile colitis is uncommon with ceftobiprole medocaril.
Like ceftobiprole medocaril, ceftaroline fosamil [79] is a new generation cephalosporin with anti-MRSA activity, which has been recently approved for the treatment of CAP but not HAP. There are no studies comparing the efficacy of ceftobiprole medocaril head-to-head with ceftaroline in patients with CAP. Such studies would help more clearly define the role of ceftobiprole medocaril in the treatment of CAP.
In summary, intravenous ceftobiprole medocaril is an effective and well tolerated option for the initial empirical treatment of patients with HAP (excluding VAP), and in those with CAP requiring hospitalization and intravenous antibacterials. MRSA and a broad-spectrum coverage with ceftobiprole medocaril monotherapy may simplify the empirical treatment relative to combination therapies.
Data selection sources:
Relevant medical literature (including published and unpublished data) on ceftobiprole medocaril was identified by searching databases including MEDLINE (from 1946) and EMBASE (from 1996) [searches last updated 15 July 2014], bibliographies from published literature, clinical trial registries/databases and websites. Additional information was also requested from the company developing the drug.
Search terms: Ceftobiprole medocaril, ceftobiprole, community-acquired pneumonia, hospital-acquired pneumonia, nosocomial pneumonia.
Study selection: Studies in patients with hospital- or community-acquired pneumonia who received ceftobiprole. When available, large, well designed, comparative trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
References
Koulenti D, Lisboa T, Brun-Buisson C, et al. Spectrum of practice in the diagnosis of nosocomial pneumonia in patients requiring mechanical ventilation in European intensive care units. Crit Care Med. 2009;37(8):2360–8.
Barbier F, Andremont A, Wolff M, et al. Hospital-acquired pneumonia and ventilator-associated pneumonia: recent advances in epidemiology and management. Curr Opin Pulm Med. 2013;19(3):216–28.
Chastre J, Blasi F, Masterton RG, et al. European perspective and update on the management of nosocomial pneumonia due to methicillin-resistant Staphylococcus aureus after more than 10 years of experience with linezolid. Clin Microbiol Infect. 2014;20(Suppl 4):19–36.
The British Thoracic Society. Guidelines for the management of community acquired pneumonia in adults. 2009. http://www.britishinfection.org/drupal/sites/default/files/Draft-CAPGuideline.pdf. Accessed 15 July 2014.
Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax. 2012;67(1):71–9.
Torres A, Ewig S, Lode H, et al. Defining, treating and preventing hospital acquired pneumonia: European perspective. Intensive Care Med. 2009;35(1):9–29.
Cilloniz C, Ewig S, Ferrer M, et al. Community-acquired polymicrobial pneumonia in the intensive care unit: aetiology and prognosis. Crit Care. 2011;15(5):R209.
Woodhead M, Blasi F, Ewig S, et al. Guidelines for the management of adult lower respiratory tract infections: summary. Clin Microbiol Infect. 2011;17(Suppl 6):1–24.
Basilea Pharmaceutica International Ltd. Zevtera 500 mg powder for concentrate for solution for infusion: summary of product characteristics. 2013. http://www.mhra.gov.uk/home/groups/spcpil/documents/spcpil/con1388122812881.pdf. Accessed 15 July 2014.
Basilea Pharmaceutica International Ltd. Basilea’s antibiotic ceftobiprole obtains regulatory approval in Europe for pneumonia [media release]. 23 Oct 2013. http://www.basilea.com/chameleon/public/584f9d1e-4298-e47c-0475-a5e5e5288ded/582542.pdf.
Zhanel GG, Lam A, Schweizer F, et al. Ceftobiprole: a review of a broad-spectrum and anti-MRSA cephalosporin. Am J Clin Dermatol. 2008;9(4):245–54.
Basilea Pharmaceutica International Ltd. Zevtera 500 mg powder for concentrate for solution for infusion: public assessment report. 2013. http://www.mhra.gov.uk/home/groups/par/documents/websiteresources/con369256.pdf. Accessed 15 July 2014.
Hebeisen P, Heinze-Krauss I, Angehrn P, et al. In vitro and in vivo properties of Ro 63-9141, a novel broad-spectrum cephalosporin with activity against methicillin-resistant staphylococci. Antimicrob Agents Chemother. 2001;45(3):825–36.
Davies TA, He W, Bush K, et al. Affinity of ceftobiprole for penicillin-binding protein 2b in Streptococcus pneumoniae strains with various susceptibilities to penicillin. Antimicrob Agents Chemother. 2010;54(10):4510–2.
Davies TA, Page MGP, Shang W, et al. Binding of ceftobiprole and comparators to the penicillin-binding proteins of Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Streptococcus pneumoniae. Antimicrob Agents Chemother. 2007;51(7):2621–4.
Henry X, Verlaine O, Amoroso A, et al. Activity of ceftaroline against Enterococcus faecium PBP5. Antimicrob Agents Chemother. 2013;57(12):6358–60.
Entenza JM, Hohl P, Heinze-Krauss I, et al. BAL9141, a novel extended-spectrum cephalosporin active against methicillin-resistant Staphylococcus aureus in treatment of experimental endocarditis. Antimicrob Agents Chemother. 2002;46(1):171–7.
Fritsche TR, Sader HS, Jones RN. Antimicrobial activity of ceftobiprole, a novel anti-methicillin-resistant Staphylococcus aureus cephalosporin, tested against contemporary pathogens: results from the SENTRY antimicrobial surveillance program (2005–2006). Diagn Microbiol Infect Dis. 2008;61(1):86–95.
Farrell D, Moet G, Sader H, et al. Ceftobiprole activity when tested against clinical bacterial pathogens from Europe, 2009 [abstract no. P1875]. Clin Microbiol Infect. 2010;16(Suppl 2):S556.
Seifert H, Gatermann S, Pfister W, et al. Susceptibility of Gram-negative pathogens to ceftobiprole, ceftazidime and cefepime isolated from centres in Austria, Germany and Switzerland [abstract no. P1269]. Clin Microbiol Infect. 2010;16(Suppl 2):S357.
Seifert H, Dryden M, Quintana A, et al. Comparative susceptibility of European Gram-negative pathogens to ceftobiprole, ceftazidime and cefepime [abstract no. P1033]. Clin Microbiol Infect. 2009;15(Suppl 4):S273.
Flamm RK, Sader H, Streit JM, et al. Activity of ceftobiprole tested against clinical isolates of staphylococci and streptococci from European surveillance (2008–2010) [abstract no. P1628]. In: 23rd European Congress of Clinical Microbiology and Infectious Diseases; 27–30 Apr 2013; Berlin.
Flamm RK, Sader HS, Streit JM, et al. Activity of ceftobiprole tested against Gram-negative clinical isolates from European medical centres [abstract no. P1627]. In: 23rd European Congress of Clinical Microbiology and Infectious Diseases; 27–30 Apr 2013; Berlin.
Flamm RK, Sader HS, Jones RN. Activity of ceftobiprole tested against pathogens associated with community-acquired bacterial pneumonia in Europe [abstract no. P1626]. In: 23rd European Congress of Clinical Microbiology and Infectious Diseases; 27–30 Apr 2013; Berlin.
Flamm RK, Sader HS, Streit JM, et al. Activity of ceftobiprole tested against pathogens associated with hospital-acquired bacterial pneumonia in Europe [abstract no. P1625]. In: 23rd European Congress of Clinical Microbiology and Infectious Diseases; 27–30 Apr 2013; Berlin.
Rossolini GM, Dryden MS, Kozlov RS, et al. Comparative activity of ceftobiprole against Gram-positive and Gram-negative isolates from Europe and the Middle East: the CLASS study. J Antimicrob Chemother. 2011;66(1):151–9.
Rios Duenas E, Rodriguez-Avial I, Picazo JJ. In vitro activity of ceftobiprole and seven other antimicrobial agents against invasive Streptococcus pneumoniae isolates in Spain. Eur J Clin Microbiol Infect Dis. 2011;30(12):1621–5.
Borbone S, Campanile F, Bongiorno D, et al. In vitro bactericidal activity of ceftobiprole against hospital- and community-associated methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 2010;65(3):591–4.
Pillar CM, Aranza MK, Shah D, et al. In vitro activity profile of ceftobiprole, an anti-MRSA cephalosporin, against recent Gram-positive and Gram-negative isolates of European origin. J Antimicrob Chemother. 2008;61(3):595–602.
Schmitz FJ, Perry J, Zbinden R, et al. Comparative susceptibility of European Gram-positive pathogens to ceftobiprole, vancomycin, teicoplanin and linezolid [abstract no. P1633]. Clin Microbiol Infect. 2009;15(Suppl 4):S464–5.
Farrell DJ, Flamm RK, Sader HS, et al. Ceftobiprole activity against over 60,000 clinical bacterial pathogens isolated in Europe, Turkey, and Israel from 2005 to 2010. Antimicrob Agents Chemother. 2014;58(7):3882–8.
Flamm RK, Farrell DJ, Streit JM, et al. Antimicrobial activity of ceftobiprole tested against staphylococci and streptococci from European countries and Israel (2013) [abstract no. eP187]. In: 24th European Congress of Clinical Microbiology and Infectious Diseases; 10–13 May 2014; Barcelona.
Flamm RK, Farrell DJ, Streit JM, et al. Ceftobiprole activity tested against bacterial isolates from hospitalised patients with pneumonia in European hospitals and Israel (2013) [abstract no. eP188]. In: 24th European Congress of Clinical Microbiology and Infectious Diseases; 10–13 May 2014; Barcelona.
Farrell DJ, Flamm RK, Sader HS, et al. Activity of ceftobiprole against methicillin-resistant Staphylococcus aureus strains with reduced susceptibility to daptomycin, linezolid or vancomycin, and strains with defined SCCmec types. Int J Antimicrob Agents. 2014;43(4):323–7.
Lascols C, Legrand P, Merens A, et al. In vitro antibacterial activity of ceftobiprole against clinical isolates from French teaching hospitals: proposition of zone diameter breakpoints. Int J Antimicrob Agents. 2011;37(3):235–9.
Bogdanovich T, Ednie LM, Shapiro S, et al. Antistaphylococcal activity of ceftobiprole, a new broad-spectrum cephalosporin. Antimicrob Agents Chemother. 2005;49(10):4210–9.
Leonard SN, Cheung CM, Rybak MJ. Activities of ceftobiprole, linezolid, vancomycin, and daptomycin against community-associated and hospital-associated methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2008;52(8):2974–6.
Lin G, Appelbaum PC. Activity of ceftobiprole compared with those of other agents against Staphylococcus aureus strains with different resistotypes by time-kill analysis. Diagn Microbiol Infect Dis. 2008;60(2):233–5.
Deshpande L, Rhomberg PR, Fritsche TR, et al. Bactericidal activity of BAL9141, a novel parenteral cephalosporin against contemporary Gram-positive and Gram-negative isolates. Diagn Microbiol Infect Dis. 2004;50(1):73–5.
Zhanel GG, Voth D, Nichol K, et al. Pharmacodynamic activity of ceftobiprole compared with vancomycin versus methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-intermediate Staphylococcus aureus (VISA) and vancomycin-resistant Staphylococcus aureus (VRSA) using an in vitro model. J Antimicrob Chemother. 2009;64(2):364–9.
Lemaire S, Glupczynski Y, Duval V, et al. Activities of ceftobiprole and other cephalosporins against extracellular and intracellular (THP-1 macrophages and keratinocytes) forms of methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2009;53(6):2289–97.
Kosowska K, Hoellman DB, Lin G, et al. Antipneumococcal activity of ceftobiprole, a novel broad-spectrum cephalosporin. Antimicrob Agents Chemother. 2005;49(5):1932–42.
Arias CA, Singh KV, Panesso D, et al. Time-kill and synergism studies of ceftobiprole against Enterococcus faecalis, including beta-lactamase-producing and vancomycin-resistant isolates. Antimicrob Agents Chemother. 2007;51(6):2043–7.
Bogdanovich T, Clark C, Ednie L, et al. Activities of ceftobiprole, a novel broad-spectrum cephalosporin, against Haemophilus influenzae and Moraxella catarrhalis. Antimicrob Agents Chemother. 2006;50(6):2050–7.
Kresken M, Korber-Irrgang B, Lauffer J, et al. In vitro activities of ceftobiprole combined with amikacin or levofloxacin against Pseudomonas aeruginosa: evidence of a synergistic effect using time-kill methodology. Int J Antimicrob Agents. 2011;38(1):70–5.
Issa NC, Rouse MS, Piper KE, et al. In vitro activity of BAL9141 against clinical isolates of Gram-negative bacteria. Diagn Microbiol Infect Dis. 2004;48(1):73–5.
Pankuch GA, Appelbaum PC. Postantibiotic effect of ceftobiprole against 12 Gram-positive organisms. Antimicrob Agents Chemother. 2006;50(11):3956–8.
Craig WA, Andes DR. In vivo pharmacodynamics of ceftobiprole against multiple bacterial pathogens in murine thigh and lung infection models. Antimicrob Agents Chemother. 2008;52(10):3492–6.
Fernandez J, Abbanat D, Shang W, et al. Synergistic activity of ceftobiprole and vancomycin in a rat model of infective endocarditis caused by methicillin-resistant and glycopeptide-intermediate Staphylococcus aureus. Antimicrob Agents Chemother. 2012;56(3):1476–84.
Entenza JM, Veloso TR, Vouillamoz J, et al. In vivo synergism of ceftobiprole and vancomycin against experimental endocarditis due to vancomycin-intermediate Staphylococcus aureus. Antimicrob Agents Chemother. 2011;55(9):3977–84.
Entenza JM, Vouillamoz J, Bizzini A, et al. In vitro synergism between ceftobiprole and vancomycin against methicillin-resistant and glycopeptide-intermediate Staphylococcus aureus [abstract no. O39]. Clin Microbiol Infect. 2010;16(Suppl 2):S9.
Werth BJ, al. E. Ceftobiprole (BPR) and ampicillin (AMP) increase daptomycin (DAP) susceptibility in DAP susceptible and resistant vancomycin resistant Enterococci (VRE) [abstract no. A-1425]. In: 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy; 10–13 Sep 2013; Denver (CO).
Barber KE, al. E. Activity of ceftobiprole (BPR) combination regimens against multiple strains of Staphylococcus aureus with differing resistance phenotypes [abstract no. E-138]. In: 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy; 10–13 Sep 2013; Denver (CO).
Lin G, Ednie LM, Appelbaum PC. Antistaphylococcal activity of ACHN-490 tested alone and in combination with other agents by time-kill assay. Antimicrob Agents Chemother. 2010;54(5):2258–61.
Rodvold KA, Nicolau DP, Lodise TP, et al. Identifying exposure targets for treatment of staphylococcal pneumonia with ceftobiprole. Antimicrob Agents Chemother. 2009;53(8):3294–301.
Laohavaleeson S, Tessier PR, Nicolau DP. Pharmacodynamic characterization of ceftobiprole in experimental pneumonia caused by phenotypically diverse Staphylococcus aureus strains. Antimicrob Agents Chemother. 2008;52(7):2389–94.
Rouse MS, Hein MM, Anguita-Alonso P, et al. Ceftobiprole medocaril (BAL5788) treatment of experimental Haemophilus influenzae, Enterobacter cloacae, and Klebsiella pneumoniae murine pneumonia. Diagn Microbiol Infect Dis. 2006;55(4):333–6.
Azoulay-Dupuis E, Bedos JP, Mohler J, et al. Efficacy of BAL5788, a prodrug of cephalosporin BAL9141, in a mouse model of acute pneumococcal pneumonia. Antimicrob Agents Chemother. 2004;48(4):1105–11.
Nerandzic MM, Donskey CJ. Effect of ceftobiprole treatment on growth of and toxin production by Clostridium difficile in cecal contents of mice. Antimicrob Agents Chemother. 2011;55(5):2174–7.
Queenan AM, Shang W, Kania M, et al. Interactions of ceftobiprole with beta-lactamases from molecular classes A to D. Antimicrob Agents Chemother. 2007;51(9):3089–95.
Banerjee R, Gretes M, Basuino L, et al. In vitro selection and characterization of ceftobiprole-resistant methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2008;52(6):2089–96.
Queenan AM, Shang W, Bush K, et al. Differential selection of single-step AmpC or efflux mutants of Pseudomonas aeruginosa by using cefepime, ceftazidime, or ceftobiprole. Antimicrob Agents Chemother. 2010;54(10):4092–7.
Murthy B, Schmitt-Hoffmann A. Pharmacokinetics and pharmacodynamics of ceftobiprole, an anti-MRSA cephalosporin with broad-spectrum activity. Clin Pharmacokinet. 2008;47(1):21–33.
Lee D-G, Murakami Y, Andes DR, et al. Inoculum effects of ceftobiprole, daptomycin, linezolid, and vancomycin with Staphylococcus aureus and Streptococcus pneumoniae at inocula of 105 and 107 CFU injected into opposite thighs of neutropenic mice. Antimicrob Agents Chemother. 2013;57(3):1434–41.
Lodise TP Jr, Pypstra R, Kahn JB, et al. Probability of target attainment for ceftobiprole as derived from a population pharmacokinetic analysis of 150 subjects. Antimicrob Agents Chemother. 2007;51(7):2378–87.
Mouton JW, Schmitt-Hoffmann A, Shapiro S, et al. Use of Monte Carlo simulations to select therapeutic doses and provisional breakpoints of BAL9141. Antimicrob Agents Chemother. 2004;48(5):1713–8.
Muller AE, Schmitt-Hoffmann AH, Punt N, et al. Monte Carlo simulations based on phase 1 studies predict target attainment of ceftobiprole in nosocomial pneumonia patients: a validation study. Antimicrob Agents Chemother. 2013;57(5):2047–53.
Muller AE, Punt N, Mouton JW. Exposure to ceftobiprole is associated with microbiological eradication and clinical cure in patients with nosocomial pneumonia. Antimicrob Agents Chemother. 2014;58(5):2512–9.
Muller E, al. E. %fT>MIC predicts the microbiological eradication at end of treatment with ceftriaxone or ceftobiprole in patients with community acquired pneumonia [abstract no. A-472]. In: 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy; 10–13 Sep 2013; Denver (CO).
Schmitt-Hoffmann A, Nyman L, Roos B, et al. Multiple-dose pharmacokinetics and safety of a novel broad-spectrum cephalosporin (BAL5788) in healthy volunteers. Antimicrob Agents Chemother. 2004;48(7):2576–80.
Schmitt-Hoffmann A, Roos B, Schleimer M, et al. Single-dose pharmacokinetics and safety of a novel broad-spectrum cephalosporin (BAL5788) in healthy volunteers. Antimicrob Agents Chemother. 2004;48(7):2570–5.
Roos B, Schmitt-Hoffmann A, Schleimer M, et al. Safety and pharmacokinetics of BAL5788 in healthy subjects with normal or impaired renal function [abstract no. A-23]. In: 43rd Annual Interscience Conference on Antimicrobial Agents and Chemotherapy; 14–17 Sep 2003; Chicago (IL).
Awad SS, Rodriguez AH, Chuang YC, et al. A phase 3 randomized double-blind comparison of ceftobiprole medocaril versus ceftazidime plus linezolid for the treatment of hospital-acquired pneumonia. Clin Infect Dis. 2014.
Nicholson SC, Welte T, File TM Jr, et al. A randomised, double-blind trial comparing ceftobiprole medocaril with ceftriaxone with or without linezolid for the treatment of patients with community-acquired pneumonia requiring hospitalisation. Int J Antimicrob Agents. 2012;39(3):240–6.
Welte T, Herrera G, Chuang Y-C, et al. Early clinical response in a randomised controlled phase 3 study of ceftobiprole versus ceftriaxone with or without linezolid in patients with community-acquired pneumonia requiring hospitalisation [abstract no. eP431]. In: 24th European Congress of Clinical Microbiology and Infectious Diseases; 10–13 May 2014; Barcelona.
Data on file, Basilea Pharmaceutica International Ltd., 2014.
Backstrom T, Panagiotidis G, Beck O, et al. Effect of ceftobiprole on the normal human intestinal microflora. Int J Antimicrob Agents. 2010;36(6):537–41.
Basilea Pharmaceutica International Ltd. Swissmedic accepts for review Basilea’s ceftobiprole marketing authorization application for the treatment of pneumonia [media release]. 16 Sep 2013. http://hugin.info/134390/R/1729288/577711.pdf.
Frampton JE. Ceftaroline fosamil: a review of its use in the treatment of complicated skin and soft tissue infections and community-acquired pneumonia. Drugs. 2013;73(10):1067–94.
Disclosure
The preparation of this review was not supported by any external funding. Yahiya Y. Syed is a salaried employee of Adis/Springer. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the author(s) on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Syed, Y.Y. Ceftobiprole Medocaril: A Review of Its Use in Patients with Hospital- or Community-Acquired Pneumonia. Drugs 74, 1523–1542 (2014). https://doi.org/10.1007/s40265-014-0273-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-014-0273-x