Summary
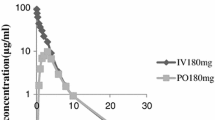
Cefpodoxime proxetil is an orally absorbed broad spectrum third generation cephalosporin antibacterial. It is a prodrug that is de-esterified in vivo to its active metabolite, cefpodoxime. After single- and multiple-dose (12-hourly) administration of cefpodoxime proxetil in the therapeutic dose range of 100 to 400mg of cefpodoxime equivalents, average peak plasma concentrations of cefpodoxime range from 1.0 to 4.5 mg/L and occur between 1.9 and 3.1 hours after administration. The half-life of cefpodoxime ranges from 1.9 to 2.8 hours. The absolute bioavailability of cefpodoxime proxetil tablets is 50%, and absorption is enhanced by concomitant administration of food. Raising gastric pH by pretreatment with antacids or H2-receptor antagonists results in reduced absorption. Binding of cefpodoxime to human plasma or serum protein is low ( 18 to 23%), suggesting that cefpodoxime should readily transfer across the capillary lining into tissues. Cefpodoxime undergoes minimal metabolism in humans. Drug not absorbed is degraded in the gastrointestinal tract and excreted in the faeces. As expected for a drug eliminated primarily by renal excretion, the disposition of cefpodoxime is altered in patients with impaired renal function; the half-life increases, while apparent plasma clearance and renal clearance decrease. The pharmacokinetics of cefpodoxime after oral administration of cefpodoxime proxetil are not affected by age.
Similar content being viewed by others
References
Backhouse C, Wade A, Williamson P, Tremblay D, Lenfant B. Multiple-dose pharmacokinetics of cefpodoxime in young adult and elderly patients. Journal of Antimicrobial Chemotherapy 26 (Suppl. E): 29–34, 1990
Bombardt PA, Cathcart KS, Bothwell BE, Closson SK. Determination of cefpodoxime levels and cefpodoxime stability in human urine by direct injection HPLC with column-switching. Journal of Liquid Chromatography 14: 1729–1746, 1991
Borin MT, Hughes GS, Patel RK, Royer ME. Pharmacokinetic and tolerance studies of cefpodoxime after single and multiple dose oral administration of cefpodoxime proxetil. Journal of Clinical Pharmacology, in press, 1991
Borin MT, Hughes GS, Spillers CR, Patel RK. Pharmacokinetics of cefpodoxime in plasma and skin blister fluid following oral dosing of cefpodoxime proxetil. Antimicrobial Agents and Chemotherapy 34: 1094–1099, 1990
Cho N, Fukunaga K, Kunii K, Kimura T, Suzuki A. Basic and clinical studies on CS-807 in the obstetric and gynecological field. Chemotherapy (Tokyo) 36 (Suppl. 1): 923–940, 1988
Couraud L, Andrews JM, Lecoeur H, Sultan E, Lenfant B. Concentrations of cefpodoxime in plasma and lung tissue after a single oral dose of cefpodoxime proxetil. Journal of Antimicrobial Chemotherapy 26 (Suppl. E): 35–40, 1990
Dumont R, Guetat F, Andrews JM, Sultan E, Lenfant B. Concentrations of cefpodoxime in plasma and pleural fluid after a single oral dose of cefpodoxime proxetil. Journal of Antimicrobial Chemotherapy 26 (Suppl. E): 41–46, 1990
Fass RJ, Helsel VL. In vitro activity of U-76,252 (CS-807), a new oral cephalosporin. Antimicrobial Agents and Chemotherapy 32: 1082–1085, 1988
Fujii A, Maeda H, Yamazaki H, Arakawa S, Kamidono S. Clinical studies on CS-807 in the urological field. Chemotherapy (Tokyo) 36 (Suppl. 1): 788–801, 1988
Fujii R, Meguro H, Arimasu O, Shiraishi H, Abe T, et al. Overall clinical evaluation of cefpodoxime proxetil against infections in pediatric fields. Japanese Journal of Antibiotics 42: 1439–1455, 1989
Fukami K, Furuta S, Fukuda K, Kiyota R, Imakiire T, et al. Basic and clinical studies on CS-807 in infections in the otorhinolaryngological field. Chemotherapy (Tokyo) 36 (Suppl. 1): 1067–1073, 1988
Gehanno P, Andrews JM, Ichou F, Sultan E, Lenfant B. Concentrations of cefpodoxime in plasma and tonsillar tissue after a single oral dose of cefpodoxime proxetil. Journal of Antimicrobial Chemotherapy 26 (Suppl. E): 47–51, 1990
Hayashi I, Ohnuma K. Clinical results of CS-807 on acute exacerbation of chronic bronchitis. Chemotherapy (Tokyo) 36 (Suppl. 1): 386–390, 1988
Hisaoka M, Ichikawa M, Kojima T. Microbiological assay for determining the CS-807 concentration in body fluids. Chemotherapy (Tokyo) 36 (Suppl. 1): 185–193, 1988
Hoffler D, Koeppe P, Corcilius M, Przyklink A. Cefpodoxime proxetil in patients with endstage renal failure on hemodialysis. Infection 18: 157–162, 1990
Hoshino M, Mogi S, Takahashi H. Clinical evaluation of CS-807 against skin and soft tissue infections. Chemotherapy (Tokyo) 36 (Suppl. 1): 1074–1078, 1988
Hughes GS, Heald DL, Barker KB, Patel RK, Spillers CR, et al. The effects of gastric pH and food on the pharmacokinetics of a new oral cephalosporin, cefpodoxime proxetil. Clinical Pharmacology and Therapeutics 46: 674–685, 1989
Hughes GS, Heald DL, Patel R, Spillers CR, Batts DH, et al. Gastric emptying and the pharmacokinetics of the cephalosporin antibiotic, cefpodoxime proxetil. Methods and Findings in Experimental and Clinical Pharmacology 12: 197–204, 1990
Ishibashi H, Ohta K, Kudo K, Kabe J. CS-807 in respiratory tract infections. Chemotherapy (Tokyo) 36 (Suppl. 1): 419–423, 1988
Iwai N, Taneda Y, Nakamura H, Miyazu M, Kasai K. Pharmacokinetic bacteriological and clinical evaluation of cefpodoxime proxetil in pediatrics. Japanese Journal of Antibiotics 42: 1571–1592, 1989
Jones RN, Barry AL. Antimicrobial activity and disk diffusion susceptibility testing of U-76,253A (R-3746), the active metabolite of the new cephalosporin ester, U-76,252 (CS-807). Antimicrobial Agents and Chemotherapy 32: 443–449, 1988
Jones RN, Barry AL, Pfaller M, Allen SD, Iyers LW, et al. Antimicrobial activity of U-76,252 (CS-807), a new orally administered cephalosporin ester, including recommendations for MIC quality control. Diagnostic Microbiology and Infectious Diseases 9: 59–63, 1988
Kobayashi K, Shimizu Y, Shiota K. CS-807 in respiratory tract infection. Chemotherapy (Tokyo) 36 (Suppl. 1): 515–521, 1988
Komai T, Kawai K, Tsubaki H, Tokui T, Kinoshita T, et al. Absorption, distribution, metabolism and excretion of CS-807, a new oral cephem antibiotic, in experimental animals. Chemotherapy (Tokyo) 36 (Suppl. 1): 229–240, 1988
Matsumoto T, Matsuda T, Urabe H. CS-807 in bacterial infections of the skin. Chemotherapy (Tokyo) 36 (Suppl. 1): 1106–1110, 1988
Molina F, Jehl F, Gallion C, Penner F, Monteil H. Determination of the third generation oral cephalosporin cefpodoxime in biological fluids by high-speed high-performance liquid chromatography. Journal of Chromatography and Biomedical Applications 563: 205–210, 1991
Naber KG, Kinzig M, Adam D, Sorgel F, Bajorski AH, et al. Concentrations of cefpodoxime in plasma, ejaculate and in prostatic fluid and adenoma tissue. Infection 19: 30–35, 1991
Nakayama I, Yamaji E, Kawamura H, Kawaguchi H, Akieda Y, et al. Clinical application of CS-807, an oral cephalosporin antibiotic, to skin and soft tissue infections. Chemotherapy (Tokyo) 36 (Suppl. 1): 620–647, 1988
Niino K, Sato H, Narita A, Nakazawa S, Suzuki H, et al. Evaluation of cefpodoxime proxetil in the pediatric field. Japanese Journal of Antibiotics 42: 1505–1518, 1989
O’Neill P, Nye K, Llouce G, Andrews J, Wise R. Pharmacokinetics and inflammatory fluid penetration of cefpodoxime proxetil in volunteers. Antimicrobial Agents and Chemotherapy 34: 232–234, 1990
Patel RK. Development and validation of microbioassay for cefpodoxime. 90th Meeting of the American Society for Microbiology, Anaheim, California, May 13–17, 1990. Abstract no. A142, 1990
Safran C. Cefpodoxime proxetil: dosage, efficacy and tolerance in adults suffering from respiratory tract infections. Journal of Antimicrobial Chemotherapy 26 (Suppl. E): 93–101, 1990
Sekine M, Sasahara K, Ichikawa M. High-performance liquid Chromatographic method for determing the CS-807 concentration in body fluids. Chemotherapy (Tokyo) 36 (Suppl. 1): 194–199, 1988
Shinkawa A, Tamura Y, Shimizu K, Miyake H. CS-807 in otorhinolaryngological infections. Chemotherapy (Tokyo) 36 (Suppl. 1): 1046–1055, 1988
Steenwyk RC, Brewer JE, Royer ME, Cathcart KS. Reversed-phase liquid Chromatographie determination of cefpodoxime in human plasma. Journal of Liquid Chromatography Clinical Analysis, in press, 1991
St Peter JV, Borin MT, Kelloway JS, Shapiro BE, Halstenson CE. Cefpodoxime proxetil disposition in patients with various degrees of renal function. Journal of Clinical Pharmacology 30: 838, 1990
Takizawa K, Ino Y, Iguchi T, Takeda Y. Antibacterial and clinical effects of a newly synthesized antibiotic, CS-807, in gynecological infections. Chemotherapy (Tokyo) 36 (Suppl. 1): 903–906, 1988
Tremblay D, Dupront A, Ho C, Coussediere D, Lenfant B. Pharmacokinetics of cefpodoxime in young and elderly volunteers after single doses. Journal of Antimicrobial Chemotherapy 26 (Suppl. E): 21–28, 1990
Uchida E, Oguchi K, Hisaoka M, Kobyashi S, Kai K, et al. Effects of ranitidine, metoclopramide, and anisotropine methylbromide on the availability of cefpodoxime proxetil (CS-807) in Japanese healthy subjects. Japanese Journal of Clinical Pharmacology and Therapeutics 19: 573–579, 1988
Ueda S, Hayashi K, Okabe T, Yoshizumi O, Yamashita T, et al. Fundamental and clinical studies on CS-807. Chemotherapy (Tokyo) 36 (Suppl. 1): 859–867, 1988
Utsui Y, Inoue M, Mitsuhashi S. In vitro and in vivo antibacterial properties of CS-807, a new oral cephalosporin. Antimicrobial Agents and Chemotherapy 31: 1085–1092, 1987
Wise R. The pharmacokinetics of the oral cephalosporins — a review. Journal of Antimicrobial Chemotherapy 26 (Suppl. E): 13–20, 1990
Yamamoto T, Yasuda SJ, Kanao M, Okada H. CS-807 in the field of obstetics and gynecology. Chemotherapy (Tokyo) 36 (Suppl. 1): 967–978, 1988
Yamasaki A, Seo K, Sanda N, Seko S, Nakano H, et al. CS-807 in urinary tract infections. Chemotherapy (Tokyo) 36 (Suppl. 1): 813–818, 1988
Yokota T, Suzuki E, Arai K. Cefpodoxime proxetil, its in vitro antibacterial activity, affinity to bacterial penicillin-binding proteins, and synergy of bactericidal activity with serum complement and mouse-cultured macrophages. Drugs Under Experimental and Clinical Research 14: 495–500, 1988
Yura J, Shinagawa N, Mizuno A, Mashita K, Taniguchi M, et al. CS-807 in surgery. Chemotherapy (Tokyo) 36 (Suppl. 1): 664–676, 1988
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Borin, M.T. A Review of the Pharmacokinetics of Cefpodoxime Proxetil. Drugs 42 (Suppl 3), 13–21 (1991). https://doi.org/10.2165/00003495-199100423-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199100423-00005