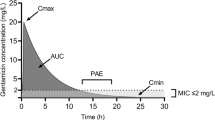
Abstract
Cefepime is a broad-spectrum fourth-generation cephalosporin with activity against Gram-positive and Gram-negative pathogens. It is generally administered as an infusion over 30–60 min or as a prolonged infusion with infusion times from 3 h to continuous administration. Cefepime is widely distributed in biological fluids and tissues with an average volume of distribution of ~ 0.2 L/kg in healthy adults with normal renal function. Protein binding is relatively low (20%), and elimination is mainly renal. About 85% of the dose is excreted unchanged in the urine, with an elimination half-life of 2–2.3 h. The pharmacokinetics of cefepime is altered under certain pathophysiological conditions, resulting in high inter-individual variability in cefepime volume of distribution and clearance, which poses challenges for population dosing approaches. Consequently, therapeutic drug monitoring of cefepime may be beneficial in certain patients including those who are critically ill, have life-threatening infections, or are infected with more resistant pathogens. Cefepime is generally safe and efficacious, with a goal exposure target of 70% time of the free drug concentration over the minimum inhibitory concentration for clinical efficacy. In recent years, reports of neurotoxicity have increased, specifically in patients with impaired renal function. This review summarizes the pharmacokinetics, pharmacodynamics, and toxicodynamics of cefepime contemporarily in the setting of increasing cefepime exposures. We explore the potential benefits of extended or continuous infusions and therapeutic drug monitoring in special populations.



Similar content being viewed by others
References
US FDA. Cefepime FDA prescribing information, side effects and uses. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/050679s042lbl.pdf
Magill SS, O’Leary E, Ray SM, Kainer MA, Evans C, Bamberg WM, Team EIPHPS, et al. Antimicrobial use in US hospitals: comparison of results from emerging infections program prevalence surveys, 2015 and 2011. Clin Infect Dis. 2020;72(10):1784–92. https://doi.org/10.1093/cid/ciaa373.
Okamoto MP, Nakahiro RK, Chin A, Bedikian A. Cefepime clinical pharmacokinetics. Clin Pharmacokinet. 1993;25(2):88–102. https://doi.org/10.2165/00003088-199325020-00002.
Georges B, Conil JM, Cougot P, Decun JF, Archambaud M, Seguin T, et al. Cefepime in critically ill patients: continuous infusion vs. an intermittent dosing regimen. Int J Clin Pharmacol Ther. 2005;43(8):360–9. https://doi.org/10.5414/cpp43360.
Jaruratanasirikul S, Sriwiriyajan S, Ingviya N. Continuous infusion versus intermittent administration of cefepime in patients with Gram-negative bacilli bacteraemia. J Pharm Pharmacol. 2002;54(12):1693–6. https://doi.org/10.1211/002235702171.
Burgess DS, Hastings RW, Hardin TC. Pharmacokinetics and pharmacodynamics of cefepime administered by intermittent and continuous infusion. Clin Ther. 2000;22(1):66–75. https://doi.org/10.1016/s0149-2918(00)87978-3.
Garrelts JC, Wagner DJ. The pharmacokinetics, safety, and tolerance of cefepime administered as an intravenous bolus or as a rapid infusion. Ann Pharmacother. 1999;33(12):1258–61. https://doi.org/10.1345/aph.19067.
Okamoto MP, Nakahiro RK, Chin A, Bedikian A, Gill MA. Cefepime: a new fourth-generation cephalosporin. Am J Hosp Pharm. 1994;51(4):463–77 (quiz 541–62).
Barbhaiya RH, Forgue ST, Gleason CR, Knupp CA, Pittman KA, Weidler DJ, et al. Pharmacokinetics of cefepime after single and multiple intravenous administrations in healthy subjects. Antimicrob Agents Chemother. 1992;36(3):552–7. https://doi.org/10.1128/aac.36.3.552.
Barbhaiya RH, Knupp CA, Tenney J, Martin RR, Weidler DJ, Pittman KA. Safety, tolerance, and pharmacokinetics of cefepime administered intramuscularly to healthy subjects. J Clin Pharmacol. 1990;30(10):900–10. https://doi.org/10.1002/j.1552-4604.1990.tb03569.x.
Walker P, Neuhauser MN, Tam VH, Willey JS, Palmer JL, Bruera E, et al. Subcutaneous administration of cefepime. J Pain Symptom Manage. 2005;30(2):170–4. https://doi.org/10.1016/j.jpainsymman.2005.03.007.
Pilmis B, Mizrahi A, Petitjean G, Le Monnier A, El Helali N. Clinical evaluation of subcutaneous administration of cefepime. Med Mal Infect. 2020;50(3):308–10. https://doi.org/10.1016/j.medmal.2019.12.006.
Nye KJ, Shi YG, Andrews JM, Wise R. Pharmacokinetics and tissue penetration of cefepime. J Antimicrob Chemother. 1989;24(1):23–8. https://doi.org/10.1093/jac/24.1.23.
Kalman D, Barriere SL, Johnson BL Jr. Pharmacokinetic disposition and bactericidal activities of cefepime, ceftazidime, and cefoperazone in serum and blister fluid. Antimicrob Agents Chemother. 1992;36(2):453–7. https://doi.org/10.1128/aac.36.2.453.
Okamoto MP, Chin A, Gill MA, Yellin AE, Berne TV, Heseltine PN, et al. Analysis of cefepime tissue penetration into human appendix. Pharmacotherapy. 1991;11(5):353–8.
Okamoto MP, Gill MA, Nakahiro RK, Chin A, Yellin AE, Berne TV, et al. Tissue concentrations of cefepime in acute cholecystitis patients. Ther Drug Monit. 1992;14(3):220–5. https://doi.org/10.1097/00007691-199206000-00008.
Chadha D, Wise R, Baldwin DR, Andrews JM, Ashby JP, Honeybourne D. Cefepime concentrations in bronchial mucosa and serum following a single 2 gram intravenous dose. J Antimicrob Chemother. 1990;25(6):959–63. https://doi.org/10.1093/jac/25.6.959.
Arkell D, Ashrap M, Andrews JM, Wise R. An evaluation of the penetration of cefepime into prostate tissue in patients undergoing elective prostatectomy. J Antimicrob Chemother. 1992;29(4):473–4. https://doi.org/10.1093/jac/29.4.473.
Koufopoulou SA, Pistos C, Giaginis C, Tsantili-Kakoulidou A. Application of the ion pair concept to the n-octanol-water partitioning of cefepime and cefpirome. Int J Pharm. 2006;316(1–2):52–7. https://doi.org/10.1016/j.ijpharm.2006.02.033.
Van der Auwera P, Santella PJ. Pharmacokinetics of cefepime: a review. J Antimicrob Chemother. 1993;32(Suppl_B):103–15. https://doi.org/10.1093/jac/32.suppl_B.103.
Barbhaiya RH, Knupp CA, Forgue ST, Matzke GR, Guay DR, Pittman KA. Pharmacokinetics of cefepime in subjects with renal insufficiency. Clin Pharmacol Ther. 1990;48(3):268–76. https://doi.org/10.1038/clpt.1990.149.
Cronqvist, J., et al., Pharmacokinetics of cefepime dihydrochloride arginine in subjects with renal impairment. Antimicrob Agents Chemother, 1992;36(12):2676-2680.
Arguedas AG, Stutman HR, Zaleska M, Knupp CA, Marks MI, Nussbaum E. Cefepime. Pharmacokinetics and clinical response in patients with cystic fibrosis. Am J Dis Child. 1992;146(7):797–802. https://doi.org/10.1001/archpedi.1992.02160190029013.
Barbhaiya RH, Knupp CA, Forgue ST, Matzke GR, Halstenson CE, Opsahl JA, et al. Disposition of the cephalosporin cefepime in normal and renally impaired subjects. Drug Metab Dispos. 1991;19(1):68–73.
Chapuis TM, Giannoni E, Majcherczyk PA, Chioléro R, Schaller MD, Berger MM, et al. Prospective monitoring of cefepime in intensive care unit adult patients. Crit Care. 2010;14(2):R51. https://doi.org/10.1186/cc8941.
Nicasio AM, Ariano RE, Zelenitsky SA, Kim A, Crandon JL, Kuti JL, et al. Population pharmacokinetics of high-dose, prolonged-infusion cefepime in adult critically ill patients with ventilator-associated pneumonia. Antimicrob Agents Chemother. 2009;53(4):1476–81. https://doi.org/10.1128/aac.01141-08.
Lipman J, Wallis SC, Rickard C. Low plasma cefepime levels in critically ill septic patients: pharmacokinetic modeling indicates improved troughs with revised dosing. Antimicrob Agents Chemother. 1999;43(10):2559–61. https://doi.org/10.1128/aac.43.10.2559.
Roos JF, Bulitta J, Lipman J, Kirkpatrick CM. Pharmacokinetic-pharmacodynamic rationale for cefepime dosing regimens in intensive care units. J Antimicrob Chemother. 2006;58(5):987–93. https://doi.org/10.1093/jac/dkl349.
Tam VH, McKinnon PS, Akins RL, Drusano GL, Rybak MJ. Pharmacokinetics and pharmacodynamics of cefepime in patients with various degrees of renal function. Antimicrob Agents Chemother. 2003;47(6):1853–61. https://doi.org/10.1128/aac.47.6.1853-1861.2003.
Lipman J, Wallis SC, Boots RJ. Cefepime versus cefpirome: the importance of creatinine clearance. Anesth Analg. 2003;97(4):1149–54.
Rhodes NJ, Grove ME, Kiel PJ, O’Donnell JN, Whited LK, Rose DT, et al. Population pharmacokinetics of cefepime in febrile neutropenia: implications for dose-dependent susceptibility and contemporary dosing regimens. Int J Antimicrob Agents. 2017;50(3):482–6. https://doi.org/10.1016/j.ijantimicag.2017.04.008.
Álvarez JC, Cuervo SI, Silva E, Díaz JA, Jiménez LL, Parra DS, et al. Pharmacokinetics and pharmacodynamics of cefepime in adults with hematological malignancies and febrile neutropenia after chemotherapy. Antibiotics (Basel). 2021;10(5). https://doi.org/10.3390/antibiotics10050504.
Sime FB, Roberts MS, Tiong IS, Gardner JH, Lehman S, Peake SL, et al. Adequacy of high-dose cefepime regimen in febrile neutropenic patients with hematological malignancies. Antimicrob Agents Chemother. 2015;59(9):5463–9. https://doi.org/10.1128/aac.00389-15.
Whited L, Grove M, Rose D, Rhodes NJ, Scheetz MH, O’Donnell JN. Pharmacokinetics of cefepime in patients with cancer and febrile neutropenia in the setting of hematologic malignancies or hematopoeitic cell transplantation. Pharmacotherapy. 2016;36(9):1003–10. https://doi.org/10.1002/phar.1807.
Paul M, Yahav D, Fraser A, Leibovici L. Empirical antibiotic monotherapy for febrile neutropenia: systematic review and meta-analysis of randomized controlled trials. J Antimicrob Chemother. 2006;57(2):176–89. https://doi.org/10.1093/jac/dki448.
Horita N, Shibata Y, Watanabe H, Namkoong H, Kaneko T. Comparison of antipseudomonal β-lactams for febrile neutropenia empiric therapy: systematic review and network meta-analysis. Clin Microbiol Infect. 2017;23(10):723–9. https://doi.org/10.1016/j.cmi.2017.03.024.
Kieft H, Hoepelman AI, Knupp CA, van Dijk A, Branger JM, Struyvenberg A, et al. Pharmacokinetics of cefepime in patients with the sepsis syndrome. J Antimicrob Chemother. 1993;32 Suppl. B:117–22. https://doi.org/10.1093/jac/32.suppl_b.117.
Liu J, Neely M, Lipman J, Sime F, Roberts JA, Kiel PJ, et al. Development of population and Bayesian models for applied use in patients receiving cefepime. Clin Pharmacokinet. 2020;59(8):1027–36. https://doi.org/10.1007/s40262-020-00873-3.
Jacobs A, Taccone FS, Roberts JA, Jacobs F, Cotton F, Wolff F, et al. β-Lactam dosage regimens in septic patients with augmented renal clearance. Antimicrob Agents Chemother. 2018. https://doi.org/10.1128/aac.02534-17.
De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med. 2002;166(1):98–104. https://doi.org/10.1164/rccm.200109-016oc.
Levitt DG. The pharmacokinetics of the interstitial space in humans. BMC Clin Pharmacol. 2003;3:3. https://doi.org/10.1186/1472-6904-3-3.
Roberts JA, Abdul-Aziz MH, Lipman J, Mouton JW, Vinks AA, Felton TW, et al. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis. 2014;14(6):498–509. https://doi.org/10.1016/s1473-3099(14)70036-2.
Gerlach AT, Wenzler E, Hunt LN, Bazan JA, Bauer KA. Pharmacokinetic/pharmacodynamic predictions and clinical outcomes of patients with augmented renal clearance and Pseudomonas aeruginosa bacteremia and/or pneumonia treated with extended infusion cefepime versus extended infusion piperacillin/tazobactam. Int J Crit Illn Inj Sci. 2019;9(3):138–43. https://doi.org/10.4103/ijciis.Ijciis_70_18.
Al-Shaer MH, Philpott CD, Droege CA, Courter JD, Healy DP, Droege ME, et al. Cefepime population pharmacokinetics and target attainment in critically ill patients on continuous renal replacement therapy. Antimicrob Agents Chemother. 2021. https://doi.org/10.1128/aac.00144-21.
Klekner A, Bagyi K, Bognar L, Gaspar A, Andrasi M, Szabo J. Effectiveness of cephalosporins in the sputum of patients with nosocomial bronchopneumonia. J Clin Microbiol. 2006;44(9):3418–21. https://doi.org/10.1128/jcm.00893-06.
Boselli E, Breilh D, Duflo F, Saux MC, Debon R, Chassard D, et al. Steady-state plasma and intrapulmonary concentrations of cefepime administered in continuous infusion in critically ill patients with severe nosocomial pneumonia. Crit Care Med. 2003;31(8):2102–6. https://doi.org/10.1097/01.Ccm.0000069734.38738.C8.
Bayat S, Louchahi K, Verdière B, Anglade D, Rahoui A, Sorin PM, et al. Comparison of 99mTc-DTPA and urea for measuring cefepime concentrations in epithelial lining fluid. Eur Respir J. 2004;24(1):150–6. https://doi.org/10.1183/09031936.04.00106803.
Breilh D, Saux MC, Delaisement C, Fratta A, Ducint D, Velly JF, et al. Pharmacokinetic population study to describe cefepime lung concentrations. Pulm Pharmacol Ther. 2001;14(2):69–74. https://doi.org/10.1006/pupt.2000.0269.
Ikawa K, Morikawa N, Hayato S, Ikeda K, Ohge H, Sueda T. Pharmacokinetic and pharmacodynamic profiling of cefepime in plasma and peritoneal fluid of abdominal surgery patients. Int J Antimicrob Agents. 2007;30(3):270–3. https://doi.org/10.1016/j.ijantimicag.2007.04.012.
Wang JF, Wang Q, Zhao LH, Shi GZ, Zhou JX. Blood-brain barrier penetration of cefepime after neurosurgery. Chin Med J (Engl). 2007;120(13):1176–8.
Lodise TP Jr, Rhoney DH, Tam VH, McKinnon PS, Drusano GL. Pharmacodynamic profiling of cefepime in plasma and cerebrospinal fluid of hospitalized patients with external ventriculostomies. Diagn Microbiol Infect Dis. 2006;54(3):223–30. https://doi.org/10.1016/j.diagmicrobio.2005.09.007.
Avedissian SN, Pais G, Joshi MD, Rhodes NJ, Scheetz MH. A translational pharmacokinetic rat model of cerebral spinal fluid and plasma concentrations of cefepime. mSphere. 2019. https://doi.org/10.1128/mSphere.00595-18.
Sáez-Llorens X, Castaño E, García R, Báez C, Pérez M, Tejeira F, et al. Prospective randomized comparison of cefepime and cefotaxime for treatment of bacterial meningitis in infants and children. Antimicrob Agents Chemother. 1995;39(4):937–40. https://doi.org/10.1128/aac.39.4.937.
Robineau O, Talagrand-Reboulh E, Brunschweiler B, Jehl F, Beltrand E, Rousseau F, et al. Low prevalence of tissue detection of cefepime and daptomycin used as empirical treatment during revision for periprosthetic joint infections: results of a prospective multicenter study. Eur J Clin Microbiol Infect Dis. 2021. https://doi.org/10.1007/s10096-021-04277-4.
Breilh D, Boselli E, Bel JC, Chassard D, Saux MC, Allaouchiche B. Diffusion of cefepime into cancellous and cortical bone tissue. J Chemother. 2003;15(2):134–8. https://doi.org/10.1179/joc.2003.15.2.134.
Zhu Q, Gao X, Brown MD, Eismont F, Gu W. Transport of vancomycin and cefepime into human intervertebral discs: quantitative analyses. Spine (Phila Pa 1976). 2019;44(17):E992–9. https://doi.org/10.1097/brs.0000000000003028.
Dzierba AL, Abrams D, Brodie D. Medicating patients during extracorporeal membrane oxygenation: the evidence is building. Crit Care. 2017;21(1):66. https://doi.org/10.1186/s13054-017-1644-y.
Leven C, Fillâtre P, Petitcollin A, Verdier MC, Laurent J, Nesseler N, et al. Ex vivo model to decipher the impact of extracorporeal membrane oxygenation on beta-lactam degradation kinetics. Ther Drug Monit. 2017;39(2):180–4. https://doi.org/10.1097/ftd.0000000000000369.
Zuppa AF, Zane NR, Moorthy G, Dalton HJ, Abraham A, Reeder RW, et al. A population pharmacokinetic analysis to study the effect of extracorporeal membrane oxygenation on cefepime disposition in children. Pediatr Crit Care Med. 2019;20(1):62–70. https://doi.org/10.1097/pcc.0000000000001786.
Cheng V, Abdul-Aziz MH, Burrows F, Buscher H, Corley A, Diehl A, et al. Population pharmacokinetics of cefepime in critically ill patients receiving extracorporeal membrane oxygenation (an ASAP ECMO study). Int J Antimicrob Agents. 2021;58(6): 106466. https://doi.org/10.1016/j.ijantimicag.2021.106466.
Maynor LM, Carl DE, Matzke GR, Gehr TW, Farthing C, Farthing D, et al. An in vivo-in vitro study of cefepime and cefazolin dialytic clearance during high-flux hemodialysis. Pharmacotherapy. 2008;28(8):977–83. https://doi.org/10.1592/phco.28.8.977.
Schmaldienst S, Traunmüller F, Burgmann H, Rosenkranz AR, Thalhammer-Scherrer R, Hörl WH, et al. Multiple-dose pharmacokinetics of cefepime in long-term hemodialysis with high-flux membranes. Eur J Clin Pharmacol. 2000;56(1):61–4. https://doi.org/10.1007/s002280050721.
Descombes E, Martins F, Hemett OM, Erard V, Chuard C. Three-times-weekly, post-dialysis cefepime therapy in patients on maintenance hemodialysis: a retrospective study. BMC Pharmacol Toxicol. 2016;17:4. https://doi.org/10.1186/s40360-016-0048-y.
Barbhaiya RH, Knupp CA, Pfeffer M, Zaccardelli D, Dukes GM, Mattern W, et al. Pharmacokinetics of cefepime in patients undergoing continuous ambulatory peritoneal dialysis. Antimicrob Agents Chemother. 1992;36(7):1387–91. https://doi.org/10.1128/aac.36.7.1387.
Elwell RJ, Frye RF, Bailie GR. Pharmacokinetics of intraperitoneal cefepime in automated peritoneal dialysis. Perit Dial Int. 2005;25(4):380–6.
Beumier M, Casu GS, Hites M, Seyler L, Cotton F, Vincent JL, et al. β-lactam antibiotic concentrations during continuous renal replacement therapy. Crit Care. 2014;18(3):R105. https://doi.org/10.1186/cc13886.
Carlier M, Taccone FS, Beumier M, Seyler L, Cotton F, Jacobs F, et al. Population pharmacokinetics and dosing simulations of cefepime in septic shock patients receiving continuous renal replacement therapy. Int J Antimicrob Agents. 2015;46(4):413–9. https://doi.org/10.1016/j.ijantimicag.2015.05.020.
Scheetz MH, Scarsi KK, Ghossein C, Hurt KM, Zembower TR, Postelnick MJ. Adjustment of antimicrobial dosages for continuous venovenous hemofiltration based on patient-specific information. Clin Infect Dis. 2006;42(3):436–7. https://doi.org/10.1086/499535 (author reply 437–8).
Jang SM, Gharibian KN, Lewis SJ, Fissell WH, Tolwani AJ, Mueller BA. A Monte Carlo simulation approach for beta-lactam dosing in critically ill patients receiving prolonged intermittent renal replacement therapy. J Clin Pharmacol. 2018;58(10):1254–65. https://doi.org/10.1002/jcph.113.7.
Huls CE, Prince RA, Seilheimer DK, Bosso JA. Pharmacokinetics of cefepime in cystic fibrosis patients. Antimicrob Agents Chemother. 1993;37(7):1414–6. https://doi.org/10.1128/aac.37.7.1414.
Okamoto MP, Gill MA, Nakahiro RK, Bedikian A, Chin A, Yellin AE, Berne TV, Knupp CA (1993) Cefepime pharmacokinetics in patients with acute cholecystitis undergoing cholecystectomy. Clin Pharm 12 (2):134-137
Barbhaiya RH, Knupp CA, Pittman KA. Effects of age and gender on pharmacokinetics of cefepime. Antimicrob Agents Chemother. 1992;36(6):1181–5. https://doi.org/10.1128/aac.36.6.1181.
Kovarik JM, ter Maaten JC, Rademaker CM, Deenstra M, Hoepelman IM, Hart HC, et al. Pharmacokinetics of cefepime in patients with respiratory tract infections. Antimicrob Agents Chemother. 1990;34(10):1885–8. https://doi.org/10.1128/aac.34.10.1885.
Hites M, Taccone FS, Wolff F, Maillart E, Beumier M, Surin R, et al. Broad-spectrum β-lactams in obese non-critically ill patients. Nutr Diabetes. 2014;4(6): e119. https://doi.org/10.1038/nutd.2014.15.
Rich BS, Keel R, Ho VP, Turbendian H, Afaneh CI, Dakin GF, et al. Cefepime dosing in the morbidly obese patient population. Obes Surg. 2012;22(3):465–71. https://doi.org/10.1007/s11695-011-0586-8.
Conil JM, Georges B, Lavit M, Seguin T, Tack I, Samii K, et al. Pharmacokinetics of ceftazidime and cefepime in burn patients: the importance of age and creatinine clearance. Int J Clin Pharmacol Ther. 2007;45(10):529–38. https://doi.org/10.5414/cpp45529.
Bonapace CR, White RL, Friedrich LV, Norcross ED, Bosso JA. Pharmacokinetics of cefepime in patients with thermal burn injury. Antimicrob Agents Chemother. 1999;43(12):2848–54. https://doi.org/10.1128/aac.43.12.2848.
Sampol E, Jacquet A, Viggiano M, Bernini V, Manelli JC, Lacarelle B, et al. Plasma, urine and skin pharmacokinetics of cefepime in burns patients. J Antimicrob Chemother. 2000;46(2):315–7. https://doi.org/10.1093/jac/46.2.315.
Capparelli E, Hochwald C, Rasmussen M, Parham A, Bradley J, Moya F. Population pharmacokinetics of cefepime in the neonate. Antimicrob Agents Chemother. 2005;49(7):2760–6. https://doi.org/10.1128/aac.49.7.2760-2766.2005.
Lima-Rogel V, Medina-Rojas EL, Del Carmen M-S, Noyola DE, Nieto-Aguirre K, López-Delarosa A, et al. Population pharmacokinetics of cefepime in neonates with severe nosocomial infections. J Clin Pharm Ther. 2008;33(3):295–306. https://doi.org/10.1111/j.1365-2710.2008.00913.x.
Zhao Y, Yao BF, Kou C, Xu HY, Tang BH, Wu YE, et al. Developmental population pharmacokinetics and dosing optimization of cefepime in neonates and young infants. Front Pharmacol. 2020;11:14. https://doi.org/10.3389/fphar.2020.00014.
Reed MD, Yamashita TS, Knupp CK, Veazey JM Jr, Blumer JL. Pharmacokinetics of intravenously and intramuscularly administered cefepime in infants and children. Antimicrob Agents Chemother. 1997;41(8):1783–7. https://doi.org/10.1128/aac.41.8.1783.
Shoji K, Bradley JS, Reed MD, van den Anker JN, Domonoske C, Capparelli EV. Population pharmacokinetic assessment and pharmacodynamic implications of pediatric cefepime dosing for susceptible-dose-dependent organisms. Antimicrob Agents Chemother. 2016;60(4):2150–6. https://doi.org/10.1128/aac.02592-15.
Al-Shaer MH, Neely MN, Liu J, Cherabuddi K, Venugopalan V, Rhodes NJ, et al. Population pharmacokinetics and target attainment of cefepime in critically ill patients and guidance for initial dosing. Antimicrob Agents Chemother. 2020. https://doi.org/10.1128/aac.00745-20.
Blumer JL, Reed MD, Knupp C. Review of the pharmacokinetics of cefepime in children. Pediatr Infect Dis J. 2001;20(3):337–42. https://doi.org/10.1097/00006454-200103000-00032.
Griffith D, Dudley MN. Animal models of infection for the study of antibiotic pharmacodynamics. In: Nightingale CH, Ambrose PG, Drusano GL, Murakawa T (eds) Antimicrobial Pharmacodynamics in Theory and Clinical Practice, 2nd edn. Informa Healthcare USA, Inc. 2007; 2001.
Craig WA, Ebert SC. Killing and regrowth of bacteria in vitro: a review. Scand J Infect Dis Suppl. 1990;74:63–70.
Turnidge JD. The pharmacodynamics of β-lactams. Clin Infect Dis. 1998;27(1):10–22. https://doi.org/10.1086/514622.
Vogelman B, Gudmundsson S, Leggett J, Turnidge J, Ebert S, Craig WA. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J Infect Dis. 1988;158(4):831–47. https://doi.org/10.1093/infdis/158.4.831.
Vogelman B, Gudmundsson S, Turnidge J, Leggett J, Craig WA. In vivo postantibiotic effect in a thigh infection in neutropenic mice. J Infect Dis. 1988;157(2):287–98. https://doi.org/10.1093/infdis/157.2.287.
Craig WA. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn Microbiol Infect Dis. 1995;22(1):89–96. https://doi.org/10.1016/0732-8893(95)00053-D.
Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26(1):1–12. https://doi.org/10.1086/516284.
Dhaese S, Heffernan A, Liu D, Abdul-Aziz MH, Stove V, Tam VH, et al. Prolonged versus intermittent infusion of β-lactam antibiotics: a systematic review and meta-regression of bacterial killing in preclinical infection models. Clin Pharmacokinet. 2020;59(10):1237–50. https://doi.org/10.1007/s40262-020-00919-6.
Johnson A, McEntee L, Farrington N, Kolamunnage-Dona R, Franzoni S, Vezzelli A, et al. Pharmacodynamics of cefepime combined with the novel extended-spectrum-β-lactamase (ESBL) inhibitor enmetazobactam for murine pneumonia caused by ESBL-producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2020;64(6):e00180-e1120. https://doi.org/10.1128/AAC.00180-20.
Melchers MJ, van Mil AC, Lagarde C, den Hartigh J, Mouton JW. Pharmacodynamics of cefepime combined with tazobactam against clinically relevant Enterobacteriaceae in a neutropenic mouse thigh model. Antimicrob Agents Chemother. 2017. https://doi.org/10.1128/aac.00267-17.
Bulitta JB, Hope WW, Eakin AE, Guina T, Tam VH, Louie A, et al. Generating robust and informative nonclinical in vitro and in vivo bacterial infection model efficacy data to support translation to humans. Antimicrob Agents Chemother. 2019;63(5):e02307-e2318. https://doi.org/10.1128/AAC.02307-18.
NIAID Workshop. Pharmacokinetics-pharmacodynamics (PKPD) for development of therapeutics against bacterial pathogens; 14–15 June, 2017; Bethesda (MD).
Lee SY, Kuti JL, Nicolau DP. Cefepime pharmacodynamics in patients with extended spectrum β-lactamase (ESBL) and non-ESBL infections. J Infect. 2007;54(5):463–8. https://doi.org/10.1016/j.jinf.2006.09.004.
Tam VH, McKinnon PS, Akins RL, Rybak MJ, Drusano GL. Pharmacodynamics of cefepime in patients with Gram-negative infections. J Antimicrob Chemothe. 2002;50(3):425–8. https://doi.org/10.1093/jac/dkf130.
McKinnon PS, Paladino JA, Schentag JJ. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents. 2008;31(4):345–51. https://doi.org/10.1016/j.ijantimicag.2007.12.009.
Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G. DALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis. 2014;58(8):1072–83. https://doi.org/10.1093/cid/ciu027.
Rhodes NJ, Kuti JL, Nicolau DP, Van Wart S, Nicasio AM, Liu J, et al. Defining clinical exposures of cefepime for Gram-negative bloodstream infections that are associated with improved survival. Antimicrob Agents Chemother. 2015;60(3):1401–10. https://doi.org/10.1128/aac.01956-15.
Yamashita Y, Kamiyama H, Yamamoto A, Kanoh H, Yuhki Y, Ueda A, et al. Relationship between PK/PD of cefepime and clinical outcome in febrile neutropenic patients with normal renal function. Yakugaku Zasshi. 2016;136(12):1641–9. https://doi.org/10.1248/yakushi.16-00168.
Li C, Du X, Kuti JL, Nicolau DP. Clinical pharmacodynamics of meropenem in patients with lower respiratory tract infections. Antimicrob Agents Chemother. 2007;51(5):1725–30. https://doi.org/10.1128/aac.00294-06.
Crandon JL, Bulik CC, Kuti JL, Nicolau DP. Clinical pharmacodynamics of cefepime in patients infected with Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2010;54(3):1111–6. https://doi.org/10.1128/AAC.01183-09.
Miglis C, Rhodes NJ, Kuti JL, Nicolau DP, Van Wart SA, Scheetz MH. Defining the impact of severity of illness on time above the MIC threshold for cefepime in Gram-negative bacteraemia: a ‘Goldilocks’ window. Int J Antimicrob Agents. 2017;50(3):487–90. https://doi.org/10.1016/j.ijantimicag.2017.04.023.
Scheetz MH, Lodise TP, Downes KJ, Drusano G, Neely M. The case for precision dosing: medical conservatism does not justify inaction. J Antimicrob Chemother. 2021;76(7):1661–5. https://doi.org/10.1093/jac/dkab086.
Barreto EF, Webb AJ, Pais GM, Rule AD, Jannetto PJ, Scheetz MH. Setting the beta-lactam therapeutic range for critically ill patients: is there a floor or even a ceiling? Crit Care Explor. 2021;3(6): e0446.
Gijsen M, Bekkers B, Maertens J, Lagrou K, Desmet S, Dreesen E, et al. Prospective assessment of breakthrough infections and neurotoxicity and their association with cefepime trough concentrations in patients with febrile neutropenia. Int J Antimicrob Agents. 2021. https://doi.org/10.1016/j.ijantimicag.2021.106472.
Taccone FS, Laterre PF, Dugernier T, Spapen H, Delattre I, Wittebole X, et al. Insufficient β-lactam concentrations in the early phase of severe sepsis and septic shock. Crit Care. 2021;14(4):R126. https://doi.org/10.1186/cc9091.
Jonckheere S, De Neve N, Verbeke J, De Decker K, Brandt I, Boel A, et al. Target-controlled infusion of cefepime in critically ill patients. Antimicrob Agents Chemother. 2019. https://doi.org/10.1128/aac.01552-19.
Khan DA, Banerji A, Bernstein JA, Bilgicer B, Blumenthal K, Castells M, et al. Cephalosporin allergy: current understanding and future challenges. J Allergy Clin Immunol Pract. 2019;7(7):2105–14. https://doi.org/10.1016/j.jaip.2019.06.001.
Shenoy ES, Macy E, Rowe T, Blumenthal KG. Evaluation and management of penicillin allergy: a review. JAMA. 2019;321(2):188–99. https://doi.org/10.1001/jama.2018.19283.
Boschung-Pasquier L, Atkinson A, Kastner LK, Banholzer S, Haschke M, Buetti N, et al. Cefepime neurotoxicity: thresholds and risk factors: a retrospective cohort study. Clin Microbiol Infect. 2020;26(3):333–9. https://doi.org/10.1016/j.cmi.2019.06.028.
Fugate JE, Kalimullah EA, Hocker SE, Clark SL, Wijdicks EF, Rabinstein AA. Cefepime neurotoxicity in the intensive care unit: a cause of severe, underappreciated encephalopathy. Crit Care. 2013;17(6):R264. https://doi.org/10.1186/cc13094.
Huwyler T, Lenggenhager L, Abbas M, Ing Lorenzini K, Hughes S, Huttner B, et al. Cefepime plasma concentrations and clinical toxicity: a retrospective cohort study. Clin Microbiol Infect. 2017;23(7):454–9. https://doi.org/10.1016/j.cmi.2017.01.005.
Singh TD, O’Horo JC, Day CN, Mandrekar J, Rabinstein AA. Cefepime is associated with acute encephalopathy in critically ill patients: a retrospective case-control study. Neurocrit Care. 2020;33(3):695–700. https://doi.org/10.1007/s12028-020-01035-w.
Haddad NA, Schreier DJ, Fugate JE, Gajic O, Hocker SE, Ice CJ, et al. Incidence and predictive factors associated with beta-lactam neurotoxicity in the critically ill: a retrospective cohort study. Neurocrit Care. 2022. https://doi.org/10.1007/s12028-022-01442-1.
Jallon P, Fankhauser L, Du Pasquier R, Coeytaux A, Picard F, Hefft S, et al. Severe but reversible encephalopathy associated with cefepime. Neurophysiol Clin. 2000;30(6):383–6. https://doi.org/10.1016/s0987-7053(00)00234-3.
Lamoth F, Buclin T, Pascual A, Vora S, Bolay S, Decosterd LA, et al. High cefepime plasma concentrations and neurological toxicity in febrile neutropenic patients with mild impairment of renal function. Antimicrob Agents Chemother. 2010;54(10):4360–7. https://doi.org/10.1128/AAC.01595-08.
Li HT, Lee CH, Wu T, Cheng MY, Tseng WJ, Chang CW, et al. Clinical, electroencephalographic features and prognostic factors of cefepime-induced neurotoxicity: a retrospective study. Neurocrit Care. 2019;31(2):329–37. https://doi.org/10.1007/s12028-019-00682-y.
Rodriguez Ruiz A, Vlachy J, Lee JW, Gilmore EJ, Ayer T, Haider HA, et al. Association of periodic and rhythmic electroencephalographic patterns with seizures in critically ill patients. JAMA Neurol. 2017;74(2):181–8. https://doi.org/10.1001/jamaneurol.2016.4990.
Appa AA, Jain R, Rakita RM, Hakimian S, Pottinger PS. Characterizing cefepime neurotoxicity: a systematic review. Open Forum Infect Dis. 2017;4(4):ofx70. https://doi.org/10.1093/ofid/ofx170.
Payne LE, Gagnon DJ, Riker RR, Seder DB, Glisic EK, Morris JG, et al. Cefepime-induced neurotoxicity: a systematic review. Crit Care. 2017;21(1):276. https://doi.org/10.1186/s13054-017-1856-1.
Barbhaiya RH, Forgue ST, Gleason CR, Knupp CA, Pittman KA, Weidler DJ, et al. Safety, tolerance, and pharmacokinetic evaluation of cefepime after administration of single intravenous doses. Antimicrob Agents Chemother. 1990;34(6):1118–22. https://doi.org/10.1128/aac.34.6.1118.
Chow KM, Szeto CC, Hui AC, Li PK. Mechanisms of antibiotic neurotoxicity in renal failure. Int J Antimicrob Agents. 2004;23(3):213–7. https://doi.org/10.1016/j.ijantimicag.2003.11.004.
Abanades S, Nolla J, Rodríguez-Campello A, Pedro C, Valls A, Farré M. Reversible coma secondary to cefepime neurotoxicity. Ann Pharmacother. 2004;38(4):606–8. https://doi.org/10.1345/aph.1D322.
Barbey F, Bugnon D, Wauters JP. Severe neurotoxicity of cefepime in uremic patients. Ann Intern Med. 2001;135(11):1011. https://doi.org/10.7326/0003-4819-135-11-200112040-00027.
Schliamser SE, Cars O, Norrby SR. Neurotoxicity of beta-lactam antibiotics: predisposing factors and pathogenesis. J Antimicrob Chemother. 1991;27(4):405–25. https://doi.org/10.1093/jac/27.4.405.
Tyler HR. Neurological aspects of uremia: an overview. Kidney Int Suppl. 1975;2:188–93.
Maganti R, Jolin D, Rishi D, Biswas A. Nonconvulsive status epilepticus due to cefepime in a patient with normal renal function. Epilepsy Behav. 2006;8(1):312–4. https://doi.org/10.1016/j.yebeh.2005.09.010.
Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19(12):1584–96. https://doi.org/10.1038/nm.3407.
Bresson J, Paugam-Burtz C, Josserand J, Bardin C, Mantz J, Pease S. Cefepime overdosage with neurotoxicity recovered by high-volume haemofiltration. J Antimicrob Chemother. 2008;62(4):849–50. https://doi.org/10.1093/jac/dkn256.
Durand-Maugard C, Lemaire-Hurtel AS, Gras-Champel V, Hary L, Maizel J, Prud’homme-Bernardy A, et al. Blood and CSF monitoring of cefepime-induced neurotoxicity: nine case reports. J Antimicrob Chemother. 2012;67(5):1297–9. https://doi.org/10.1093/jac/dks012.
Smith NL, Freebairn RC, Park MA, Wallis SC, Roberts JA, Lipman J. Therapeutic drug monitoring when using cefepime in continuous renal replacement therapy: seizures associated with cefepime. Crit Care Resusc. 2012;14(4):312–5.
Tanaka A, Takechi K, Watanabe S, Tanaka M, Suemaru K, Araki H. Comparison of the prevalence of convulsions associated with the use of cefepime and meropenem. Int J Clin Pharm. 2013;35(5):683–7. https://doi.org/10.1007/s11096-013-9799-3.
Kwon J, Choi JY, Bae EK. Cefepime-induced aphasic status epilepticus mimicking acute stroke. J Epilepsy Res. 2014;4(2):85–7. https://doi.org/10.14581/jer.14018.
Gangireddy VG, Mitchell LC, Coleman T. Cefepime neurotoxicity despite renal adjusted dosing. Scand J Infect Dis. 2011;43(10):827–9. https://doi.org/10.3109/00365548.2011.581308.
Nakagawa R, Sato K, Uesaka Y, Mitsuki T, Kondo K, Wake A, et al. Cefepime-induced encephalopathy in end-stage renal disease patients. J Neurol Sci. 2017;376:123–8. https://doi.org/10.1016/j.jns.2017.03.018.
Rhodes NJ, Kuti JL, Nicolau DP, Neely MN, Nicasio AM, Scheetz MH. An exploratory analysis of the ability of a cefepime trough concentration greater than 22 mg/L to predict neurotoxicity. J Infect Chemother. 2016;22(2):78–83. https://doi.org/10.1016/j.jiac.2015.10.009.
Lau C, Marriott D, Gould M, Andresen D, Reuter SE, Penm J. A retrospective study to determine the cefepime-induced neurotoxicity threshold in hospitalized patients. J Antimicrob Chemother. 2020;75(3):718–25. https://doi.org/10.1093/jac/dkz476.
Lau C, Marriott D, Schultz HB, Gould M, Andresen D, Wicha SG, et al. Assessment of cefepime toxico-dynamics: comprehensive examination of pharmacokinetic/pharmacodynamic targets for cefepime-induced neurotoxicity and evaluation of current dosing guidelines. Int J Antimicrob Agents. 2021. https://doi.org/10.1016/j.ijantimicag.2021.106443.
Scheetz MH, McKoy JM, Parada JP, Djulbegovic B, Raisch DW, Yarnold PR, et al. Systematic review of piperacillin-induced neutropenia. Drug Saf. 2007;30(4):295–306. https://doi.org/10.2165/00002018-200730040-00002.
Neftel KA, Hauser SP, Müller MR. Inhibition of granulopoiesis in vivo and in vitro by beta-lactam antibiotics. J Infect Dis. 1985;152(1):90–8. https://doi.org/10.1093/infdis/152.1.90.
Lim PP, Chong CP, Aziz NA. Cefepime-associated thrombocytopenia in a critically ill patient. Int J Clin Pharm. 2011;33(6):902–4. https://doi.org/10.1007/s11096-011-9571-5.
Neu HC. Safety of cefepime: a new extended-spectrum parenteral cephalosporin. Am J Med. 1996;100(6a):68s–75s. https://doi.org/10.1016/s0002-9343(96)00110-6.
Wong BB, Ko GJ. Neutropenia in patients receiving long-term cefepime therapy for osteomyelitis. Am J Health Syst Pharm. 2003;60(21):2229–32. https://doi.org/10.1093/ajhp/60.21.2229.
Hernández R, Delpiano L, Amador J, Arias M, Carrasco DJ. Neutropenia associated with the use of cefepime in pediatric patients with cystic fibrosis. Rev Chilena Infectol. 2019;36(1):112–4. https://doi.org/10.4067/s0716-10182019000100112.
Malincarne L, Francisci D, Martinelli L, Masini G, Baldelli F. A case of severe cefepime-related neutropenia in a 15-year-old patient. Scand J Infect Dis. 2010;42(2):156–7. https://doi.org/10.3109/00365540903380503.
Foong KS, Hsueh K, Bailey TC, Luong L, Iqbal A, Hoehner C, et al. A cluster of cefepime-induced neutropenia during outpatient parenteral antimicrobial therapy. Clin Infect Dis. 2019;69(3):534–7. https://doi.org/10.1093/cid/ciy1112.
Medrano-Casique N, Tong HY, Borobia AM, Carcas AJ, Frías J, Ramírez E. Non-chemotherapy-induced agranulocytosis detected by a prospective pharmacovigilance program in a tertiary Hhspital. Basic Clin Pharmacol Toxicol. 2015;117(6):399–408. https://doi.org/10.1111/bcpt.12418.
Jacobs JW, Stump JA, Perez AN, Sharma D, Booth GS. Probable cefepime-induced immune mediated hemolytic anaemia. Transfus Med. 2021. https://doi.org/10.1111/tme.12826.
Barbhaiya RH, Forgue ST, Shyu WC, Papp EA, Pittman KA. High-pressure liquid chromatographic analysis of BMY-28142 in plasma and urine. Antimicrob Agents Chemother. 1987;31(1):55–9. https://doi.org/10.1128/aac.31.1.55.
Kessler RE, Bies M, Buck RE, Chisholm DR, Pursiano TA, Tsai YH, et al. Comparison of a new cephalosporin, BMY 28142, with other broad-spectrum beta-lactam antibiotics. Antimicrob Agents Chemother. 1985;27(2):207–16. https://doi.org/10.1128/aac.27.2.207.
Al-Shaer MH, Alghamdi WA, Graham E, Peloquin CA. Meropenem, cefepime, and piperacillin protein binding in patient samples. Ther Drug Monit. 2020;42(1):129–32. https://doi.org/10.1097/ftd.0000000000000675.
Dorn C, Schießer S, Wulkersdorfer B, Hitzenbichler F, Kees MG, Zeitlinger M. Determination of free clindamycin, flucloxacillin or tedizolid in plasma: pay attention to physiological conditions when using ultrafiltration. Biomed Chromatogr. 2020;34(6): e4820. https://doi.org/10.1002/bmc.4820.
Bugnon D, Giannoni E, Majcherczyk P, Glauser MP, Moreillon P. Pitfalls in cefepime titration from human plasma: plasma- and temperature-related drug degradation in vitro. Antimicrob Agents Chemother. 2002;46(11):3654–6. https://doi.org/10.1128/aac.46.11.3654-3656.2002.
Kratzer A, Liebchen U, Schleibinger M, Kees MG, Kees F. Determination of free vancomycin, ceftriaxone, cefazolin and ertapenem in plasma by ultrafiltration: impact of experimental conditions. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;961:97–102. https://doi.org/10.1016/j.jchromb.2014.05.021.
U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM). Bioanalytical Method Validation Guidance for Industry 2018. https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf.
Jonckheere S, De Neve N, De Beenhouwer H, Berth M, Vermeulen A, Van Bocxlaer J, et al. A model-based analysis of the predictive performance of different renal function markers for cefepime clearance in the ICU. J Antimicrob Chemother. 2016;71(9):2538–46. https://doi.org/10.1093/jac/dkw171.
Georges B, Conil JM, Seguin T, Dieye E, Cougot P, Decun JF, et al. Cefepime in intensive care unit patients: validation of a population pharmacokinetic approach and influence of covariables. Int J Clin Pharmacol Ther. 2008;46(4):157–64. https://doi.org/10.5414/cpp46157.
Tang Girdwood SC, Tang PH, Murphy ME, Chamberlain AR, Benken LA, Jones RL, et al. Demonstrating feasibility of an opportunistic sampling approach for pharmacokinetic studies of β-lactam antibiotics in critically ill children. J Clin Pharmacol. 2021;61(4):565–73. https://doi.org/10.1002/jcph.1773.
Abdul-Aziz MH, Alffenaar JC, Bassetti M, Bracht H, Dimopoulos G, Marriott D, et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: a Position Paper. Intensive Care Med. 2020;46(6):1127–53. https://doi.org/10.1007/s00134-020-06050-1.
Guilhaumou R, Benaboud S, Bennis Y, Dahyot-Fizelier C, Dailly E, Gandia P, et al. Optimization of the treatment with beta-lactam antibiotics in critically ill patients-guidelines from the French Society of Pharmacology and Therapeutics (Société Française de Pharmacologie et Thérapeutique-SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Société Française d’Anesthésie et Réanimation-SFAR). Crit Care. 2019;23(1):104. https://doi.org/10.1186/s13054-019-2378-9.
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45(3):486–552. https://doi.org/10.1097/ccm.0000000000002255.
Bjergum MW, Barreto EF, Scheetz MH, Rule AD, Jannetto PJ. Stability and validation of a high-throughput LC-MS/MS method for the quantification of cefepime, meropenem, and piperacillin and tazobactam in serum. J Appl Lab Med. 2021. https://doi.org/10.1093/jalm/jfab036.
D’Cunha R, Bach T, Young BA, Li P, Nalbant D, Zhang J, et al. Quantification of cefepime, meropenem, piperacillin, and tazobactam in human plasma using a sensitive and robust liquid chromatography-tandem mass spectrometry method, part 2: stability evaluation. Antimicrob Agents Chemother. 2018. https://doi.org/10.1128/aac.00861-18.
D’Cunha R, Bach T, Young BA, Li P, Nalbant D, Zhang J, et al. Quantification of cefepime, meropenem, piperacillin, and tazobactam in human plasma using a sensitive and robust liquid chromatography-tandem mass spectrometry method, part 1: assay development and validation. Antimicrob Agents Chemother. 2018. https://doi.org/10.1128/aac.00859-18.
Ohmori T, Suzuki A, Niwa T, Ushikoshi H, Shirai K, Yoshida S, Ogura S, Itoh Y. Simultaneous determination of eight β-lactam antibiotics in human serum by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(15–16):1038–42. https://doi.org/10.1016/j.jchromb.2011.03.001.
Zander J, Maier B, Suhr A, Zoller M, Frey L, Teupser D, et al. Quantification of piperacillin, tazobactam, cefepime, meropenem, ciprofloxacin and linezolid in serum using an isotope dilution UHPLC-MS/MS method with semi-automated sample preparation. Clin Chem Lab Med. 2015;53(5):781–91. https://doi.org/10.1515/cclm-2014-0746.
Deshpande AD, Baheti KG, Chatterjee NR. Degradation of β-lactam antibiotics. Curr Sci. 2004;87(12):1684–95.
Rigo-Bonnin R, Ribera A, Arbiol-Roca A, Cobo-Sacristán S, Padullés A, Murillo Ò, et al. Development and validation of a measurement procedure based on ultra-high performance liquid chromatography-tandem mass spectrometry for simultaneous measurement of β-lactam antibiotic concentration in human plasma. Clin Chim Acta. 2017;468:215–24. https://doi.org/10.1016/j.cca.2017.03.009.
Colin P, De Bock L, T’Jollyn H, Boussery K, Van Bocxlaer J. Development and validation of a fast and uniform approach to quantify β-lactam antibiotics in human plasma by solid phase extraction-liquid chromatography-electrospray-tandem mass spectrometry. Talanta. 2013;103:285–93. https://doi.org/10.1016/j.talanta.2012.10.046.
Bauer KA, West JE, O’Brien JM, Goff DA. Extended-infusion cefepime reduces mortality in patients with Pseudomonas aeruginosa infections. Antimicrob Agents Chemother. 2013;57(7):2907–12. https://doi.org/10.1128/aac.02365-12.
Rhodes NJ, Liu J, McLaughlin MM, Qi C, Scheetz MH. Evaluation of clinical outcomes in patients with Gram-negative bloodstream infections according to cefepime MIC. Diagn Microbiol Infect Dis. 2015;82(2):165–71. https://doi.org/10.1016/j.diagmicrobio.2015.03.005.
Trotman RL, Williamson JC, Shoemaker DM, Salzer WL. Antibiotic dosing in critically ill adult patients receiving continuous renal replacement therapy. Clin Infect Dis. 2005;41(8):1159–66. https://doi.org/10.1086/444500.
Heintz BH, Matzke GR, Dager WE. Antimicrobial dosing concepts and recommendations for critically ill adult patients receiving continuous renal replacement therapy or intermittent hemodialysis. Pharmacotherapy. 2009;29(5):562–77. https://doi.org/10.1592/phco.29.5.562.
Chaijamorn W, Charoensareerat T, Srisawat N, Pattharachayakul S, Boonpeng A. Cefepime dosing regimens in critically ill patients receiving continuous renal replacement therapy: a Monte Carlo simulation study. J Intensive Care. 2018;6:61. https://doi.org/10.1186/s40560-018-0330-8.
Philpott CD, Droege CA, Droege ME, Healy DP, Courter JD, Ernst NE, et al. Pharmacokinetics and pharmacodynamics of extended-infusion cefepime in critically ill patients receiving continuous renal replacement therapy: a prospective, open-label study. Pharmacotherapy. 2019;39(11):1066–76. https://doi.org/10.1002/phar.2332.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This review received no specific grant from any funding agency. Erin F. Barreto is supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health under Award Number K23AI143882 (PI; EFB). Gideon Stitt is supported by a National Institute of Child Health & Human Development (NICHD)-funded postdoctoral fellowship (T32GM008562). Kevin J. Downes is supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) of the National Institutes of Health under Award Number K23HD091365. Kevin J. Downes has received research support from Merck & Co., Inc. unrelated to the current work. The funding sources had no role in data collection, interpretation; writing the report; or the decision to submit the report for publication. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIAID, NICHD, or NIH.
Conflicts of interest/Competing interests
Marc H. Scheetz reports a research contract with Allecra. Kevin J. Downes receives research support from Merck, Inc., unrelated to the current project. Erin F. Barreto is a consultant for FAST Biomedical and Wolters Kluwer, unrelated to the current project. All other authors have no other related conflicts of interest to declare.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code dvailability
Not applicable.
Authors’ contributions
All authors contributed to the drafting of the manuscript, provided meaningful and substantial edits, and critically reviewed/approved the final version of the manuscript.
Rights and permissions
About this article
Cite this article
Pais, G.M., Chang, J., Barreto, E.F. et al. Clinical Pharmacokinetics and Pharmacodynamics of Cefepime. Clin Pharmacokinet 61, 929–953 (2022). https://doi.org/10.1007/s40262-022-01137-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-022-01137-y