Summary
The pharmacokinetics of single oral doses of zileuton 200 to 800mg, its R(+) and S(−) enantiomers, and its Af-dehydroxylated and glucuronide metabolites have been investigated in a randomised study in 16 normal male healthy volunteers.
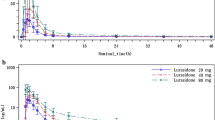
Zileuton was 93.4% bound to plasma proteins. The overall dispositional pharmacokinetics of zileuton racemate appeared to be linear. The mean dosenormalised area under the concentration-time curve from zero to infinity (AUC0-∞) remained constant, while the mean dose-normalised peak plasma concentration (Cmax) decreased with the increase in dose, possibly because of dissolution rate-limited absorption at the higher doses.
The R(+) and S(−) enantiomers of zileuton may have similar absorption profiles, although the apparent total plasma clearance of the S(−) enantiomer was 49 to 76% higher than the corresponding values for the R(+) enantiomer. The AUC-∞ of each enantiomer increased proportionately with dose.
The pharmacokinetics of the N-dehydroxylated metabolite of zileuton were highly variable, with a more than dose-proportional increase in the mean dosenormalised Cmax and area under the concentration-time curve from zero to 24 hours.
The elimination of the glucuronide metabolites of the R(+) and S(−) enantiomers of zileuton was formation rate-limited. The mean percentage of the administered zileuton dose recovered in urine as glucuronide metabolites ranged from 73.1 to 76.5% and showed no dose-related differences. The renal clearances of the glucuronide metabolites of zileuton exceeded the normal glomerular filtration rate, suggesting that these metabolites may be excreted through renal tubular secretion in addition to filtration.
Similar content being viewed by others
References
Batt DG. 5-Lipoxygenase inhibitors and their anti-inflammatory activities. Prog Med Chem 1992; 29: 1–63
Lewis RA, Austen KF, Soberman RJ. Leukotrienes and other products of the 5-lipoxygenase pathway: biochemistry and relation to pathobiology in human diseases. N Engl J Med 1990; 323: 645–55
Bell RL, Young PR, Albert D, et al. The discovery and development of zileuton: an orally active 5-lipoxygenase inhibitor. Int J Immunopharmacol 1992; 14: 505–10
Carter GW, Young PE, Albert DH, et al. 5-Lipoxygenase inhibitory activity of zileuton. Journal of Pharmacology and Experimental Therapeutics 1991; 256: 929–37
Collawn C, Rubin P, Perez N, et al. Phase II study of the safety and efficacy of a 5-lipoxygenase inhibitor in patients with ulcerative colitis. Am J Gastroenterol 1992; 87: 342–6
Hui KP, Taylor IK, Taylor GW, et al. Effect of a 5-lipoxygenase inhibitor on leukotriene generation and airway responses after allergen challenge in asthmatic patients. Thorax 1991; 46: 184–9
Israel E, Dermarkarian R, Rosenberg M, et al. The effects of a 5-lipoxygenase inhibitor on asthma induced by cold, dry air. N Engl J Med 1990; 323: 1740–4
Knapp HR. Reduced allergen-induced nasal congestion and leukotriene synthesis with an orally active 5-lipoxygenase inhibitor. N Engl J Med 1990; 323: 1745–8
Rubin P, Dubé L, Braeckman R, et al. Pharmacokinetics, safety, and ability to diminish leukotriene synthesis by zileuton, an inhibitor of 5-lipoxygenase. Agents Actions 1991; 35:103–16
Sirois P, Borgeat P, Lauziere M, et al. Effect of zileuton (A-64077) on the 5-lipoxygenase activity of human whole blood ex vivo. Agents Actions 1991; 34: 117–20
Awni WM, Granneman R, Locke C, et al. Population pharmacokinetics of zileuton in patients with rheumatoid arthritis. Abstract. Pharm Res 1993; 10: S401
Machinist JM. Metabolism and disposition of Abbott-64077-14C in humans following a single 400mg oral dose. Drug Metab Dispos. In press
Sweeney DJ, Bouska J, Machinist J, et al. Glucuronidation of zileuton (A-64077) by human hepatic microsomes. Drug Metab Dispos 1992; 20: 328–9
Machinist JM, Kukulka MJ, Bopp BA. In vitro plasma protein binding of zileuton and its N-denydroxylated metabolite. Clin Pharmacokinet 1995; 29 Suppl. 2: 34–41
Gibaldi M, Perrier D. Pharmacokinetics. 2nd ed. New York: Marcel Dekker, Inc., 1982
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wong, S.L., Awni, W.M., Cavanaugh, J.H. et al. The Pharmacokinetics of Single Oral Doses of Zileuton 200 to 800mg, its Enantiomers, and its Metabolites, in Normal Healthy Volunteers. Clin-Pharmacokinet 29 (Suppl 2), 9–21 (1995). https://doi.org/10.2165/00003088-199500292-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-199500292-00004