Abstract
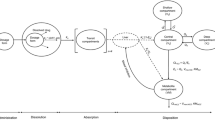
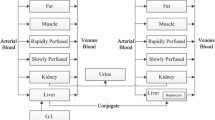
A population PK model was developed in order to simultaneously describe citalopram and its major metabolite, n-desmethyl citalopram, plasma concentrations in two different strain of rats after intravenous (IV) and oral (PO) administration of citalopram. Citalopram was administered to Sprague–Dawley (SD) rats at doses: 0.3, 1, 3, and 10 mg/kg IV and 10 mg/kg PO. The compound was dosed orally to Wistar rats at doses: 0.3, 1, 3, 10, 30 and 60 mg/kg. Plasma samples were collected for citalopram and metabolite. Pharmacokinetic analyses were conducted using NONMEM 7.2. Values below the quantification limit (BLQ <0.1 ng/mL) were included in the analyses and treated as censored information. The disposition of citalopram was best described by a 3-compartment model and its desmethyl metabolite by a 2-compartment model. Several models for the absorption rate were explored (e.g. first, zero order and combined first and zero order absorption, Michaelis–Menten, lag time) in combination with dose and/or time dependent covariate effects. Dose dependent oral bioavailability properties were also identified in this analysis. Citalopram IV clearance and metabolite formation rate were adequately described as linear processes. Metabolite clearance was adequately described using a Michaelis–Menten clearance with different parameters depending on the strain. This analysis demonstrates a very complex absorption/metabolism process explaining the highly non-linear pharmacokinetics observed across all the doses. This is the first combined parent/metabolite population PK analysis in both SD and Wistar rats over a wide range of IV and PO dosages for citalopram, a compound that exhibits highly nonlinear oral pharmacokinetics in rats.







Similar content being viewed by others
Abbreviations
- PK:
-
Pharmacokinetic
- PO:
-
per os (oral administration)
- IV:
-
Intravenous
- SD:
-
Sprague–Dawley
- BLQ:
-
Below the limit of quantification
- SSRI:
-
Selective serotonin reuptake inhibitor
- MM:
-
Michaelis–Menten
References
Pollock BG (2001) Citalopram: a comprehensive review. Expert Opin Pharmacother 2:681–698
Burghardt N, Sullivan G, McEwen B, Gorman J, LeDoux J (2004) The selective serotonin reuptake inhibitor citalopram increases fear after acute treatment but reduces fear with chronic treatment: a comparison with tianeptine. Biol Psychiatry 55:1171–1178
Bigos KL, Pollock BG, Aizenstein HJ, Fisher PM, Bies RR, Hariri AR (2008) Acute 5-HT reuptake blockade potentiates human amygdala reactivity. Neuropsychopharmacology. 33:3221–3225
Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM (2006) Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry 59:816–820
Rocha A, Marques MP, Coelho EB, Lanchote VL (2007) Enantioselective analysis of citalopram and demethylcitalopram in human and rat plasma by chiral LC-MS/MS: application to pharmacokinetics. Chirality 19:793–801
Hiemkeand C, Hartter S (2000) Pharmacokinetics of selective serotonin reuptake inhibitors. Pharmacol Ther 85:11–28
Ramirez E, Laosa O, Guerra P, Duque B, Mosquera B, Borobia AM, Lei SH, Carcas AJ, Frias J (2010) Acceptability and characteristics of 124 human bioequivalence studies with active substances classified according to the Biopharmaceutic Classification System. Br J Clin Pharmacol 70:694–702
Baumann P (1996) Pharmacology and pharmacokinetics of citalopram and other SSRIs. Int Clin Psychopharmacol 11(Suppl 1):5–11
Jawien W, Majcherczyk J, Kulza M, Florek E, Piekoszewski W (2013) How to combine noncompartmental pharmacokinetics with the population analysis. A study of tobacco smoke’s influence on the bioavailability of racemic citalopram in rats. Pharmacol Rep 65:517–524
Beal SL (2001) Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn 28:481–504
Savic RM, Jonker DM, Kerbusch T, Karlsson MO (2007) Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn 34:711–726
Ludden TM, Beal SL, Sheiner LB (1994) Comparison of the Akaike Information Criterion, the Schwarz criterion and the F test as guides to model selection. J Pharmacokinet Biopharm 22:431–445
Lindbom L, Ribbing J, Jonsson EN (2004) Perl-speaks-NONMEM (PsN)—a Perl module for NONMEM related programming. Comput Methods Programs Biomed 75:85–94
Acknowledgments
We thank Claire F Brittain, Andrew P McCarthy and Wesley F Seidel for the useful comments and fruitful discussions. We also want to thank Gemma L Dickinson for her review and William H Landschulz for his support to the Disease and Therapeutic Response Modeling program and to this project. NVM and RRB were supported by Eli Lilly and Company through the Indiana Clinical and Translational Sciences Institute (CTSI).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Velez de Mendizabal, N., Jackson, K., Eastwood, B. et al. A population PK model for citalopram and its major metabolite, N-desmethyl citalopram, in rats. J Pharmacokinet Pharmacodyn 42, 721–733 (2015). https://doi.org/10.1007/s10928-015-9448-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-015-9448-7