Abstract
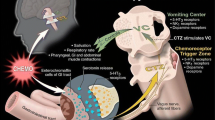
Nausea and vomiting are common adverse effects of chemotherapy, radiation therapy, anaesthesia and surgery. The incidence of chemotherapy-induced nausea and vomiting (CINV) is estimated to vary from 30 to 90%, depending on the type of chemotherapeutic agent used. Radiation-induced emesis varies with anatomical site radiated but is estimated to have an overall incidence of approximately 40%. The incidence of postoperative nausea and vomiting (PONV) depends on the type of anaesthesia and surgery, but overall is estimated to be 20–30%. Evidence-based medicine and meta-analysis have been used to direct medical therapy to help determine equivalence, optimal dose, timing, safety and efficacy of antiemetic medications. Concepts such as the number needed to treat and number needed to harm are helpful to guide the clinician regarding the benefits and risks of a particular treatment.
The serotonin 5-HT3 receptor antagonists ondansetron, granisetron, tropisetron and dolasetron have been important additions to the antiemetic armamentarium. The 5-HT3 receptor antagonists are similar in chemical structure, efficacy and adverse effect profile. They appear to have no important differences among themselves in clinical outcomes for CINV and PONV. Headache, dizziness, constipation and diarrhoea are their most common adverse effects, and when they occur they are usually mild and easily managed. Haemodynamic changes and extrapyramidal adverse effects are uncommon. ECG changes such as prolonged corrected QT (QTc) interval are infrequent, dose-related and overall judged to be clinically insignificant. As most studies with the 5-HT3 antagonists have been conducted on relatively healthy patients, caution should be exercised when these drugs are used in susceptible patients with co-morbidities. The clinician must weigh the benefit of administering an antiemetic for CINV or PONV against the risk of occurrence of an adverse event.














Similar content being viewed by others
References
Jamrozik K. Why evidence-based medicine. Best Pract Res Clin Anaesthesiol 2001; 15(4): 505–18
Sackett DL, Rosenberg WMC, Gray JA, et al. Evidence-based medicine: what it is and what it isn’t. BMJ 1996; 312: 71–2
Jones B, Jarvis P, Lewis JA, et al. Trials to assess equivalence: the importance of rigorous methods. BMJ 1996; 313: 36–9
Laupacis A, Sackett DL, Roberts RS. An assessment of clinically useful measures of the consequences of treatment. N Engl J Med 1988; 318: 1728–33
Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ 1995; 310: 452–4
Tramèr MR. A rational approach to the control of postoperative nausea and vomiting: evidence from systematic reviews. Part I. Efficacy and harm of antiemetic interventions, and methodological issues. Acta Anesthesiol Scand 2001; 45: 4–13
Tramèr MR. A rational approach to the control of postoperative nausea and vomiting: evidence from systematic reviews. Part II. Recommendations for prevention and treatment, and research agenda. Acta Anesthesiol Scand 2001; 45: 14–9
Tramèr MR, editor. Evidence-based resource in anaesthesia and analgesia. London; BMJ Books, 2000
ASHP Commission on Therapeutics. ASHP therapeutic guidelines on the pharmacologic management of nausea and vomiting in adult and pediatric patients receiving chemotherapy or radiation therapy or undergoing surgery. Am J Health Syst Pharm 1999; 56: 729–64
Hesketh P. Comparative review of 5-HT3 receptor antagonists in the treatment of acute chemotherapy-induced nausea and vomiting. Cancer Invest 2000; 18: 163–73
Aapro MS. 5-HT3 receptor antagonists: an overview of their present status and future potential in cancer therapy-induced emesis. Drugs 1991; 42(4): 551–68
Gregory RE, Ettinger DS. 5-HT3 receptor antagonists for the prevention of chemotherapy-induced nausea and vomiting: a comparison of their pharmacology and clinical efficacy. Drugs 1998; 55(2): 173–89
Veyrat-Follet C, Farinotti R, Palmer JL. Physiology of chemotherapy-induced emesis and antiemetic therapy: predictive models for evaluation of new compounds. Drugs 1997; 53(2): 206–34
Merrifield KR, Chaffee BJ. Recent advances in the management of nausea and vomiting caused by antineoplastic agents. Clin Pharm 1989; 8: 187–99
Bunce KT, Tyers MB. The role of 5-HT in postoperative nausea and vomiting. Br J Anaesth 1992; 69Suppl. 1: 60S–2S
Hesketh PJ, Kris MG, Grunberg SM, et al. Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol 1997; 15: 103–9
Eberhart LH, Hogel J, Seeling W, et al. Evaluation of three risk scores to predict postoperative nausea and vomiting. Acta Anaesthesiol Scand 2000; 44(4): 480–8
Watcha MF, White PF. Postoperative nausea and vomiting: its etiology, treatment and prevention. Anesthesiology 1992; 77: 162–84
Koivuranta M, Laara E, Snare L, et al. A survey of postoperative nausea and vomiting. Anaesthesia 1997; 52: 443–9
Apfel C, Laara E, Koivuranta M, et al. A simplified risk score for predicting post-anesthetic nausea and vomiting. Anesthesiology 1999; 91: 693–700
Sinclair DR, Chung F, Mezei G. Can postoperative nausea and vomiting be predicted? Anesthesiology 1999; 91: 109–18
Rose JB, Watcha MF. Postoperative nausea and vomiting in paediatric patients. Br J Anaesth 1999; 83: 104–17
Gold BS, Kitz DS, Lecky HJ, et al. Unanticipated admission to the hospital following ambulatory surgery. JAMA 1989; 262: 3003–10
Fancourt-Smith PF, Hornstein J, Jenkins LC. Hospital admissions from the Surgical Day Care Centre of Vancouver General Hospital 1977-1987. Can J Anaesth 1990; 37(6): 699–704
Tramèr MR, Reynolds DJ, Moore RA, et al. When placebo controlled trials are essential and equivalence trials are inadequate. BMJ 1998; 317(7162): 875–80
Henzi I, Walder B, Tramèr MR. Dexamethasone for the prevention of postoperative nausea and vomiting: a quantitative systematic review. Anesth Analg 2000; 90(1): 186–94
Fortney JT, Gan TJ, Graczyk S, et al. A comparison of the efficacy, safety and patient satisfaction of ondansetron versus droperidol as antiemetics for elective outpatient surgical procedures. Anesth Analg 1998; 86: 731–8
Milne RJ, Hell RC. Ondansetron: therapeutic use as an antiemetic. Drugs 1991; 41: 574–95
Wilde MI, Markham A. Ondansetron: a review of its pharmacology and preliminary clinical findings in novel applications. Drugs 1996; 52: 773–94
Zofran (ondansetron HCl): prescribing information. Research Triangle Park (NC): GlaxoSmithKline, 2001
Frazer NM, Palmer JL. Tolerability and pharmacokinetics of intra-muscular ondansetron 4mg [abstract]. Anesthesiology 1992; 77(3A): A439
Jarosinski PF, Hirschfeld S. Precipitation of ondansetron in alkaline solution. N Engl J Med 1991; 325(18): 1315–6
Grunberg SM, Groshen S, Robinson D, et al. Correlation of anti-emetic efficacy and plasma levels of ondansetron. Eur J Cancer 1990; 26: 879–82
Dixon CM, Colthup PV, Serabjit-Singh CJ, et al. Multiple forms of cytochrome P450 are involved in the metabolism of ondansetron in humans. Drug Metab Dispos 1995; 23: 1225–30
Kaiser R, Sezen O, Papies A, et al. Patient-tailored antiemetic treatment with 5-hydroxytryptamine type 3 receptor antagonists according to cytochrome P-450 2D6 genotype. J Clin Oncol 2002; 20: 2805–11
Figg WD, Dukes GE, Pritchard JF. Pharmacokinetics of ondansetron in patients with hepatic insufficiency. J Clin Pharmacol 1996; 36: 206–15
Kovac A. Prevention and treatment of postoperative nausea and vomiting. Drugs 2000; 59(2): 213–43
Gyermek L. Pharmacology of serotonin as related to anesthesia. J Clin Anesth 1996; 8: 402–25
Talley NJ, Phillips SF, Haddad A, et al. GR 38032F (ondansetron), a selective 5-HT3 receptor antagonist, slows colonic transit in healthy man. Dig Dis Sci 1990; 35: 477–80
Talley NJ, Phillips SF, Haddad A, et al. Effect of selective 5-HT3 antagonist (Gr 38032F) on small intestinal transit and release of gastrointestinal peptides. Dig Dis Sci 1989; 34: 1511–5
Gore S, Gilmore IT, Haigh CG, et al. Colonic transit in man is slowed by ondansetron (GR38032F), a selective 5-hydroxytryptamine receptor (type 3) antagonist. Aliment Pharmacol Ther 1990; 4: 139–44
Figg WD, Graham CL, Hak LJ, et al. Ondansetron: a novel antiemetic agent. South Med J 1993; 86: 497–501
Heyman JS, Young ML, Bagshaw RJ, et al. Cardiovascular stability with rapid intravenous infusion of ondansetron. Can J Anaesth 1993; 40: 448–52
Tramèr MR, Reynolds DJM, Moore RA, et al. Efficacy, dose-response, and safety of ondansetron in prevention of postoperative nausea and vomiting. Anesthesiology 1997; 87: 1277–89
Dershwitz M, diBiase PM, Rosow CE, et al. Ondansetron does not affect alfentanil-induced ventilatory depression or sedation. Anesthesiology 1992; 77(3): 447–52
Kovac A, Steer P, Hutchison M, et al. Effect of ondansetron on recovery time, sedation level and discharge from ambulatory surgery [abstract]. Anesthesiology 1991; 75(3A): A7
Pearson KS, From RP, Ostman LP, et al. Psychomotor effects of intravenous ondansetron in female outpatients [abstract]. Anesthesiology 1991; 75(3A): A8
Beck TM, Hesketh PJ, Madajewica S, et al. Stratified, randomized, double-blind comparison of intravenous ondansetron administered as a multiple-dose regiment versus two single-dose regimens in the prevention of cisplatin-induced nausea and vomiting. J Clin Oncol 1992; 10: 1969–75
Pearman MH. Single dose intravenous ondansetron in the prevention of postoperative nausea and vomiting. Anaesthesia 1994; 49: 11–5
Rung GW, Claybon L, Hord A, et al. Intravenous ondansetron for postsurgical opioid-induced nausea and vomiting. Anesth Analg 1997; 84: 832–8
Smith RN. Safety of ondansetron. Eur J Cancer Clin Oncol 1989; 25Suppl. 1: S47–50
McKenzie R, Kovac A, O’Connor T, et al. Comparison of ondansetron versus placebo to prevent postoperative nausea and vomiting in women undergoing ambulatory gynecologic surgery. Anesthesiology 1993; 78: 21–8
Scuderi P, Wetchler B, Sung YF, et al. Treatment of postoperative nausea and vomiting after outpatient surgery with the 5-HT3 antagonist ondansetron. Anesthesiology 1993; 78: 15–20
Claybon L. Single dose intravenous ondansetron for the 24-hour treatment of postoperative nausea and vomiting. Anaesthesia 1994; 49: 24–9
Kovac AL, Pearman MH, Khalil SN, et al. Ondansetron prevents postoperative emesis in male outpatients. J Clin Anesth 1996; 8: 644–51
Kovac AL, O’Connor TA, Pearman MH, et al. Efficacy of repeat intravenous dosing of ondansetron in controlling postoperative nausea and vomiting: a randomized, double-blind, placebo-controlled multicenter trial. J Clin Anesth 1999; 11: 453–9
Khalil S, Rodarte A, Weldon C, et al. Intravenous ondansetron in established postoperative emesis in children. Anesthesiology 1996; 85: 270–6
Furst SR, Rodarte A. Prophylactic antiemetic treatment with ondansetron in children undergoing tonsillectomy. Anesthesiology 1994; 81: 799–803
Rose JB, Brenn BR, Corddry DH, et al. Preoperative oral ondansetron for pediatric tonsillectomy. Anesth Analg 1996; 83: 558–62
Plosker GL, Goa KL. Granisetron: a review of its pharmacological properties and therapeutic use as an antiemetic. Drugs 1991; 42: 805–24
Hunter AE, Prentice HG, Pothecary K, et al. Granisetron, a selective 5-HT3 receptor antagonist, for the prevention of radiation induced emesis during total body irradiation. Bone Marrow Transplant 1991; 7: 439–41
Yarker YE, McTavish D. Granisetron: an update of its therapeutic use in nausea and vomiting induced by antineoplastic therapy. Drugs 1994; 48(5): 761–93
Addelman M, Erlichman C, Fine S, et al. Phase I/II trial of granisetron: a novel 5-hydroxytryptamine antagonist for the prevention of chemotherapy-induced nausea and vomiting. J Clin Oncol 1990; 8: 337–41
Chevallier B. Efficacy and safety of granisetron compared with high-dose metoclopramide plus dexamethasone in patients receiving high-dose cisplatin in a single-blind study. Eur J Cancer 1990; 26: S33–6
Marty M. A comparative study of the use of granisetron, a selective 5-HT3 antagonist, versus a standard anti-emetic regimen of chlorpromazine plus dexamethasone in the treatment of cytostatic-induced emesis. Eur J Cancer 1990; 26: S28–32
Kytril injection (granisetron HCl): package insert. Nutley, NJ: Roche Laboratories, 2000
Bloomer JC, Baldwin SJ, Smith GJ, et al. Characteristics of the cytochrome P450 enzymes involved in the in vitro metabolism of granisetron. Br J Clin Pharmacol 1994; 38: 557–66
Pintens H. Granisetron (BRL43694) in the treatment of cytostatic drug-induced emesis: a summary. Cancer Treat Rev 1990; 17: 307–10
Wilson AJ, Diemunsch P, Lindeque BG, et al. Single-dose iv granisetron in the prevention of postoperative nausea and vomiting. Br J Anaesth 1996; 76: 515–8
Fujii Y, Tanaka H, Toyooka H. Optimal antiemetic dose of granisetron for preventing postoperative nausea and vomiting. Can J Anaesth 1994; 51: 794–7
Fujii Y, Tanaka H, Toyooka H. Reduction of postoperative nausea and vomiting with granisetron. Can J Anaesth 1994; 41: 291–4
Fujii Y, Tanaka H, Toyooka H. Granisetron: dexamethasone combination reduces postoperative nausea and vomiting. Can J Anaesth 1995; 42: 387–90
Cieslak GD, Watcha MF, Phillips MB, et al. The dose-response relationship of granisetron for the prophylaxis of pediatric postoperative emesis. Anesthesiology 1996; 85: 1076–85
Taylor AM, Rosen M, Diemunsch PA, et al. A double-blind, parallel-group, placebo-controlled, dose-ranging, multicenter study of intravenous granisetron in the treatment of postoperative nausea and vomiting in patients undergoing surgery with general anesthesia. J Clin Anesth 1997; 9(8): 658–63
Mikawa K, Takao Y, Nichina K, et al. Optimal dose of granisetron for prophylaxis against postoperative emesis after gynecological surgery. Anesth Analg 1997; 85: 652–6
Watanabe H, Hasegawa A, Shinozaki T, et al. Possible cardiac side effects of granisetron, an antiemetic agent, in patients with bone and soft-tissue sarcomas receiving cytotoxic chemotherapy. Cancer Chemother Pharmacol 1995; 35: 278–82
Diemunsch P, Wilson J, Chitour K, et al. A double blind, parallel group, placebo controlled dose ranging study of intravenous granisetron in the prevention of postoperative nausea and vomiting (PONV) [abstract]. Anesthesiology 1994; 81: A1280
de Bruijn KM. Tropisetron: a review of the clinical experience. Drugs 1992; 43: 11–22
Lee CR, Plosker GL, McTavish D. Tropisetron: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential as an antiemetic. Drugs 1993; 46: 925–43
Simpson K, Spencer CM, McClellan KJ. Tropisetron: an update of its use in the prevention of chemotherapy-induced nausea and vomiting. Drugs 2000; 59: 1297–316
Seinen H, Zonnenberg BA, Tija P, et al. The effect of three dose levels of ICS 205-930 (a selective 5HT-3 antagonist) on cisplatin-induced nausea and vomiting. Eur J Cancer Clin Oncol 1989; 25: 1333–5
Bleiberg H, Van Belle S, Paridaens R, et al. Compassionate use of a 5-HT3 receptor antagonist, tropisetron, in patients refractory to standard antiemetic treatment. Drugs 1992; 43: 27–32
Sorbe B, Berglind A-M. Tropisetron, a new 5-HT3 receptor antagonist, in the prevention of radiation-induced nausea, vomiting and diarrhoea. Drugs 1992; 43: 33–9
Alon E, Kocian R, Nett PC, et al. Tropisetron for the prevention of postoperative nausea and vomiting in women undergoing gynecologic surgery. Anesth analg 1966; 82: 338–41
Alon E, Buchser E, Herrera E, et al. Tropisetron for treating established postoperative nausea and vomiting: a randomized, double-blind, placebo-controlled study. Anesth Analg 1998; 86: 617–23
Lee CR, Plosker GL, McTavish D. Tropisetron: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential as an antiemetic. Drugs 1993; 46: 925–43
Bruntsch U, Drechsler S, Eggert J, et al. Prevention of chemotherapy-induced nausea and vomiting by tropisetron (Novoban) alone or in combination with other antiemetic agents. Semin Oncol 1994 Oct; 21(5 Suppl. 9): 7–11
de Bruijn KM. The development of tropisetron in its clinical perspective. Ann Oncol 1993; 4Suppl. 3: S19–23
Bruntsch U, Rüfenacht E, Parker I, et al. Tropisetron in the prevention of chemotherapy-induced nausea and vomiting in patients responding poorly to previous conventional antiemetic therapy. Ann Oncol 1993; 4Suppl. 3: S25–9
Balfour JA, Goa KL. Dolasetron: a review of its pharmacology and therapeutic potential in the management of nausea and vomiting induced by chemotherapy, radiotherapy or surgery. Drugs 1997; 54: 273–98
Hui YF, Ignoffo RJ. Dolasetron: a new 5-hydroxytryptamine3 receptor antagonist. Cancer Pract 1997; 5: 324–8
Anzemet (dolasetron mesylate): prescribing information. Kansas City (MO): Aventis Pharmaceuticals, 2002
Reith MK, Sproles GD, Cheng LK. Human metabolism of dolasetron mesylate, a 5-HT3 receptor antagonist. Drug Metab Dispos 1995; 23: 806–8
Dow J, Berg C. Steroselectivity of the carbonyl reduction of dolasetron in rats, dogs and humans. Chirality 1995; 7: 342–8
Boxenbaum H, Gillespie T, Heck K, et al. Human dolasetron pharmacokinetics: I. disposition following single-dose intravenous administration to normal male subjects. Biopharm Drug Dispos 1992; 13: 693–701
Boxenbaum H, Gillespie T, Heck K, et al. Human dolasetron pharmacokinetics: II. disposition following single-dose oral administration to normal male subjects. Biopharm Drug Dispos 1993; 14: 131–41
Shah A, Lanman R, Bhargava V, et al. Pharmacokinetics of dolasetron following single- and multiple-dose intravenous administration to normal male subjects. Biopharm Drug Dispos 1995; 16: 177–89
Dimmitt DC, Shah AK, Arumughan T, et al. Pharmacokinetics of oral and intravenous dolasetron mesylate in patients with renal impairment. J Clin Pharmacol 1998; 38: 798–806
McDermott S, Songer S, Benedict C, et al. Safety profile of dolasetron. Eur Hosp Pharm 1996; 2Suppl. 1: S25–31
Dixon RM, Cramer M, Shah AK, et al. Single-dose, placebo-controlled, phase I study of oral dolasetron. Pharmacotherapy 1996; 16(2): 245–52
Hunt TL, Cramer M, Shah A, et al. A double-blind, placebo-controlled, dose-ranging safety evaluation of single-dose intravenous dolasetron in healthy male volunteers. J Clin Pharmacol 1995 Jul; 35(7): 705–12
Hunt TL, Cramer M, Christy-Bittel J, et al. Multiple-dose, placebo-controlled, phase I study of oral dolasetron. Pharmacotherapy 1996; 16: 253–60
Lerman J, Sims C, Sikich N, et al. Pharmacokinetics of the active metabolite (MDL 74,156) of dolasetron mesylate after oral or intravenous administration to anesthetized children. Clin Pharmacol Ther 1996; 60: 485–92
Hesketh P, Navari R, Grote T, et al. Double-blind, randomized comparison of the antiemetic efficacy of intravenous dolasetron mesylate and intravenous ondansetron in the prevention of acute cisplatin-induced emesis in patients with cancer. J Clin Oncol 1996; 14: 2242–9
Chevallier B, Bleiberg H, Fauser AA, et al. Dolasetron in the control of chemotherapy-induced nausea and vomiting. Eur Hosp Pharm 1996; 2Suppl. 1: S38–42
Audhuy B, Cappelaer EP, Martin M, et al. A double-blind randomized comparison of the antiemetic efficacy of two intravenous doses of dolasetron mesylate and granisetron in patients receiving dose cisplatin therapy. Eur J Cancer 1996; 32A(5): 807–13
Diemunsch P, d’Hollander A, Feiss P, et al. The prevention and treatment of postoperative nausea and vomiting with dolasetron. Eur Hosp Pharm 1996; 21Suppl. 1: S34–7
Warriner CB, Knox D, Belo S, et al. Prophylactic oral dolasetron mesylate reduces nausea and vomiting after abdominal hysterectomy. Can J Anaesth 1997; 44: 1167–73
Graczyk SG, McKenzie R, Kallar S. Intravenous dolasetron for the prevention of postoperative nausea and vomiting after outpatient laparoscopic gynecologic surgery. Anesth Analg 1997; 84: 325–30
Diemunsch P, Leeser J, Feiss P, et al. Intravenous dolasetron mesilate ameliorates postoperative nausea and vomiting. Can J Anaesth 1997; 44: 173–81
Kovac AL, Scuderi PE, Boerner TF, et al. Treatment of postoperative nausea and vomiting with single intravenous doses of dolasetron mesylate: multicenter trial. Dolasetron Mesylate PONV Treatment Study Group. Anesth Analg 1997; 84: 546–52
Whitmore JB, Kris MG, Hesketh PJ, et al. Rationale for the use of a single fixed intravenous dolasetron dose for the prevention of cisplatin-induced nausea and vomiting. Pooled analysis of 14 clinical trials. Support Care Cancer 1998; 6(5): 473–8
Chevallier B, Cappelaere P, Splinter T, et al. A double-blind, multicentre comparison of intravenous dolasetron mesilate and metoclopramide in the prevention of nausea and vomiting in cancer patients receiving high-dose cisplatin chemotherapy. Support Care Cancer 1997; 5(1): 22–30
Graczyk SG, McKenzie R, Kallar S, et al. Intravenous dolasetron for the prevention of nausea and vomiting after outpatient laparoscopic gynecologic surgery. Anesth Analg 1997; 84: 325–330
Diemunsch P, D’Hollander A, Paxton L, et al. Intravenous dolasetron mesylate in the prevention of postoperative nausea and vomiting in females undergoing gynecological surgery. J Clin Anesth 1997; 9(5): 365–73
Diemunsch P, Lesser J, Feiss P, et al. Intravenous dolasetron mesilate ameliorates postoperative nausea and vomiting. Can J Anaesth 1997; 44(2): 173–81
Keefe DL. The cardiotoxic potential of the 5-HT3 receptor antagonist antiemetics: Is there cause for concern? Oncologist 2002; 7: 65–72
Hesketh P, Navari R, Grote T, et al. Double-blind, randomized comparison of the antiemetic efficacy of intravenous dolasetron mesylate and intravenous ondansetron in the prevention of acute cisplatin-induced emesis in patients with cancer. Dolasetron Comparative Chemotherapy-induced Emesis Prevention Group. J Clin Oncol 1996; 14(8): 2242–9
Jantunen IT, Kataja VV, Muhonen TT, et al. Effects of granisetron with doxorubicin or epirubicin on ECG intervals. Cancer Chemother Pharmacol 1996; 37: 502–4
Lofters WS, Pater JL, Zee B, et al. Phase III double-blind comparison of dolasetron mesylate and ondansetron and an evaluation of the additive role of dexamethasone in the prevention of acute and delayed nausea and vomiting due to moderately emetogenic chemotherapy. J Clin Oncol 1997; 15(8): 2966–73
Ballard HS, Bottino G, Bottino J. Ondansetron and chest pain [letter]. Lancet 1992; 340: 1107
Baltzer L, Kris MG, Hinkley L, et al. Reversible electrocardiographic interval prolongations following the specific serotonin antagonists ondansetron (OND) and dolasetron mesylate (DM): a possible drug class effect without sequelae? [abstract 1489]. Proc Am Soc Clin Oncol 1994; 13: 544
Benedict CR, Arbogast R, Martin L, et al. Single-blind study of the effects of intravenous dolasetron mesylate versus ondansetron on electrocardiographic parameters in normal volunteers. J Cardiovasc Pharmacol 1996; 28: 53–9
Kuryshev YA, Brown AM, Wang L, et al. Interactions of the 5-hydroxytryptamine 3 antagonist class of antiemetic drugs with human cardiac ion channels. J Pharmacol Ther 2000; 295: 614–20
Yancik R. Cancer burden in the aged: an epidemiologic and demographic overview. Cancer 1997; 80: 1273–83
Lindsey AM, Larson PJ, Dodd MJ, et al. Comorbidity, nutrition intake, social support, weight and functional status over time in older cancer patients receiving radiotherapy. Cancer Nurs 1994; 17: 113–24
Wei JY. Cardiovascular comorbidity in the older cancer patient. Semin Oncol 1995; 22: 9–10
Kamataki T, Yokoi T, Fujita K, et al. Preclinical approach for identifying drug interactions. Cancer Chemother Pharmacol 1998; 42Suppl: S50–3
Bertz RJ, Granneman GR. Use of in vitro and in vivo data to estimate the likelihood of metabolic pharmacokinetic interactions. Clin Pharmacokinet 1997; 32(3): 210–58
Woosley RL. Drugs that prolong QTc and/or induce torsades de pointes [online]. Available from URL: http://www.torsades.org [Accessed 2002 Dec 26]
Moss AJ, Schwartz PJ, Crampton RS, et al. The long QT syndrome: prospective longitudinal study of 328 families. Circulation 1991; 84: 1136–44
Keating M, Atkinson D, Dunn C, et al. Linkage of a cardiac arrhythmia the long-QT syndrome and the Harvey-ras-1 gene. Science 1991; 252: 704–6
Vincent MG, Timothy KW, Leppert M, et al. The spectrum of symptoms and QT intervals in carriers of the gene for the long QT syndrome. N Engl J Med 1992; 327: 846–52
Schwartz PT, Locati EH, Moss AJ, et al. Left cardiac sympathetic denervation in the therapy of congenital long QT syndrome: a worldwide report. Circulation 1991; 84: 503–11
Lowenthal RM, Eaton K. Toxicity of chemotherapy. Hematol Oncol Clin North Am 1996; 10: 967–90
Eberhart LHJ, Morin AM, Seeling W, et al. Meta-analysis of controlled randomized studies on droperidol for prevention of postoperative phas nausea and vomiting. Anasthesiol Intensivmed Notfallmed Schmerzther 1999; 34: 528–36
Tang J, Watcha MF, White PF. A comparison of costs and efficacy of ondansetron and droperidol as prophylactic antiemetic therapy for elective outpatient gynecologic procedures. Anesth Analg 1996; 83: 304–13
Henzi I, Sonderegger J, Tramèr MR. Efficacy, dose-response and adverse effects of droperidol for prevention of postoperative nausea and vomiting. Can J Anaesth 2000; 47: 537–51
Lischke V, Behne M, Doelken P, et al. Droperidol causes a dose-dependent prolongation of the QT interval. Anesth Analg 1994; 79: 983–6
Food and Drug Administration. FDA strengthens warnings for droperidol [online]. Available from URL: http://www.fda.gov/bbs/topics/ANSWERS/2001/ANS01123.html [Accessed 2002 Dec 26]
McCormick CG. FDA alert: current FDA report on droperidol status and basis for black box warning. Am Soc Anesthesiologists Newsletter 2002; 66(4): 19–20
Lopez-Olaondo L, Carrascosa F, Pueyo FJ. Advances in antiemetic pharmacology. Review Course Lectures, European Society of Anesthesiologists 8th Annual Meeting; 2000 Apr; Vienna, Austria: 251–8
Chang N, Rappaport B. FDA alert: update on droperidol and the FDA. Am Soc Anesth Newsletter 2002; 66(12): 25.
Hochhaus G, Barth J, al Fayoumi S, et al. Pharmacokinetics and pharmacodynamics of dexamethasone sodium-m-sulfobenzoate (DS) after intravenous and intramuscular administration: a comparison with dexamethasone phosphate (DP). J Clin Pharmacol 2001; 41(4): 425–34
Henzi I, Walder B, Tramèr MR. Dexamethasone for the prevention of postoperative nausea and vomiting: a quantitative systematic review. Anesth Analg 2000; 90(1): 186–94
Aouad MT, Siddik SS, Rizk LB, et al. The effect of dexamethasone on postoperative vomiting after tonsillectomy. Anesth Analg 2001; 92(3): 636–40
Wang JJ, Ho ST, Tzeng JI, et al. The effect of timing of dexamethasone administration on its efficacy as a prophylactic antiemetic for postoperative nausea and vomiting. Anesth Analg 2000; 91: 136–9
McKenzie R, Tantisira B, Karambelkar DJ, et al. A recent revolution in patient symptom control. Cancer Treat Rev 1991; 18: 95–135
McKenzie R, Tantisira B, Karambelkar DJ, et al. Comparison of ondansetron with ondansetron plus dexamethasone in the prevention of postoperative nausea and vomiting. Anesth Analg 1994; 79: 961–4
McKenzie R, Riley TJ, Tantisira B, et al. Effect of propofol for induction and ondansetron with or without dexamethasone for the prevention of nausea and vomiting after major gynecologic surgery. J Clin Anesth 1997; 9: 15–20
Fujii Y, Toyooka H, Tanaka H. Prevention of postoperative nausea and vomiting with a combination of granisetron and droperidol. Anesth Analg 1998; 86: 613–6
Fujii Y, Tanaka H, Toyooka H. The effects of dexamethasone on antiemetics in female patients undergoing gynecologic surgery. Anesth Analg 1997; 85(4): 913–7
Fujii Y, Toyooka H, Tanaka H. Prophylactic antiemetic therapy with granisetron-dexamethasone combination in women undergoing breast surgery. Acta Anaesthesiol Scand 1998; 42: 1038–42
Fujii Y, Toyooka H, Tanaka H. Prophylactic antiemetic therapy with a combination of granisetron and dexamethasone in patients undergoing middle ear surgery. Br J Anaesth 1998; 81: 754–6
Fujii Y, Saitoh Y, Tanaka Y, et al. Granisetron/dexamethasone combination for preventing postoperative nausea and vomiting after laparoscopic cholecystectomy. Eur J Anaesthesiol 2000; 17: 64–8
Fujii Y, Tanaka H, Kobayashi N. Granisetron/dexamethasone combination for the prevention of postoperative nausea and vomiting after thyroidectomy. Aneasth Intensive Care 2000; 28: 266–9
Fujii Y, Saitoh Y, Tanaka H, et al. Granisetron/dexamethasone combination for reducing nausea and vomiting during spinal anesthesia for cesarean section. Anesth Analg 1999; 88: 1346–50
Lopez-Olaondo L, Carrascosa F, Pueyo FJ, et al. Combination of ondansetron and dexamethasone in the prophylaxis of postoperative nausea and vomiting. Br J Anaesth 1996; 76: 835–40
Coloma M, White PF, Markowitz SD, et al. Dexamethasone in combination with dolasetron for prophylaxis in the ambulatory setting. Anesthesiology 2002; 96: 1346–50
McKenzie R, Uy NT, Riley TJ, et al. Droperidol/ondansetron combination controls nausea and vomiting after tubal banding. Anesth Analg 1996; 83: 1218–22
Fujii Y, Saitoh Y, Tanaka H, et al. Prevention of PONV with granisetron, droperidol or metoclopramide in patients with postoperative emesis. Can J Anaesth 1998; 45(2): 153–6
Fujii Y, Toyooka H, Tanaka H, et al. Prevention of PONV with granisetron, droperidol and metoclopramide in female patients with history of motion sickness. Can J Anaesth 1997; 44: 820–4
Fujii Y, Toyooka H, Tanaka H. Prevention of postoperative nausea and vomiting in female patients during menstruation: comparison of droperidol, metoclopramide and granisetron. Br J Anaesth 1998; 80(2): 248–9
Koivuranta M, Jokela R, Kiviluomak K, et al. The antiemetic efficacy of a combination of ondansetron and droperidol. Anaesthesia 1997; 52(9): 863–8
Pueyo FJ, Carrascosa F, Lopez L, et al. Combination of ondansetron and droperidol in the prophylaxis of postoperative nausea and vomiting. Anesth Analg 1996; 83: 117–22
Steinbrook RA, Freiberger D, Gosnell JL, et al. Prophylactic antiemetics for laparoscopic cholecystectomy: ondansetron versus droperidol plus metoclopramide. Anesth Analg 1996; 83: 1081–3
Sanchez-Ledesma MJ, Lopez-Olaondo L, Pueyo FJ, et al. A comparison of three antiemetic combinations for the prevention of postoperative nausea and vomiting. Anesth Analg 2002
Eberhart LH, Morin AM, Georgieff M. Dexamethasone for prophylaxis of postoperative nausea and vomiting: a meta-analysis of randomized controlled studies. Anaesthetist 2000; 49: 713–20
Acknowledgments
Dr Kovac has disclosed that in the past he has received grant support from GlaxoWellcome (now GlaxoSmithKline), Roche Pharmaceuticals and Hoechst Marion Roussel (now Aventis Pharmaceuticals) and participated on the Speakers Bureau for GlaxoWellcome, Roche Pharmaceuticals and Abbott Laboratories. There were no sources of funding for the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kovac, A.L. Benefits and Risks of Newer Treatments for Chemotherapy-Induced and Postoperative Nausea and Vomiting. Drug-Safety 26, 227–259 (2003). https://doi.org/10.2165/00002018-200326040-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002018-200326040-00003