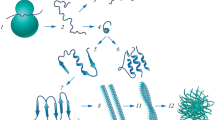
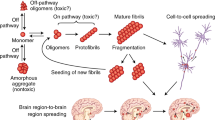
Abstract
Developing effective treatments for neurodegenerative diseases is one of the greatest medical challenges of the 21st century. Although many of these clinical entities have been recognized for more than a hundred years, it is only during the past twenty years that the molecular events that precipitate disease have begun to be understood. Protein aggregation is a common feature of many neurodegenerative diseases, and it is assumed that the aggregation process plays a central role in pathogenesis. In this process, one molecule (monomer) of a soluble protein interacts with other monomers of the same protein to form dimers, oligomers, and polymers. Conformation changes in three-dimensional structure of the protein, especially the formation of β-strands, often accompany the process. Eventually, as the size of the aggregates increases, they may precipitate as insoluble amyloid fibrils, in which the structure is stabilized by the β-strands interacting within a β-sheet. In this review, we discuss this theme as it relates to the two most common neurodegenerative conditions—Alzheimer’s and Parkinson’s diseases.
Similar content being viewed by others
Introduction
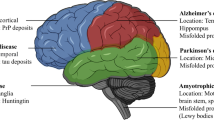
Extracellular fibrous amyloid deposits or intracellular inclusions containing abnormal protein fibrils characterize many neurodegenerative diseases, including Alzheimer’s, Parkinson’s, and Huntington’s diseases, amyotrophic lateral sclerosis, frontal temporal dementia, and the human prion diseases (1). Burgeoning evidence suggests that accumulation of proteins capable of forming amyloid deposits may represent a common pathological mechanism for these diverse illnesses (2,3). In each of these diseases, misfolding of a particular protein can lead to its aggregation, involving a process in which monomers interact to form dimers, oligomers, and eventually insoluble fibrillar deposits. Alzheimer’s and Parkinson’s diseases, representative examples that account for the majority of cases of neurodegenerative diseases, are reviewed here with an emphasis on the fundamental importance of aggregation as the pathological trigger. For each disease, we first outline the major characteristics and then present the evidence for the crucial role of soluble oligomers (rather than the end-stage insoluble fibrils) of the aggregating protein—β-amyloid protein (Aβ) in Alzheimer’s disease and α-synuclein in Parkinson’s disease. Finally, we discuss future therapeutic and diagnostic approaches, based on current understanding of Aβ and α-synuclein pathobiology.
Alzheimer’s Disease: Incidence and Symptoms
First identified by Alois Alzheimer in 1906, Alzheimer’s disease is an irreversible, progressive brain disease that slowly destroys memory and cognitive skills (4). It is the most common cause of dementia, accounting for more than half of all such cases, and currently affects more than 24 million people worldwide, with 4.6 million new cases each year (5). Age is the single biggest known risk factor, with the incidence of the disease increasing from one in ten of those over 65 to almost half of those over 85 (6,7). There is no strong sex or race effect, but because women tend to live longer than men there are more women with Alzheimer’s disease. The average duration of the disease is about 8 years, but it can last in excess of 20 years.
The disease is divided into two categories based on the age of onset. Early-onset Alzheimer’s disease is extremely rare, accounting for only ∼2% of all cases. It develops between the ages of ∼30 and 60 years, and more than half of all such cases are genetic, with a strong Mendelian inheritance pattern (8). Late-onset Alzheimer’s disease is by far the most common form of the disease. It too has a genetic predisposition but appears to involve several gene polymorphisms, some yet to be identified, that individually or in combination increase one’s risk for developing Alzheimer’s disease (9). A genetic component for late-onset disease is supported by the finding that the age at onset of Alzheimer’s disease is significantly more variable for concordant nonidentical twins than concordant identical twins, providing evidence that genetic background strongly influences the timing of the disease (10). Genetic factors that predispose to Alzheimer’s disease are difficult to identify because their inheritance does not cause the disease phenotype, but rather modulates the age of onset.
The disease progression is similar for both early- and late-onset Alzheimer’s disease and is arbitrarily split into three overlapping stages: early/mild, moderate, and severe. The initial onset of Alzheimer’s disease is insidious, with memory loss the earliest and most frequently cited symptom. In a living person, there is no precise diagnostic test that confirms Alzheimer’s disease. A diagnosis of probable Alzheimer’s disease is achieved by excluding other conditions that might explain the observed symptoms. Currently the most important diagnostic tool for clinicians is neuropsychological and mental status testing. The Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) criteria for diagnosing dementia requires loss of two or more domains, including memory, language, calculation, orientation, and judgment (11). Neuropsychological tests, such as the Mini-Mental State Examination (MMSE), can provide clinical insight into the patient’s cognitive changes. Patients are considered to have mild cognitive impairment, not Alzheimer’s disease, if they present with memory loss but have only minimal impairment in other cognitive domains and are not functionally impaired at work or home. Thereafter, computed tomography or magnetic resonance imaging (MRI) can be used to discriminate between other forms of dementia. An experienced physician can diagnose Alzheimer’s disease with up to 90% accuracy. However, a definitive diagnosis of Alzheimer’s disease requires not only the presence of severe dementia but also postmortem confirmation of two histopathological features, tangles and plaques (Figure 1A) (12).
Pathological hallmarks of Alzheimer’s and Parkinson’s diseases. (A) Tangles and plaques in Alzheimer’s disease. Neurofibrillary tangles are intraneuronal and consist of paired helical filaments, the subunit of which is a microtubule-associated protein called tau that has been phosphorylated at multiple sites (dark staining structures). Amyloid plaques are extracellular and are largely composed of a ∼4-kDa protein called the amyloid β-protein (Aβ) (round diffuse structures). See Acknowledgements for source information on panel A. (B) Lewy bodies in Parkinson’s disease. Nerve cell with 3 Lewy bodies that are double-stained for α-synuclein (brown) and ubiquitin (blue). Where only α-synuclein is stained, the color appears as pale reddish-brown, but where ubiquitin also is stained, the superposition of color gives a dark black and brown appearance. The blue staining is not seen on its own, because all the ubiquitin-immunoreactive structures are also positive for α-synuclein. The halo of each Lewy body is strongly immunoreactive for ubiquitin, whereas both the core and the halo of each Lewy body are immunoreactive for α-synuclein. Bar, 10 µm. Panel B has been reproduced from Figure 3 of Spillantini et al. (96), © 1993–2005 by the National Academy of Sciences of the USA, all rights reserved.
Emerging Diagnostic Tools
Techniques for imaging the brain are progressing, and both the sensitivity and specificity for diagnosis of Alzheimer’s disease are constantly improving. For instance, MRI of the hippocampal formation can be used to identify atrophy found in patients with mild cognitive impairment and Alzheimer’s disease, as such atrophy is absent in the normal elderly. Similarly, changes in the volume of the fusiform gyrus can be used to distinguish between mild cognitive impairment and Alzheimer’s disease, whereas common cerebral alterations such as enlarged ventricular spaces are used to support the diagnosis of Alzheimer’s disease (13). Positron emission tomography (PET) allows for visualization of metabolism in various regions of the brain by using 18F-2-deoxy-2-fluoro-D-glucose (FDG) as a surrogate marker of glucose metabolism. In patients with early Alzheimer’s disease, decreased metabolism in parieto-temporal association cortex and cingulate gyrus and more marked changes in the medial temporal region and parieto-temporal association cortex are detected. As the condition progresses, abnormal PET-FDG is also obvious in the frontal association cortex. In addition, changes detected in regional cerebral perfusion studies by single photon emission computed tomography (SPECT) can distinguish between mild Alzheimer’s disease and forms of vascular dementia (14).
As will be discussed below, accumulation of Aβ in the brain, manifesting as β-sheet rich plaques, is a hallmark of Alzheimer’s disease. Recently, researchers at the University of Pittsburgh developed a thioflavin T analog, Pittsburgh compound B (PIB), which binds β-sheet-rich fibrils (15). This compound crosses the blood-brain barrier and binds amyloid deposits in the brain parenchyma, where binding of PIB labeled with carbon-11 can be detected by PET imaging. As one would expect, an inverse correlation exists between FDG-PET imaging of glucose metabolism in the parietal cortex and PIB binding (16). This novel in vivo imaging technique provides promise for more definitive diagnosis of Alzheimer’s disease by detecting the pathognomonic Aβ accumulation; following the progression of Alzheimer’s disease in individual patients; and, tracking changes in plaque burden in response to amyloid-lowering therapeutics (see below).
Neuropathological Hallmarks of Alzheimer’s Disease
Microscopically, the Alzheimer brain is characterized by the presence of extracellular amyloid plaques and intraneuronal neurofibrillary tangles. Amyloid plaques display a broad range of morphologic and biochemical characteristics and contain numerous proteins, the principal of which is Aβ (17,18). Aβ is a ∼4-kDa protein with a common core sequence but heterogeneous N- and C-termini. The most common form of Aβ is 40 amino acids long and is called Aβ40. Aβ42, a less abundant form of this protein that differs only by having two additional amino acid residues at the C-terminus, is particularly associated with disease (19). Compact, neuritic amyloid plaques contain thioflavin S and Congo red-positive fibrillar deposits with both Aβ40 and Aβ42 present (20). Diffuse plaques, on the other hand, are not fibrillar and consist almost exclusively of Aβ42. These immature deposits may be detected in the brains of young patients with Down’s syndrome before the manifestation of Alzheimer’s disease-type dementia or in brain regions that do not display the complete extent of Alzheimer’s disease pathology described above (21). As a result, diffuse plaques are considered precursors to mature, neuritic plaques. Dilated, dystrophic neurites, activated microglia, and reactive astrocytes can be found within and immediately surrounding neuritic plaques (22). The processes of neurons found herein display abnormal signs of enlarged lysosomes and numerous mitochondria.
Neurons bearing neurofibrillary tangles, composed of hyperphosphorylated forms of the microtubule-associated protein, tau, are also frequently found proximate to amyloid deposits (23,24), and their temporal and spatial appearance more closely reflects disease severity than does the appearance of amyloid plaques (25,26). Neurofibrillary tangles are not specific to Alzheimer’s disease, however, and are found in other disorders (for example, subacute sclerosing panencephalitis and progressive supranuclear palsy) not associated with the cognitive dysfunction and memory impairment that characterize Alzheimer’s disease (27). Indeed a growing body of genetic and biochemical evidence suggests that neurofibrillary tangles are downstream of Aβ. Specifically, experimental evidence suggests that abnormal Aβ accumulation triggers tau pathology (28,29), and tau has been proposed as an essential mediator of Aβ-induced neurotoxicity (30); however, the steps connecting Aβ to tau remain undefined. Aβ has been shown to induce the calpain-mediated cleavage of tau, leading to the generation of a toxic 17-kDa fragment (31), and to induce abnormal tau phosphorylation at disease-relevant sites (32,33); a recent study even suggested that tau phosphorylation is the limiting factor in Aβ-induced neurotoxicity (34). Similarly, tau appears to play a central role in the memory deficits apparent in certain transgenic mouse models of Alzheimer’s disease (35). Together, these results suggest that Aβ plays an initiating role in a pathogenic cascade that requires altered metabolism of tau to result in disease.
A Molecular Explanation of Alzheimer’s Disease: The Aβ Hypothesis
Considerable genetic, animal-modeling, and biochemical data have emerged to suggest that Aβ plays a central role in initiating Alzheimer’s disease. Aβ is derived from the amyloid precursor protein (APP) by the action of two aspartyl proteases called β- and γ-secretases (Figure 2) (36–38). APP is first cleaved by β-secretase shedding its large ectodomain and leaving a membrane-bound C-terminal stub (39,40). This 99-amino acid stub is subsequently cleaved by γ-secretase and Aβ is released (41) (Figure 2). Depending on the exact point of cleavage by γ-secretase, two main forms of Aβ, comprising either 40 or 42 amino acid residues, are produced. The proportion of Aβ42 to Aβ40 formed is particularly noteworthy, because the longer form of Aβ is far more prone to oligomerize and form fibrils than the more abundantly produced Aβ40 peptide. Production of Aβ is a normal process, but in a small number of individuals the overproduction of Aβ, or an increased proportion of the 42-amino acid form, appears sufficient to cause early-onset Alzheimer’s disease (see Table 1).
Production of Aβ by proteolytic cleavage from APP followed by association of Aβ to form oligomers and fibrils, showing potential targets for anti-amyloid therapies. Aβ, the gray shaded box, is cleaved from APP by sequential action of 2 proteases; β-secretase carries out the initial cleavage to form the N-terminus of Aβ; γ-secretase then cleaves the C99 stub to produce the C-terminus of Aβ. The parallel dotted lines represent a membrane bilayer in which part of the C-terminal region of APP is anchored. Hence γ-secretase activity is a protease that cleaves a substrate within a membrane. Production of Aβ by secretase action leads to Aβ monomer, the concentration of which in the steady state is a balance between formation and degradation. Monomers can associate to form small oligomers that increase in size and eventually lead to fibril formation. One anti-amyloid strategy is to inhibit the enzymatic action of either secretase (shown by a black cross). A second strategy is to remove soluble and deposited Aβ using antibodies (shown as semicircles).
The evidence in support of a causative role for Aβ in Alzheimer’s disease is as follows:
-
1.
Localization of the APP gene to chromosome 21 and the observation that Alzheimer’s disease-like neuropathology is invariably seen in Down’s syndrome (trisomy 21) (Table 1) (42,43). This point is further supported by detection of a rare case of Down’s syndrome in which the distal location of the chromosome 21q breakpoint left the patient diploid for the APP gene (44). This individual showed no signs of dementia, and amyloid deposition was essentially absent from the brain upon death at age 78. In addition, duplication of APP is also associated with early-onset Alzheimer’s disease (42).
-
2.
Synthetic Aβ peptides are toxic to hippocampal and cortical neurons, both in culture and in vivo (45–47).
-
3.
Inherited mutations in the APP gene that immediately flank or localize within the Aβ region and increase the amount or aggregation properties of Aβ are sufficient to precipitate early-onset Alzheimer’s disease. Mutations lying outside the Aβ domain are proximate to the β- and γ-cleavage sites and elevate Aβ production or increase the Aβ42/Aβ 40 ratio (48–51). The five point mutations that lie within the Aβ sequence are clustered around the central hydrophobic core of Aβ and cause an increase in steady-state levels of Aβ and/or an increased propensity of the resultant Aβ to aggregate.
-
4.
Inherited mutations within the presenilin 1 and 2 genes increase the Aβ42/Aβ40 ratio throughout life and cause very early and aggressive forms of Alzheimer’s disease. In this regard, presenilin has been found to contribute the active site of the protease (γ-secretase) that generates the C-terminus of Aβ (Figure 2) (19,52). 5. In humans, Apo E, which codes for apolipoprotein E, has three common alleles, ε2, ε3, and ε4, and genetic epidemiological studies show that the ε4 allele is a major risk factor for developing late-onset Alzheimer’s disease, whereas the ε2 allele appears to be protective. Importantly, ε4 is associated with more extensive and fulminant Aβ deposition than is ε2 (53,54).
-
6.
Mice transgenic for mutant human APP show a time-dependent increase in extracellular Aβ and develop certain neuropathological and behavioral changes similar to those seen in Alzheimer’s disease (55).
-
7.
Finally, injection of synthetic Aβ into the brains of tau transgenic mice accelerates tau hyperphosphorylation and leads to tangle formation reminiscent of the other hallmark that characterizes Alzheimer’s disease (Figure 1A), whereas reducing endogenous expression of tau ameliorates behavioral deficits in APP transgenic mice (35).
Aβ Toxicity: The Importance of Structure
Aβ is a natural product present in the brains and cerebrospinal fluid (CSF) of normal subjects (36,56,57). The presence of Aβ itself does not lead to neurodegeneration, but neuronal injury ensues as a result of the ordered self-association of Aβ molecules. Within the amyloid plaques that characterize Alzheimer’s disease, Aβ is organized into fibrils 6–10 nm in diameter, whereas in vitro, Aβ readily assembles into very similar amyloid fibrils. Many studies have demonstrated that when synthetic Aβ is pre-incubated to form amyloid fibrils, such preparations are directly toxic to neurons (46,58).
One important caveat when considering the activity of Aβ assemblies is the dynamic nature of the aggregation process. Initial studies clearly demonstrated that aggregation of Aβ was essential for toxicity, but characterization of the assemblies used was limited and it was assumed that, because amyloid fibrils were detectable, it was fibrils that mediated the observed toxicity. Yet this ignored the fact that in patients dying with Alzheimer’s disease there is a relatively weak correlation between the severity of dementia and the density of fibrillar amyloid (59).
In contrast, robust correlations between the levels of soluble Aβ and the extent of synaptic loss and severity of cognitive impairment have been demonstrated (60,61); the term soluble Aβ refers to all forms of Aβ that remain in aqueous solution following high speed centrifugation of brain extracts. To date, most studies of soluble Aβ brain levels have employed assays that cannot identify the aggregation state of the species detected (62). Thus, although one cannot attribute the effects to a specific assembly form of Aβ, the solubility of the species in aqueous buffer following ultracentrifugation (typically >100,000g for >1 h) would indicate that the preparations used are free of fibrillar assemblies. Furthermore, in very recent studies, antibodies reported to be specific for oligomeric, but not monomeric or fibrillar, Aβ revealed abundant anti-oligomer reactivity in soluble extracts of Alzheimer’s disease brain, but none in age-matched controls (63).
Identification of Neurotoxic, Nonfibrillar Aβ Aggregates
SDS-stable dimers and trimers (so-called low-n oligomers) of Aβ have been detected in the buffer-soluble fraction of human cerebral cortex and in human CSF (57,64). Similar oligomers are also formed by a fibroblast cell line genetically manipulated to express mutant human APP (7PA2 cells). These cells produce and secrete significant amounts of SDS-stable low-n oligomers of Aβ that migrate in denaturing SDS-polyacrylamide gels with molecular weights consistent with dimers, trimers, and occasionally tetramers (65,66). Because of the easy maintenance and fast growth rate of these cells, 7PA2 culture medium has provided a convenient tool to investigate the biological activities of low-n Aβ oligomers. This led to the discovery that Aβ oligomers can inhibit hippocampal long-term potentiation (an electrophysiological measure of synaptic plasticity) (67,68), impair complex learned behavior in the live rat (69), and reduce the density of dendritic spines in cultured hippocampal neurons (70,71).
Additional support for a role for prefibrillar Aβ assemblies in Alzheimer’s disease pathogenesis comes from studies using synthetic Aβ peptides. The first nonfibrillar assemblies identified were protofibrils; these heterogeneous structures range from spherical assemblies of ∼5 nm diameter to short, flexible rods of up to 200 nm in length (72,73). The principal difference between protofibrils and mature fibrils is size and relative solubility; fibrils are frequently several microns long and are often associated with other fibrils, whereas protofibrils tend not to be associated with other protofibrils and seldom exceed 150 nm in length. Consequently, unlike fibrils, they do not sediment upon low-speed centrifugation (73,74). Protofibrils can be generated under a variety of biochemical conditions and appear to behave as true fibril intermediates in that they can both form fibrils and dissociate to lower-molecular-weight species. Acute application of protofibrils in vivo rapidly alters synaptic physiology, whereas chronic application causes cell death (75). A second soluble, nonfibrillar assembly of synthetic Aβ called Aβ-derived diffusible ligands (ADDLs), appear as spheres with a diameter ∼5 nm and migrate in polyacrylamide gels at ∼4, 8, 16, and 18 kDa. ADDLs are formed only under certain specific in vitro conditions but can cause neuronal death and block long-term potentiation in ex vivo preparations (76,77). A recent study reported that synthetic ADDL preparations can bind excitatory synapses (78) and cause a reduction in spine density (79) similar to the findings observed with soluble Aβ oligomers secreted in cell culture.
Together these results provide compelling evidence that soluble nonfibrillar forms of Aβ are potent neurotoxins. Indeed, in the human brain it is likely that multiple Aβ assemblies that are in dynamic equilibrium simultaneously alter neuronal, astrocytic, and microglial function, and that different toxic effects may occur virtually concurrently in various regions of the cerebral cortex. Thus removal or neutralization of such toxic species is an attractive therapeutic strategy.
Candidate Aβ-Based Therapies and Diagnostics
To date, there is no effective treatment that can prevent progression of Alzheimer’s disease; available drugs can only delay worsening of symptoms. Therefore there is urgent need for therapies that alter the progression of Alzheimer’s disease. The Aβ hypothesis posits that increased steady-state levels and consequent Aβ assembly is the primary event driving Alzheimer’s disease pathogenesis (Figure 3). The rest of the disease process is believed to result from this aberrant assembly. A number of different anti-amyloid therapies are under development; two examples are discussed and illustrated in Figure 2: decreasing the production of soluble Aβ monomer and removing soluble and deposited Aβ.
Reduction of Aβ levels is particularly attractive because it may be possible to titrate Aβ down to concentrations that will not support oligomerization. It would be anticipated that cell-penetrant agents that could reduce intracellular and/or extracellular monomer levels below the critical concentration needed for oligomerization would thus prevent Aβ from assembling into toxic structures. The development of potent highly selective inhibitors of β- and γ-secretases that can readily enter the brain and lower Aβ production (Figure 2) is being actively pursued. Similarly, efforts are also ongoing to develop small molecules that can upregulate the enzymes that control Aβ degradation and thus lower Aβ levels by increasing Aβ catabolism.
Anti-Aβ immunotherapy employs antibodies that recognize multiple different toxic Aβ assemblies by both directly neutralizing them and preventing their toxic effect, by promoting microglial clearance, and/or by redistributing Aβ from the brain to the systemic circulation. This approach has already been shown to reduce cerebral Aβ levels, decrease amyloidassociated gliosis and neuritic dystrophy, and alleviate memory impairment in transgenic mouse models of Alzheimer’s disease (80). More importantly, Alzheimer’s disease patients that were immunized with aggregated Aβ showed diminished cognitive decline and slowed disease progression compared with patients that received placebo (81). Unfortunately, this phase IIa trial had to be stopped prematurely because 18 of the 298 patients who had been immunized developed meningoencephalitis. Notably, in four cases that have since come to autopsy (two affected with encephalitis and two not), all showed evidence of clearance of amyloid deposits. Thus in the first clinical test of the Aβ hypothesis it appears that (as in preclinical studies of mouse models) targeted removal of cortical Aβ beneficially modifies Alzheimer’s disease progression. Efforts are ongoing to develop an equally effective immunization protocol that avoids induction of encephalitis. Thus there is good reason to believe that therapies directed at preventing the generation of toxic Aβ assemblies will soon come to the clinic and that, unlike current therapies, they will actually halt further deterioration and offer the potential of restoring normal cognitive function.
With the advancement of potentially disease-modifying therapies, there is an urgent need to develop methods for use in early ante mortem diagnosis. This is required, not only from a clinical standpoint, but also because it affects the integrity of clinical trials and epidemiological research. Currently there are at least four methods that have evolved from our better understanding of the disease process: analysis of Aβ species in CSF (82); visualization of amyloid plaques by PET as discussed above (15,83); measurement of Aβ in peripheral blood (84); and measurement of total tau and/or phospho-tau in CSF (85,86).
Given the genetic evidence supporting a prominent role for Aβ42 in disease, many studies have investigated the diagnostic utility of measuring Aβ42 in CSF. For Alzheimer’s disease patients, Aβ42 levels in CSF are typically reduced to around 50% of the level found in controls. The mean sensitivity and specificity to discriminate between Alzheimer’s disease and normal aging are both >85% (87). However, decreased CSF Aβ 42 is found in certain patients with frontotemporal dementia and vascular dementia, and measurement of CSF Aβ 42 alone is insufficient to discriminate between Alzheimer’s disease and these dementias (88). CSF Aβ40 is unchanged or slightly increased in Alzheimer’s disease (89); consequently a decrease in the ratio of Aβ42/Aβ40 in CSF has been found in Alzheimer’s disease, and this decrease seems more pronounced than the reduction of CSF Aβ42 alone (90). Alzheimer’s disease is also associated with a significant increase in CSF tau and phosphotau levels (85,86), and combining measurement of total tau, Aβ42, and phospho-tau identifies incipient Alzheimer’s disease in patients with mild cognitive impairment with very high accuracy (90,91).
The reduced level of CSF Aβ42 in Alzheimer’s disease is believed to be caused by deposition of Aβ42 in senile plaques, hence leaving lower levels of Aβ 42 to diffuse into CSF. Accordingly, studies have found a strong correlation between low Aβ42 in CSF and high retention of PIB (83). Factors that may contribute to reduced Aβ42 levels, in addition to deposition in senile plaques, include formation of Aβ 42 oligomers that escape ELISA detection and binding of Aβ42 to other proteins that block the antibody recognition of Aβ. For instance, ELISA measurements of plasma Aβ levels in Alzheimer’s disease have yielded conflicting data and this could, in part, reflect an inability to measure Aβ oligomers. This possible confounder might differ for antibodies used in different ELISA protocols and could explain some of the contradictory results. Development of anti-Aβ dimer/oligomer-specific antibodies should obviate concerns about epitope masking due to Aβ self-association and may provide a useful system to measure Aβ dimer/oligomer levels in both CSF and plasma. Indeed, a small number of preliminary studies suggests that measurement of Aβ oligomers will be of benefit (63,92). If this holds true in larger studies, one would anticipate that combining measurement of disease-linked assembly forms (oligomers) of Aβ together with measurement of tau in CSF and PIB binding in brain will provide a highly specific and sensitive means of measuring both early and incipient Alzheimer’s disease.
Parkinson’s Disease: Incidence, Symptoms, and Diagnosis
Named after the English physician who first described the clinical syndrome in “An Essay on the Shaking Palsy” published in 1817 [reprinted in (93)], Parkinson’s disease is an irreversible, progressive neurodegenerative disease that impairs movement. Incidence increases markedly with age; hence young-onset Parkinson’s disease, defined as occurrence before age 40, accounts for just 5% of newly diagnosed cases (Parkinson’s Disease Society). Upon reaching the 65–69 age range, 0.6% of the population are affected, increasing to 2.6% of those aged 85–89 (94), making it the second most common neurodegenerative illness after Alzheimer’s disease. The majority of cases of Parkinson’s disease are idiopathic and sporadic. However, studies within the past few years indicate that a substantial number of cases do have a genetic component. The three principal signs of parkinsonism, the clinical phenotype that defines the disease, are resting tremor, often beginning in one finger, bradykinesia, and rigidity. Whereas dementia is the defining feature of Alzheimer’s disease, cognitive ability remains intact in most Parkinson’s disease sufferers, at least in the early stages of the disease.
Pathology and Mechanism of Disease
Pathologically, the most obvious brain structure affected by Parkinson’s disease is a part of the substantia nigra called the pars compacta. Because patients suffering from other neurological disorders can display parkinsonian features, a definitive diagnosis of Parkinson’s disease can be confirmed only by post mortem histopathological examination of the substantia nigra for loss of pigmented neurons and presence of Lewy bodies in remaining neurons. Identification of Lewy bodies has been facilitated by immunostaining for particular proteins, initially for ubiquitin and more recently for α-synuclein, now regarded as the major protein constituent (95). Such staining reveals filaments that, when purified and examined by immunoelectron microscopy, can be seen to contain α-synuclein (96) (Figure 1B) and resemble filaments formed when purified recombinant α-synuclein is allowed to aggregate in vitro (97).
In the mid-twentieth century, Arvid Carlsson showed that dopamine acted as the neurotransmitter in neurons of the substantia nigra pars compacta, work for which he shared the Nobel Prize in 2000. These neurons operate in a pathway that controls voluntary movement. This involves signals being relayed from the cerebral cortex through the basal ganglia back to the cortex and then on to muscles. Neurons from the substantia nigra pars compacta project axons that release dopamine in synapses on interneurons in the striatum. As the dopamine-containing neurons die, failure to complete this circuit results in inability to coordinate movement. Neuromelanin, the black pigment that gives the substantia nigra its name, is a byproduct from the metabolic pathway for dopamine synthesis. When the symptoms of Parkinson’s disease first become apparent more than 70% of the dopamine-containing neurons have already been lost, releasing their neuromelanin and hence turning the tissue less black. It is now apparent, however, that many regions of the brain are affected in Parkinson’s disease, and indeed in the early stages it may affect only a lower region of the brain stem called the medulla oblongata, spreading gradually upward through the basal ganglia into the cortical areas (98).
One of the most obvious questions when considering the etiology of Parkinson’s disease is why dopaminergic neurons are particularly vulnerable. Until recently no clear genetic links were apparent, and one hypothesis was that the metabolic pathways for dopamine synthesis might be at the root of the problem (99). There is evidence that dopamine metabolites can increase levels of reactive oxygen species that damage cells, especially mitochondria; for example, neuromelanin binds heavy metals that can lead to free radical production (100). Another hypothesis, that postulated exposure to environmental toxins as the cause, was given impetus by two apparently diverse findings that were both subsequently linked to mitochondrial dysfunction. First, during the 1970s and 80s, some users of illegal recreational drugs developed parkinsonism that was traced to the ingestion of a contaminant called 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (101). Itself relatively innocuous, this compound is lipid-soluble and so crosses the blood-brain barrier, where it is oxidized by monoamine oxidase-B, present in astrocytes, to the toxic 1-methyl-4-phenylpyridinium (MPP+) ion. The unique susceptibility of dopaminergic neurons to this toxin is due to it being taken up by the dopamine transporter. Once inside the neuron, it is concentrated in the mitochondria, where it binds NADH dehydrogenase, inhibiting complex I of the electron transport chain and leading to mitochondrial dysfunction (102). Second, epidemiological studies had suggested a link between long-term exposure to agricultural pesticides, such as rotenone and paraquat. Rotenone, a plant derivative used as an insecticide, is known to be an inhibitor of NADH dehydrogenase. Chronic infusion of either rotenone or MPTP in rodents results in parkinsonism-like behavior and pathology, including the formation of inclusion bodies, and indeed these are among the best animal models for the human disease (103). It has also been suggested that exposure to paraquat may alter the expression and aggregation of α-synuclein (see below).
The view that Parkinson’s disease was almost entirely a sporadic disease due to metabolic dysfunction caused by environmental toxins has altered dramatically during the past 10 years. The first hereditary connection was a link to the protein α-synuclein, and so its genetic locus on chromosome 4 became known as PARK1. Nevertheless, mutations in the gene for α-synuclein account for only a tiny percentage of Parkinson’s disease cases. Several additional loci, named PARK2 through PARK12, have since been implicated (Online Mendelian Inheritance in Man; Johns Hopkins University, Baltimore; MIM no. 168600, 2007). The most promising candidates are listed in Table 2.
α-Synuclein
In 1997, a mutation in the α-synuclein gene leading to a single amino acid substitution (Ala53Thr) was detected in a family with an early-onset autosomal dominant form of Parkinson’s disease with high penetrance (104). Spurred by this report, researchers searched for and detected α-synuclein in Lewy bodies (Figure 1B) and in so doing further implicated α-synuclein as a major player in Parkinson’s disease pathogenesis (105). Subsequently, two additional disease-causing mutations were identified in the α-synuclein gene, each causing a different single amino acid substitution (106,107).
α-Synuclein belongs to a family of three closely related proteins (the others being β- and γ-synucleins) that are encoded by three distinct genes. In humans, minor spliced variants of α-synuclein occur, but the predominant form in the brain is a small, soluble protein of 140 residues that accounts for about 1% of total protein in neurons, where it is localized in presynaptic nerve terminals. The primary structure of α-synuclein is characterized by three distinct regions (108):
-
the N-terminal region (1–60) contains several degenerative repeats of an 11-amino acid sequence related to an α-helical lipid-binding motif of apolipoproteins that mediates protein binding to phospholipid vesicles
-
the central region (61–95) is composed of extremely hydrophobic amino acid residues, and part of this region has been implicated in association of monomers into larger aggregates that ultimately form amyloid fibrils (109)
-
the C-terminal region (96–140) is hydrophilic and rich in the amino acid residue proline and the acidic residues glutamate and aspartate; its size and sequence vary markedly between species, and it has been suggested to confer chaperone activity to α-synuclein (110)
Until recently, the function of α-synuclein remained unclear, and indeed mice deficient in α- or β-synuclein, or both, survive with no very obvious brain defects (111). α-Synuclein lacks secondary or tertiary structure, so it belongs to the family of natively unfolded proteins, many of which act as chaperones. It is known to interact with several other proteins, whereas exposure to lipid micelles induces a structural change to α-helix in the N-terminal region (112). In the presynaptic nerve terminals, α-synuclein associates with membranes of synaptic vesicles and may have a regulatory role in inhibiting neurotransmitter release (113). Support for a chaperone function for α-synuclein has come from genetically manipulated mice in which cysteine-string protein-α (CSPα) was deleted (114). CSPα is a synaptic vesicle protein that acts as a cochaperone for SNARE proteins. α-Synuclein appears to be able to act as an auxiliary chaperone, complementing the chaperone activity of CSPα.
A Molecular Explanation of Parkinson’s Disease: The α-Synuclein Hypothesis
In an analogous manner to the involvement of Aβ in Alzheimer’s disease, considerable genetic, animal-modeling, and biochemical data have emerged to suggest that α-synuclein plays a central role in initiating Parkinson’s disease.
-
1.
Solutions of α-synuclein, or synthetic peptide fragments derived from it, associate into oligomers that eventually aggregate to produce amyloid fibrils whose morphology resembles that of fibrils purified from Lewy bodies (97,115).
-
2.
The three mutant forms described above, each involving a single amino acid substitution, are associated with autosomal dominant Parkinson’s disease. The mutations increase the aggregation rate of the resultant α-synuclein (115,116).
-
3.
Duplication or triplication of the α-synuclein gene on one chromosome, giving 50% or 100% increase in expression of protein, causes parkinsonism (117–119). Age of onset of disease and its severity are proportional to gene copy number.
-
4.
α-Synuclein is the principal constituent of Lewy bodies. Much of this α-synuclein has been posttranslationally modified by sequence truncation at the C-terminus, phosphorylation at Ser129 (120,121), or nitration (122); these modifications have been shown to increase the rate at which oligomers are formed (121,123). Dopamine and related catecholamines have been shown to interact with α-synuclein and stabilize the protofibril stage of aggregation (124,125), providing a possible explanation for the increased susceptibility of dopaminergic neurons. Levels of soluble oligomers of α-synuclein are also affected by fatty acids, being upregulated by polyunsaturated fatty acids (126).
-
5.
α-Synuclein aggregation is also increased in the presence of metals and pesticides, which may be environmental risk factors (127). For example, exposure to paraquat induced increased expression and aggregation of α-synuclein in mice. The authors speculate that these increased levels of α-synuclein may form part of the neuronal response to toxic insult, but that the increasing protein concentration or its interaction with paraquat may lead to the production of deleterious aggregates (128). Iron levels are elevated in substantia nigra from Parkinson’s disease patients (129), and in vitro it has been demonstrated that, in the presence of iron, solutions of α-synuclein give rise to reactive oxygen species that could be toxic to cells (100).
-
6.
Oligomers of α-synuclein are toxic to some cell cultures, including the dopaminergic SH-SY5Y human neuroblastoma cell line (130). As is the case for Alzheimer’s disease, there is mounting evidence that the toxic species is not the final fibril, but early aggregates, which are soluble oligomers on the pathway to fibril formation (131). A recent study demonstrated that the affinity of α-synuclein for a phospholipid membrane is a function of its degree of aggregation, with tightest binding by an intermediate formed during the conversion from monomeric to fibrillar state (132).
-
7.
Increased human α-synuclein expression in transgenic flies (133) and mice (134) is accompanied by neuronal dysfunction and loss of synaptic terminals and/or neurons, the formation of lesions similar to those found in Parkinson’s disease brain, and the development of motor abnormalities.
-
8.
Expression of mutant forms of α-synuclein in cells promotes mitochondrial defects and cell death and enhances susceptibility to oxidative stress. On the other hand, mice deficient in synuclein are resistant to toxicity induced by MPTP and other mitochondrial toxins (135).
Taken together, all these studies provide strong evidence for a central role for α-synuclein in the pathogenesis of Parkinson’s disease and, as is the case for Alzheimer’s disease, recent evidence implicates small aggregates rather than insoluble fibrils as the toxic species, though the exact mechanism of toxicity remains unclear (131,136).
Genetic evidence also implicates several other proteins, listed in Table 2, some of which have been found to be associated with mitochondria. Because these subcellular organelles are also the initial site of damage by environmental toxins, sporadic and hereditary Parkinson’s disease may converge in that both types of disease have at their root mitochondrial dysfunction. The PARK2 locus codes for the enzyme parkin, which is a ubiquitin E3-ligase. Parkin can protect cells against mutant α-synuclein, and an obvious explanation might be that α-synuclein is a parkin substrate. This has not been convincingly demonstrated, however; rather, a connection with mitochondrial function may be involved, because parkin-null mutants in both fly (137) and mouse (138) models had compromised mitochondrial function and increased oxidative damage. PINK1 and DJ-1 have both been shown to be required for normal mitochondrial function, and mutations in either of these proteins can decrease mitochondrial viability. Loss of PINK1 kinase activity adversely affects mitochondria under stressful conditions (139), whereas DJ-1 may protect against intracellular oxidative conditions (140) and also prevent α-synuclein aggregation (141). Mitochondrial deficiency will ultimately result in decreased ATP production, reducing proteasome activity and allowing excessive amounts of cellular proteins, including α-synuclein, to build up. Indeed, one of the other major protein constituents of Lewy bodies is ubiquitin, suggesting a general malfunction of the ubiquitin-proteasome system. LRRK2 is a kinase (142) whose connection with Parkinson’s disease pathology remains unclear. The proteins mentioned above and their potential interactions in the pathways leading to Parkinson’s disease are illustrated in Figure 4. Whether any particular individual succumbs to Parkinson’s pathology may depend on a complex set of circumstances that include genetic factors, environmental exposures, and loss of cellular protective mechanisms. As suggested by Sulzer (143), development of pathology may require “multiple hits” that involve some combination of these factors; this might explain the difficulties encountered in explaining low penetrant inheritance.
Summary of α-synuclein’s role in Parkinson’s disease pathology. Cellular α-synuclein levels or chemical structure may be altered by overexpression, mutations, or chemical modifications (such as phosphorylation, nitration, oxidation, exposure to metal ions or toxins, or adduct formation with dopamine quinone). The increased oxidative environment of dopaminergic neurons is likely to exacerbate some of these processes, making these cells especially vulnerable. The end result is increased oligomer formation that may damage mitochondrial membranes. The amyloid-containing Lewy body is likely to be much less toxic than this precursor, and it may be the case that cells that rapidly produce Lewy bodies survive best. Other mutations in parkin, PINK1, and DJ-1 are likely to compromise the ability of mitochondria to resist stress.
Emerging Diagnostic Tools
Despite progress in understanding the underlying disease mechanism, there remains an urgent need to develop methods for use in diagnosis of Parkinson’s disease. To date, there is no serological or urine test that can confirm the diagnosis of Parkinson’s disease. Routine blood, CSF, and urine tests yield normal results. Neither CT nor MRI scans of the head show abnormalities in idiopathic Parkinson’s disease, but may sometimes help to diagnose a secondary parkinsonism due to tumor, infarction, etc. Deoxyglucose PET scans of the brain reveal an abnormal pattern of increased glucose metabolism in the globus pallidus, and fluorodopa (F-dopa) PET shows loss of striatal dopamine characteristic of Parkinson’s disease (144). The PET/F-dopa measures dopamine function and SPECT/B-CIT tags the dopamine transporter. It has to be kept in mind, however, that PET/F-dopa is an indirect measure of striatal dopamine levels, and dopa decarboxylase activity only poorly correlates with nigral cell counts. The need for an accurate diagnostic method is amply demonstrated by studies indicating that clinical diagnosis during life is correct only about 75% of the time, even in specialist research centers (145). This is serious not only from a clinical diagnostic standpoint, but also because it affects the efficacy of clinical trials and epidemiological research. At present, the most advanced imaging biomarker for Parkinson’s disease is F-dopa. The amyloid-binding ligand PIB (see above) has been shown to bind filaments of α-synuclein generated in vitro but does not interact with sections from Parkinson’s brain that contain Lewy bodies, suggesting that the amyloid in these in vivo lesions is less accessible (146). Beyond ensuring optimal use of existing imaging biomarkers, it is critical to foster the development of new biomarkers. Given the likely multiple etiologies of Parkinson’s disease and clear heterogeneity in expression of the clinical manifestations and progression, several biomarkers will probably be necessary to fully understand the disorder. To this end, two recent approaches have yielded some positive indicators, though neither is yet at the stage of providing an established diagnostic protocol. In the first approach, microarrays were used to detect changes in mRNA expression in blood cells, and a set of 22 genes was found to have differential expression in patient samples compared with control samples (147). The second approach was based on high-performance liquid chromatographic assays for a large number (many hundreds) of low-molecularweight analytes in plasma, so-called metabolomic profiling, that identified several potential biomarkers (148).
Current and Future Therapies
The realization that Parkinson’s disease was related to a deficit in dopamine production soon led to the first effective treatment, because dopamine levels can be supplemented pharmacologically. Dopamine is synthesized in neurons from L-tyrosine by a two-step process. Tyrosine hydroxylase catalyses the synthesis of 3,4-dihydroxy-L-phenylalanine (L-dopa or levodopa); this then is decarboxylated by dopa decarboxylase. Dopamine cannot cross the blood-brain barrier, but its metabolic precursor, L-dopa, can. This is usually given in conjunction with carbidopa, an inhibitor of peripheral decarboxylase that cannot cross the blood-brain barrier but prevents metabolism of L-dopa before it reaches its target tissue, where it is converted to dopamine by neuronal decarboxylase. Other current therapies include drugs, such as monoamine oxidase B inhibitors that reduce dopamine metabolism or dopamine receptor agonists, and physical interventions, such as deep brain stimulation with electrical pulses or surgical ablation of regions of the basal ganglia. However, L-dopa remains the standard treatment for Parkinson’s disease, although it has undesirable side effects and efficacy wanes after treatment for several years.
In the longer term, transplantation of stem cells may eventually allow regeneration of neurons, and this might be the ultimate goal for treatment of most neurodegenerative diseases, though in the more immediate future other approaches are required. Because amyloidogenic proteins display toxic properties only when they form oligomers, one strategy is to prevent them from forming aggregates. Recently, simvastatin has been shown to reduce α-synuclein aggregation in cell cultures, providing a possible clue to a reported epidemiological link between simvastatin and a reduced incidence of Parkinson’s disease (149). Molecules that can bind and disrupt fibril formation have been discovered for several amyloidogenic proteins. Their mode of action seems to be insertion into the newly forming protein assemblies, which are held together by interactions between β-strands in an incipient β-sheet, preventing additional β-strands from being added. Several peptides derived from the central hydrophobic region (residues 68–72) of α-synuclein have been shown to prevent aggregation of the full-length protein (150). A cell-permeable version of one of these inhibitors was able to reduce toxicity in cells transfected with mutant A53T α-synuclein (151). The flavinoid compound, baicalein, has also been shown to inhibit α-synuclein aggregation and indeed to disaggregate existing fibrils (152). However, a potential danger in this approach is that although fibril formation might be prevented, smaller assemblies might be stabilized, as seems to be the case with baicalein. Because there is now good evidence that even low-n oligomers are toxic (see above regarding Aβ oligomers in Alzheimer’s disease), this strategy could well be counterproductive, as it might shift the equilibrium of aggregation away from fibrils to more hazardous oligomeric species. Another approach would be to remove synuclein using antibodies in a manner analogous to the Alzheimer’s disease therapy discussed above. Immunization of a human α-synuclein transgenic mouse to generate anti-α-synuclein antibodies reduced the deposits of α-synuclein in intracellular neuronal cell bodies (153). However, this approach is at least 5 years behind the analogous putative Alzheimer’s therapy, which itself has encountered severe technical difficulties due to development of encephalitis.
Conclusions
During the past few years there has been mounting evidence that the underlying pathology in several neurodegenerative diseases arises from the production of soluble oligomeric assemblies of a protein characteristic of each disease. In the case of Alzheimer’s and Parkinson’s diseases, these proteins are Aβ and α-synuclein, respectively. It is clear that such oligomers are deleterious to neurons in their vicinity, though the molecular mechanisms by which damage occurs remain to be established. Emerging therapies are likely to be based on preventing such assemblies from forming and/or persisting. When oligomeric assemblies grow in size, they can form insoluble deposits of amyloid fibrils. Emerging diagnostic tools are likely to include the early detection of these fibrils.
References
Ross CA, Poirier MA. (2004) Protein aggregation and neurodegenerative disease. Nat. Med. 10(Suppl):S10–7.
Dobson CM. (2004) Protein chemistry: in the footsteps of alchemists. Science 304:1259–62.
Kelly JW. (2006) Structural biology: proteins downhill all the way. Nature 442:255–6.
Alzheimer A. (1906) Über einen eigenartigen schweren Erkrankungsprozeβ der Hirnrinde. Neurologisches Zentralblatt 23:1129–36.
Ferri CP, et al. (2005) Global prevalence of dementia: a Delphi consensus study. Lancet 366:2112–7.
Evans DA, et al. (1989) Prevalence of Alzheimer’s disease in a community population of older persons: higher than previously reported. JAMA 262:2551–6.
Kukull WA, Bowen JD. (2002) Dementia epidemiology. Med. Clin. North Am. 86:573–90.
Rossor MN, Fox NC, Freeborough PA, Harvey RJ. (1996) Clinical features of sporadic and familial Alzheimer’s disease. Neurodegeneration 5:393–7.
Chai CK. (2007) The genetics of Alzheimer’s disease. Am. J. Alzheimers Dis. Other Dement. 22:37–41.
Gatz M, et al. (2006) Role of genes and environments for explaining Alzheimer disease. Arch. Gen. Psychiatry 63:168–74.
Kawas CH. (2003) Clinical practice: early Alzheimer’s disease. N. Engl. J. Med. 349:1056–63.
Nussbaum RL, Ellis CE. (2003) Alzheimer’s disease and Parkinson’s disease. N. Engl. J. Med. 348:1356–64.
de Leon MJ, et al. (2004) MRI and CSF studies in the early diagnosis of Alzheimer’s disease. J. Intern. Med. 256:205–23.
Masdeu JC, Zubieta JL, Arbizu J. (2005) Neuroimaging as a marker of the onset and progression of Alzheimer’s disease. J. Neurol. Sci. 236:55–64.
Klunk WE, et al. (2004) Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann. Neurol. 55:306–19.
Mathis CA, Klunk WE, Price JC, DeKosky ST. (2005) Imaging technology for neurodegenerative diseases: progress toward detection of specific pathologies. Arch. Neurol. 62:196–200.
Glenner GG, Wong CW. (1984) Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 120:885–90.
Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. (1985) Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc. Natl. Acad. Sci. U. S. A. 82:4245–9.
Bentahir M, et al. (2006) Presenilin clinical mutations can affect gamma-secretase activity by different mechanisms. J. Neurochem. 96:732–42.
Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. (1994) Visualization of Abeta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43). Neuron 13:45–53.
Lemere CA, Blusztajn JK, Yamaguchi H, Wisniewski T, Saido TC, Selkoe DJ. (1996) Sequence of deposition of heterogeneous amyloid beta-peptides and APO E in Down syndrome: implications for initial events in amyloid plaque formation. Neurobiol. Dis. 3:16–32.
Meda L, Baron P, Scarlato G. (2001) Glial activation in Alzheimer’s disease: the role of Abeta and its associated proteins. Neurobiol. Aging 22:885–93.
Wood JG, Mirra SS, Pollock NJ, Binder LI. (1986) Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (τ). Proc. Natl. Acad. Sci. U. S. A. 83:4040–3.
Kosik KS, Joachim CL, Selkoe DJ. (1986) Microtubule-associated protein tau (τ) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc. Natl. Acad. Sci. U. S. A. 83:4044–8.
Braak H, Braak E. (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82:239–59.
Thal DR, Capetillo-Zarate E, Del Tredici K, Braak H. (2006) The development of amyloid beta protein deposits in the aged brain. Sci. Aging Knowledge Environ. 2006:re1.
Iwatsubo T, Hasegawa M, Ihara Y. (1994) Neuronal and glial tau-positive inclusions in diverse neurologic diseases share common phosphorylation characteristics. Acta Neuropathol. 88:129–36.
Gotz J, Chen F, van Dorpe J, Nitsch RM. (2001) Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science 293:1491–5.
Lewis J, et al. (2001) Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science 293:1487–91.
Alexander GE, Chen K, Pietrini P, Rapoport SI, Reiman EM. (2002) Longitudinal PET evaluation of cerebral metabolic decline in dementia: a potential outcome measure in Alzheimer’s disease treatment studies. Am. J. Psychiatry 159:738–45.
Park SY, Ferreira A. (2005) The generation of a 17 kDa neurotoxic fragment: an alternative mechanism by which tau mediates beta-amyloid-induced neurodegeneration. J. Neurosci. 25:5365–75.
Busciglio J, Lorenzo A, Yeh J, Yankner BA. (1995) Beta-amyloid fibrils induce tau phosphorylation and loss of microtubule binding. Neuron 14:879–88.
Greenberg SM, Koo EH, Selkoe DJ, Qiu WQ, Kosik KS. (1994) Secreted beta-amyloid precursor protein stimulates mitogen-activated protein kinase and enhances tau phosphorylation. Proc. Natl. Acad. Sci. U. S. A. 91:7104–8.
Leschik J, Welzel A, Weissmann C, Eckert A, Brandt R. (2007) Inverse and distinct modulation of tau-dependent neurodegeneration by presenilin 1 and amyloid-beta in cultured cortical neurons: evidence that tau phosphorylation is the limiting factor in amyloid-beta-induced cell death. J. Neurochem. 101:1303–15.
Roberson ED, et al. (2007) Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science 316:750–4.
Haass C, et al. (1992) Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature 359:322–5.
Seubert P, et al. (1992) Isolation and quantification of soluble Alzheimer’s beta-peptide from biological fluids. Nature 359:325–7.
Shoji M, et al. (1992) Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science 258:126–9.
Cai H, Wang Y, McCarthy D, Wen H, Borchelt DR, Price DL, Wong PC. (2001) BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nat. Neurosci. 4:233–4.
Vassar R, et al. (1999) Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286:735–41.
Schroeter EH, et al. (2003) A presenilin dimer at the core of the gamma-secretase enzyme: insights from parallel analysis of Notch 1 and APP proteolysis. Proc. Natl. Acad. Sci. U. S. A. 100:13075–80.
Mann DM, Yates PO, Marcyniuk B. (1984) Alzheimer’s presenile dementia, senile dementia of Alzheimer type and Down’s syndrome in middle age form an age related continuum of pathological changes. Neuropathol. Appl. Neurobiol. 10:185–207.
Olson MI, Shaw CM. (1969) Presenile dementia and Alzheimer’s disease in mongolism. Brain 92:147–56.
Prasher VP, Farrer MJ, Kessling AM, Fisher EM, West RJ, Barber PC, Butler AC. (1998) Molecular mapping of Alzheimer-type dementia in Down’s syndrome. Ann. Neurol. 43:380–3.
Busciglio J, Lorenzo A, Yankner BA. (1992) Methodological variables in the assessment of beta amyloid neurotoxicity. Neurobiol. Aging 13:609–12.
Pike CJ, Walencewicz AJ, Glabe CG, Cotman CW. (1991) In vitro aging of beta-amyloid protein causes peptide aggregation and neurotoxicity. Brain Res. 563:311–4.
Pike CJ, Burdick D, Walencewicz AJ, Glabe CG, Cotman CW. (1993) Neurodegeneration induced by beta-amyloid peptides in vitro: the role of peptide assembly state. J. Neurosci. 13:1676–87.
Chartier-Harlin MC, et al. (1991) Early-onset Alzheimer’s disease caused by mutations at codon 717 of the beta-amyloid precursor protein gene. Nature 353:844–6.
Citron M, et al. (1992) Mutation of the beta-amyloid precursor protein in familial Alzheimer’s disease increases beta-protein production. Nature 360:672–4.
Goate A, et al. (1991) Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature 349:704–6.
Levy E, et al. (1990) Mutation of the Alzheimer’s disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science 248:1124–6.
Kumar-Singh S, et al. (2006) Mean age-of-onset of familial Alzheimer disease caused by presenilin mutations correlates with both increased Abeta42 and decreased Abeta40. Hum. Mutat. 27:686–95.
Corder EH, et al. (1993) Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261:921–3.
Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. (1993) Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl. Acad. Sci. U. S. A. 90:1977–81.
Games D, Buttini M, Kobayashi D, Schenk D, Seubert P. (2006) Mice as models: transgenic approaches and Alzheimer’s disease. J. Alzheimers Dis. 9:133–49.
Vigo-Pelfrey C, Lee D, Keim P, Lieberburg I, Schenk DB. (1993) Characterization of beta-amyloid peptide from human cerebrospinal fluid. J. Neurochem. 61:1965–8.
Walsh DM, Tseng BP, Rydel RE, Podlisny MB, Selkoe DJ. (2000) The oligomerization of amyloid beta-protein begins intracellularly in cells derived from human brain. Biochemistry 39:10831–9.
Deshpande A, Mina E, Glabe C, Busciglio J. (2006) Different conformations of amyloid beta induce neurotoxicity by distinct mechanisms in human cortical neurons. J. Neurosci. 26:6011–8.
Terry RD, et al. (1991) Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 30:572–80.
Lue LF, et al. (1999) Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am. J. Pathol. 155:853–62.
McLean CA, et al. (1999) Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann. Neurol. 46:860–6.
Morishima-Kawashima M, Ihara Y. (1998) The presence of amyloid beta-protein in the detergent-insoluble membrane compartment of human neuroblastoma cells. Biochemistry 37:15247–53.
Georganopoulou DG, Chang L, Nam JM, Thaxton CS, Mufson EJ, Klein WL, Mirkin CA. (2005) Nanoparticle-based detection in cerebral spinal fluid of a soluble pathogenic biomarker for Alzheimer’s disease. Proc. Natl. Acad. Sci. U. S. A. 102:2273–6.
Enya M, et al. (1999) Appearance of sodium dodecyl sulfate-stable amyloid beta-protein (Abeta) dimer in the cortex during aging. Am. J. Pathol. 154:271–9.
Podlisny MB, Ostaszewski BL, Squazzo SL, Koo EH, Rydell RE, Teplow DB, Selkoe DJ. (1995) Aggregation of secreted amyloid beta-protein into sodium dodecyl sulfate-stable oligomers in cell culture. J. Biol. Chem. 270:9564–70.
Walsh DM, Klyubin I, Fadeeva JV, Rowan MJ, Selkoe DJ. (2002) Amyloid-beta oligomers: their production, toxicity and therapeutic inhibition. Biochem. Soc. Trans. 30:552–7.
Walsh DM, et al. (2002) Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416:535–9.
Wang Q, Walsh DM, Rowan MJ, Selkoe DJ, Anwyl R. (2004) Block of long-term potentiation by naturally secreted and synthetic amyloid beta-peptide in hippocampal slices is mediated via activation of the kinases c-Jun N-terminal kinase, cyclin-dependent kinase 5, and p38 mitogen-activated protein kinase as well as metabotropic glutamate receptor type 5. J. Neurosci. 24:3370–8.
Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. (2005) Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat. Neurosci. 8:79–84.
Calabrese B, Shaked GM, Tabarean IV, Braga J, Koo EH, Halpain S. (2007) Rapid, concurrent alterations in pre- and postsynaptic structure induced by naturally-secreted amyloid-beta protein. Mol. Cell Neurosci. 35:183–93.
Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. (2007) Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J. Neurosci. 27:2866–75.
Harper JD, Lieber CM, Lansbury PT Jr. (1997) Atomic force microscopic imaging of seeded fibril formation and fibril branching by the Alzheimer’s disease amyloid-beta protein. Chem. Biol. 4:951–9.
Walsh DM, Lomakin A, Benedek GB, Condron MM, Teplow DB. (1997) Amyloid beta-protein fibrillogenesis: detection of a protofibrillar intermediate. J. Biol. Chem. 272:22364–72.
Walsh DM, et al. (1999) Amyloid beta-protein fibrillogenesis: structure and biological activity of protofibrillar intermediates. J. Biol. Chem. 274:25945–52.
Hartley DM, et al. (1999) Protofibrillar intermediates of amyloid beta-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J. Neurosci. 19:8876–84.
Lambert MP, et al. (1998) Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. U. S. A. 95:6448–53.
Wang HW, et al. (2002) Soluble oligomers of beta amyloid (1-42) inhibit long-term potentiation but not long-term depression in rat dentate gyrus. Brain Res. 924:133–40.
Lacor PN, et al. (2004) Synaptic targeting by Alzheimer’s-related amyloid beta oligomers. J. Neurosci. 24:10191–200.
Lacor PN, et al. (2007) Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J. Neurosci. 27:796–807.
Schenk D, et al. (1999) Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400:173–7.
Gilman S, et al. (2005) Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology 64:1553–62.
Andreasen N, et al. (1999) Cerebrospinal fluid beta-amyloid(1–42) in Alzheimer disease: differences between early- and late-onset Alzheimer disease and stability during the course of disease. Arch. Neurol. 56:673–80.
Fagan AM, et al. (2006) Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann. Neurol. 59:512–9.
Irizarry MC. (2004) Biomarkers of Alzheimer disease in plasma. NeuroRx 1:226–34.
Kahle PJ, et al. (2000) Combined assessment of tau and neuronal thread protein in Alzheimer’s disease CSF. Neurology 54:1498–504.
Buerger K, et al. (2002) Differential diagnosis of Alzheimer disease with cerebrospinal fluid levels of tau protein phosphorylated at threonine 231. Arch. Neurol. 59:1267–72.
Blennow K. (2004) Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. NeuroRx 1:213–25.
Riemenschneider M, et al. (2002) Tau and Abeta42 protein in CSF of patients with frontotemporal degeneration. Neurology 58:1622–8.
Fukuyama R, Mizuno T, Mori S, Nakajima K, Fushiki S, Yanagisawa K. (2000) Age-dependent change in the levels of Abeta40 and Abeta42 in cerebrospinal fluid from control subjects, and a decrease in the ratio of Abeta42 to Abeta40 level in cerebrospinal fluid from Alzheimer’s disease patients. Eur. Neurol. 43:155–60.
Hansson O, Zetterberg H, Buchhave P, Andreasson U, Londos E, Minthon L, Blennow K. (2007) Prediction of Alzheimer’s disease using the CSF Abeta42/Abeta40 ratio in patients with mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 23:316–20.
Herukka SK, Hallikainen M, Soininen H, Pirttila T. (2005) CSF Abeta42 and tau or phosphorylated tau and prediction of progressive mild cognitive impairment. Neurology 64:1294–7.
Pitschke M, Prior R, Haupt M, Riesner D. (1998) Detection of single amyloid beta-protein aggregates in the cerebrospinal fluid of Alzheimer’s patients by fluorescence correlation spectroscopy. Nat. Med. 4:832–4.
Parkinson J. (2002) An essay on the shaking palsy. 1817. J. Neuropsychiatry Clin. Neurosci. 14:223–36.
de Rijk MC, et al. (2000) Prevalence of Parkinson’s disease in Europe: a collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology 54:S21–3.
Shults CW. (2006) Lewy bodies. Proc. Natl. Acad. Sci. U. S. A. 103:1661–8.
Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. (1998) alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc. Natl. Acad. Sci. U. S. A. 95:6469–73.
Crowther RA, Jakes R, Spillantini MG, Goedert M. (1998) Synthetic filaments assembled from C-terminally truncated alpha-synuclein. FEBS Lett. 436:309–12.
Braak H, Rub U, Gai WP, Del Tredici K. (2003) Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural Transm. 110:517–36.
Jenner P. (1989) Clues to the mechanism underlying dopamine cell death in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry Suppl:22–8.
Turnbull S, Tabner BJ, El-Agnaf OM, Moore S, Davies Y, Allsop D. (2001) alpha-Synuclein implicated in Parkinson’s disease catalyses the formation of hydrogen peroxide in vitro. Free Radic. Biol. Med. 30:1163–70.
Langston JW, Ballard P, Tetrud JW, Irwin I. (1983) Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219:979–80.
Ramsay RR, Salach JI, Dadgar J, Singer TP. (1986) Inhibition of mitochondrial NADH dehydrogenase by pyridine derivatives and its possible relation to experimental and idiopathic parkinsonism. Biochem. Biophys. Res. Commun. 135:269–75.
Dauer W, Przedborski S. (2003) Parkinson’s disease: mechanisms and models. Neuron 39:889–909.
Polymeropoulos MH, et al. (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276:2045–7.
Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. (1997) Alpha-synuclein in Lewy bodies. Nature 388:839–40.
Kruger R, et al. (1998) Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat. Genet. 18:106–8.
Zarranz JJ, et al. (2004) The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann. Neurol. 55:164–73.
George JM, Jin H, Woods WS, Clayton DF. (1995) Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron 15:361–72.
Bodles AM, Guthrie DJ, Greer B, Irvine GB. (2001) Identification of the region of non-Abeta component (NAC) of Alzheimer’s disease amyloid responsible for its aggregation and toxicity. J. Neurochem. 78:384–95.
Park SM, Jung HY, Kim TD, Park JH, Yang CH, Kim J. (2002) Distinct roles of the N-terminal-binding domain and the C-terminal-solubilizing domain of alpha-synuclein, a molecular chaper-one. J. Biol. Chem. 277:28512–20.
Chandra S, et al. (2004) Double-knockout mice for alpha- and beta-synucleins: effect on synaptic functions. Proc. Natl. Acad. Sci. U. S. A. 101:14966–71.
Bussell R Jr, Eliezer D. (2003) Astructural and functional role for 11-mer repeats in alpha-synuclein and other exchangeable lipid binding proteins. J. Mol. Biol. 329:763–78.
Larsen KE, et al. (2006) Alpha-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis. J. Neurosci. 26:11915–22.
Chandra S, Gallardo G, Fernandez-Chacon R, Schluter OM, Sudhof TC. (2005) Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell 123:383–96.
El-Agnaf OM, Jakes R, Curran MD, Wallace A. (1998) Effects of the mutations Ala30 to Pro and Ala53 to Thr on the physical and morphological properties of alpha-synuclein protein implicated in Parkinson’s disease. FEBS Lett. 440:67–70.
Greenbaum EA, et al. (2005) The E46K mutation in alpha-synuclein increases amyloid fibril formation. J. Biol. Chem. 280:7800–7.
Chartier-Harlin MC, et al. (2004) Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet 364:1167–9.
Ibanez P, et al. (2004) Causal relation between alpha-synuclein gene duplication and familial Parkinson’s disease. Lancet 364:1169–71.
Singleton AB, et al. (2003) alpha-Synuclein locus triplication causes Parkinson’s disease. Science 302:841.
Anderson JP, et al. (2006) Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J. Biol. Chem. 281:29739–52.
Fujiwara H, et al. (2002) alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat. Cell Biol. 4:160–4.
Giasson BI, et al. (2000) Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science 290:985–9.
Yamin G, Uversky VN, Fink AL. (2003) Nitration inhibits fibrillation of human alpha-synuclein in vitro by formation of soluble oligomers. FEBS Lett. 542:147–52.
Conway KA, Rochet JC, Bieganski RM, Lansbury PT Jr. (2001) Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science 294:1346–9.
Mazzulli JR, Armakola M, Dumoulin M, Parastatidis I, Ischiropoulos H. (2007) Cellular oligomerization of alpha-synuclein is determined by the interaction of oxidized catechols with a C-terminal sequence. J. Biol. Chem. 282:31621–30.
Sharon R, Bar-Joseph I, Frosch MP, Walsh DM, Hamilton JA, Selkoe DJ. (2003) The formation of highly soluble oligomers of alpha-synuclein is regulated by fatty acids and enhanced in Parkinson’s disease. Neuron 37:583–95.
Uversky VN, Li J, Bower K, Fink AL. (2002) Synergistic effects of pesticides and metals on the fibrillation of alpha-synuclein: implications for Parkinson’s disease. Neurotoxicology 23:527–36.
Manning-Bog AB, McCormack AL, Li J, Uversky VN, Fink AL, Di Monte DA. (2002) The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice: paraquat and alpha-synuclein. J. Biol. Chem. 277:1641–4.
Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. (2004) Iron, brain ageing and neurodegenerative disorders. Nat. Rev. Neurosci. 5:863–73.
El-Agnaf OM, et al. (1998) Aggregates from mutant and wild-type alpha-synuclein proteins and NAC peptide induce apoptotic cell death in human neuroblastoma cells by formation of beta-sheet and amyloid-like filaments. FEBS Lett. 440:71–5.
Volles MJ, Lansbury PT Jr. (2003) Zeroing in on the pathogenic form of alpha-synuclein and its mechanism of neurotoxicity in Parkinson’s disease. Biochemistry 42:7871–8.
Smith DP, Tew DJ, Hill AF, Bottomley SP, Masters CL, Barnham KJ, Cappai R. (2008) Formation of a high affinity lipid-binding intermediate during the early aggregation phase of alpha-synuclein. Biochemistry 47:1425–34.
Feany MB, Bender WW. (2000) A Drosophila model of Parkinson’s disease. Nature 404:394–8.
Masliah E, et al. (2000) Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science 287:1265–9.
Klivenyi P, et al. (2006) Mice lacking alpha-synuclein are resistant to mitochondrial toxins. Neurobiol. Dis. 21:541–8.
Cookson MR, van der Brug M. (2007) Cell systems and the toxic mechanism(s) of alpha-synuclein. Exp. Neurol. 209:5–11.
Greene JC, Whitworth AJ, Andrews LA, Parker TJ, Pallanck LJ. (2005) Genetic and genomic studies of Drosophila parkin mutants implicate oxidative stress and innate immune responses in pathogenesis. Hum. Mol. Genet. 14:799–811.
Palacino JJ, et al. (2004) Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J. Biol. Chem. 279:18614–22.
Valente EM, et al. (2004) Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science 304:1158–60.
Canet-Aviles RM, et al. (2004) The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc. Natl. Acad. Sci. U. S. A. 101:9103–8.
Zhou W, Zhu M, Wilson MA, Petsko GA, Fink AL. (2006) The oxidation state of DJ-1 regulates its chaperone activity toward alpha-synuclein. J. Mol. Biol. 356:1036–48.
Zimprich A, et al. (2004) Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 44:601–7.
Sulzer D. (2007) Multiple hit hypotheses for dopamine neuron loss in Parkinson’s disease. Trends Neurosci. 30:244–50.
Rueger MA, et al. (2007) Role of in vivo imaging of the central nervous system for developing novel drugs. Q. J. Nucl. Med. Mol. Imaging 51:164–81.
Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. (1992) What features improve the accuracy of clinical diagnosis in Parkinson’s disease: a clinicopathologic study. Neurology 42:1142–6.
Ye L, et al. (2008) In vitro high affinity alpha-synuclein binding sites for the amyloid imaging agent PIB are not matched by binding to Lewy bodies in postmortem human brain. J. Neurochem. 2008, Feb 18 [Epub ahead of print]
Scherzer CR, et al. (2007) Molecular markers of early Parkinson’s disease based on gene expression in blood. Proc. Natl. Acad. Sci. U. S. A. 104:955–60.
Bogdanov M, Matson WR, Wang L, Matson T, Saunders-Pullman R, Bressman SS, Flint Beal M. (2008) Metabolomic profiling to develop blood biomarkers for Parkinson’s disease. Brain 131:389–96.
Wolozin B, Wang SW, Li NC, Lee A, Lee TA, Kazis LE. (2007) Simvastatin is associated with a reduced incidence of dementia and Parkinson’s disease. BMC Med. 5:20.
Bodles AM, El-Agnaf OM, Greer B, Guthrie DJ, Irvine GB. (2004) Inhibition of fibril formation and toxicity of a fragment of alpha-synuclein by an N-methylated peptide analogue. Neurosci. Lett. 359:89–93.
Amer DA, Irvine GB, El-Agnaf OM. (2006) Inhibitors of alpha-synuclein oligomerization and toxicity: a future therapeutic strategy for Parkinson’s disease and related disorders. Exp. Brain Res. 173:223–33.
Zhu M, Rajamani S, Kaylor J, Han S, Zhou F, Fink AL. (2004) The flavonoid baicalein inhibits fibrillation of alpha-synuclein and disaggregates existing fibrils. J. Biol. Chem. 279:26846–57.
Masliah E, et al. (2005) Effects of alpha-synuclein immunization in a mouse model of Parkinson’s disease. Neuron 46:857–68.
Acknowledgments
This work was supported by Wellcome Trust grant 067660 (D.M.W.), by a Marie-Curie short-term fellowship (G.M.S.), Michael J. Fox Foundation (O.M.E.-A.), and by the Research and Development Office, Health and Personal Social Services, Northern Ireland (G.B.I.). Thanks to Professor Peter Maxwell and Dr. Brian Wisdom for critical reading of the manuscript.
We thank Dr. Cindy Lemere for providing the image shown in Figure 1A.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Irvine, G.B., El-Agnaf, O.M., Shankar, G.M. et al. Protein Aggregation in the Brain: The Molecular Basis for Alzheimer’s and Parkinson’s Diseases. Mol Med 14, 451–464 (2008). https://doi.org/10.2119/2007-00100.Irvine
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2119/2007-00100.Irvine