Abstract
Objective
Comparison of global end-diastolic volume index (GEDVI) obtained by femoral and jugular transpulmonary thermodilution (TPTD) indicator injections using the EV1000/VolumnView® device (Edwards Lifesciences, Irvine, USA).
Methods
In an 87-year-old woman with hypovolemic shock and equipped with both jugular and femoral vein access and monitored with the EV1000/VolumeView® device, we recorded 10 datasets, each comprising duplicate TPTD via femoral access and duplicate TPTD (20 ml cold saline) via jugular access.
Results
Mean femoral GEDVI ((674.6±52.3) ml/m2) was significantly higher than jugular GEDVI ((552.3±69.7) ml/m2), with P=0.003. Bland-Altman analysis demonstrated a bias of (+122±61) ml/m2, limits of agreement of −16 and +260 ml/m2, and a percentage error of 22%. Use of the correction-formula recently suggested for the PiCCO® device significantly reduced bias and percentage error. Similarly, mean values of parameters derived from GEDVI such as pulmonary vascular permeability index (PVPI; 1.244±0.101 vs. 1.522±0.139; P<0.001) and global ejection fraction (GEF; (24.7±1.6)% vs. (28.1±1.8)%; P<0.001) were significantly different in the case of femoral compared to jugular indicator injection. Furthermore, the mean cardiac index derived from femoral indicator injection ((4.50±0.36) L/(min·m2)) was significantly higher (P=0.02) than that derived from jugular indicator injection ((4.12±0.44) L/(min·m2)), resulting in a bias of (+0.38±0.37) L/(min·m2) and a percentage error of 19.4%.
Conclusions
Femoral access for indicator injection results in markedly altered values provided by the EV1000/VolumeView®, particularly for GEDVI, PVPI, and GEF.
中文概要
目 的
临床上因各种原因(如穿刺位置烧伤、感染、颈静脉和锁骨下静脉血栓形成)在行经肺热稀释检测时只能通过股静脉注射指示剂,用PiCCO®设备时会使检测结果产生偏差,而用EV1000/VolumeView®是否同样会产生偏差目前尚不清楚。本研究的主要目的是观察股静脉注射指示剂对EV1000/VolumeView®检测数据的影响。
方 法
通过EV1000/VolumeView®设备对一例低容量休克患者(87 岁,女性)进行经肺热稀释检测,分别通过颈内静脉和股静脉注射冰盐水指示剂,对所测得的两组数据进行对比,分析股静脉注射指示剂对全心舒张末容积指数(GEDVI)、血管外肺水指数(ELWI)、心指数(CI)、肺血管通透性指数(PVPI)和全心射血分数(GEF)等参数的影响。
结 论
经股静脉注射指示剂同样会影响EV1000/VolumeView®设备所检测的热稀释数据,尤其会高估GEDVI 和CI 值,而PVPI 和GEF 也会受到影响,用适合PiCCO®的校正公式校正数据后则可以明显减少偏差。
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The usefulness of transpulmonary thermodilution (TPTD) for the measurement of cardiac index (CI), global end-diastolic volume index (GEDVI), extra vascular lung water index (EVLWI), pulmonary vascular permeability index (PVPI), and global ejection fraction (GEF) has been demonstrated in a number of studies (Michard et al., 2003; Kuzkov et al., 2006; Goepfert et al., 2007; Renner et al., 2007; Malbrain et al., 2010; Jozwiak et al., 2013; Sun et al., 2015). Usually TPTD is performed by indicator injection via the jugular or subclavian vein. However, under certain circumstances, superior vena cava access is not feasible due to thrombosis of the jugular or subclavian vein, polytrauma, burns, infection, or use of the superior vena cava access for dialysis catheters. In these cases, femoral access can be used for TPTD. Femoral access accounts for up to 20%–35% of all catheter insertions (Deshpande et al., 2005; Gowardman et al., 2008). However, two studies (Schmidt et al., 2007; Saugel et al., 2010) comparing jugular and femoral TPTD indicator bolus injections using PiCCO® device (Pulsion Medical Systems AG, Munich, Germany) have demonstrated significant over-estimation particularly of GEDVI due to the additional volume of the inferior vena cava participating in the indicator dilution in the case of femoral injection. Both studies suggested correction for this volume. One of these studies provided correction formulae for femoral GEDVI (GEDVIfem), femoral EVLWI (EVLWIfem), and femoral CI (CIfem) based on data from 48 TPTDs in 24 patients (Saugel et al., 2010). This study was able to demonstrate the usefulness particularly of the correction of GEDVIfem in another 6 subsequent patients.
Recently, in addition to the PiCCO® device, another commercially available device for TPTD has been introduced, EV1000/VolumeView® (Edwards Lifesciences, Irvine, USA) (Bendjelid et al., 2010; 2013; Kiefer et al., 2012; Cho et al., 2015). Based on TPTD, the EV1000/VolumeView® uses similar methodologies and algorithms as the PiCCO® device, which resulted in a good agreement of the data obtained simultaneously by the PiCCO system and the EV1000/VolumeView® in one animal and one clinical evaluation study (Bendjelid et al., 2010; Kiefer et al., 2012). Nevertheless, regarding calculation of GEDVI and EVLWI there is a certain difference between these devices: calculations based on the “downslope time” used by the PiCCO® system have been replaced by a “proprietary function” of the maximum ascending and descending slopes of the thermodilution curve in the EV1000/VolumeView®.
To the best of our knowledge, there are no reports on the bias induced by femoral indicator injection using the EV1000/VolumeView® device.
Therefore, we compared GEDVI, CI, and EVLWI, PVPI, and GEF obtained by femoral as well as by jugular TPTD-indicator injection using the EV1000/VolumeView® device in a patient equipped with both jugular and femoral venous access. Furthermore, we evaluated the usefulness of the above-mentioned correction formulae for femoral TPTD-indicator injection developed for the PiCCO® device (Saugel et al., 2010).
2 Case history
An 87-year old woman (65 kg; 170 cm) was transferred to the intensive care unit (ICU) because of hypovolemic shock and oliguria due to diuretics over-dosage. The patient had been admitted to another hospital 8 h before due to pre-renal acute renal failure (creatinine 7.0 mg/dl) with hyperkalaemia (8.6 mmol/L). Therefore, the patient had undergone emergency dialysis. On admission to our hospital she was equipped with a femoral dialysis catheter. For fluid resuscitation an additional jugular central venous catheter was placed in the right jugular vein. With informed and written consent of the representative of the patient, we recorded 10 datasets, each comprising duplicate TPTD via femoral vein access and immediately subsequent duplicate TPTD via jugular vein access within 15 h after admission using the EV1000/VolumeView® device. An injection volume of 20 ml cold saline (4 °C) was used for TPTD. During the 15-h period with 10 TPTDs, the patient was always spontaneously breathing and always had sinus rhythm. After 15 h of controlled resuscitation, the patient could be transferred to the normal ward. Renal function recovered and the patient could be discharged after 5 d with a serum creatinine level of 1.0 mg/dl.
Endpoints, statistics: comparison of jugular GEDVI (GEDVIjug), jugular CI (CIjug), jugular EVLWI (EVLWIjug), jugular PVPI (PVPIjug), and jugular GEF (GEFjug), to uncorrected GEDVIfem, CIfem, EVLWIfem, femoral PVPI (PVPIfem) and femoral GEF (GEFfem).
In a second step, femoral TPTD-derived parameters were corrected by the formulae suggested by Saugel et al. (2010) and compared to jugular indicator injection-derived parameters:
where IBW is ideal body weight, CVPfem is femoral central venous pressure, and H is height.
The Wilcoxon-test for paired samples was used to compare parameters derived from femoral TPTD to the corresponding data obtained by jugular TPTD. Bland-Altman-analysis modified for repeated measurements was performed to demonstrate the differences of TPTD-derived parameters after femoral and jugular bolus injections in dependence of mean measurement levels (Bland and Altman, 1999). To correct for a limited number of repeated measurements in a single patient, agreement between the two measurement methods was evaluated by calculating the systematic error (bias) with the 95% limit of individual agreement (LOA) as bias ±2.26 standard deviation (SD) with 2.26 corresponding to the 0.975-quantile of the t-distribution for n=10 measurements. The percentage errors of hemodynamic parameters were calculated as demonstrated by Critchley and Critchley (1999).
Software: IBM SPSS Statistics 23 (SPSS Inc., Chicago, IL, USA).
3 Results
During the 15-h observation period and between the 10 TPTD measurements with a mean interval of 100 min, the patient underwent a number of therapeutic interventions including fluid resuscitation and changes in the noradrenaline dosage (between 0 and 600 µg/h). This also resulted in slight changes in non-TPTD-derived parameters such as heart rate ((106±7) min−1; range: 93–117 min−1; coefficient of variation (CV): 0.06), mean arterial pressure ((82±8) mmHg; rang: 68–97 mmHg; CV: 0.10), and CVP ((1.8±1.7) mmHg; rang: 0–5 mmHg; CV: 0.96). All changes in heart rate occurred during continuous sinus rhythm.
3.1 Global end-diastolic volume index
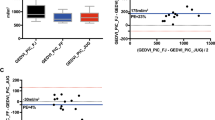
Fig. 1 demonstrates the time course of GEDVIjug, GEDVIfem, and corrected GEDVIfem (GEDVIfem, corrected). GEDVIfem ((674.6±52.3) ml/m2) was significantly higher than GEDVIjug ((552.3±69.7) ml/m2) (P=0.003). Bland-Altman analyses (Fig. 2) of GEDVIjug and GEDVIfem demonstrated a bias of (+122±61) ml/m2, limits of agreement of −16 and +260 ml/m2, and a percentage error of 22%. Correction of GEDVIfem using the above-mentioned formula resulted in a mean GEDVIfem, corrected of (586.3±34.9) ml/m2, which was not significantly different from GEDVIjug ((552.3±69.7) ml/m2; P=0.074; Fig. 3). The bias GEDVIfem, corrected−GEDVIjug of (34.0±58.7) ml/m2 (limits of agreement of −97 and +167 ml/m2; percentage error of 23%) was significantly lower than the bias using uncorrected GEDVIfem (122.3±60.7) ml/m2; P=0.005).
3.2 Cardiac index
Similarly CIfem ((4.50±0.36) L/(min·m2)) was significantly higher than CIjug ((4.12±0.44) L/(min·m2); P=0.02), resulting in a bias of (+0.38±0.37) L/(min·m2) (limits of agreement of −0.46 and +1.22 L/(min·m2); percentage error of 19.4%; Fig. 4). Application of the above-mentioned correction formula resulted in a significant reduction (P=0.005) of the bias to −0.098 L/(min·m) (limits of agreement of −0.93 and +0.74 L/(min·m2); percentage error of 20.5%; Fig. 5). Furthermore, corrected CIfem (CIfem, corrected) and CIjug were not significantly different ((4.02±0.35) vs. (4.12±0.44) L/(min·m2); P=0.799).
3.3 Extra vascular lung water index
EVLWIfem ((5.32±0.82) ml/kg) was not significantly different (P=0.154) from EVLWIjug ((5.15± 1.10) ml/kg), resulting in a small bias of (0.17± 0.59) ml/kg (limits of agreement of −1.16 and +1.5 ml/kg; percentage error of 25.5%). Corrected EVLWIfem (EVLWIfem, corrected (5.41±0.72) ml/kg) was neither significantly different compared to EVLWIjug (P=0.203) nor to EVLWIfem (P=0.139).
3.4 Pulmonary vascular permeability index and global ejection fraction
Furthermore, femoral TPTD-derived values of PVPI (PVPI=EVLW/(0.25×GEDVI)) and GEF (GEF=4×stroke volume/GEDVI) were significantly lower than those for jugular indicator injection (PVPI: 1.244±0.101 vs. 1.522±0.139, P<0.001; GEF: (24.7± 1.6)% vs. (28.1±1.8)%, P<0.001).
4 Discussion
In this report, we present data on TPTD with the EV1000/VolumeView® device using indicator injection via inferior vena cava access. In general, the hemodynamic parameters provided and the normal ranges given by the manufacturer of the EV1000/VolumeView® device are congruent to the PiCCO® device. Identical normal values as for the PiCCO and the data of two evaluation studies suggest that the normal route for indicator bolus application using the EV1000/VolumeView® is via jugular or subclavian access (Kiefer et al., 2012).
4.1 Physiological background
With injection of the indicator bolus via a femoral catheter, the total indicator distribution volume is augmented by larger parts of the inferior vena cava compared to indicator injection closest to the right atrium using jugular or subclavian access. This necessarily results in an overestimation of GEDVI.
The differences between femoral and jugular vein injection in particular for GEDVI can be explained by the mathematical fundamentals of single indicator TPTD (Calbet and Boushel, 2015):
-
(1)
The total volume participating in single indicator TPTD is called intrathoracic thermo-volume (ITTV), which is calculated by multiplication of mean transit time (MTT) with cardiac output (CO): ITTV=MTT×CO.
-
(2)
MTT is the time from injection until half of the indicator has passed the thermistor tip. In single indicator TPTD, GEDVI is calculated by subtraction of pulmonary thermo-volume (PTV) from ITTV: GEDVI=ITTV−PTV.
-
(3)
PTV is composed of pulmonary blood volume (PBV) and EVLWI. PTV can be calculated by multiplication of CO and the time of the exponential decrease of the thermodilution curve (“downslope time” (td): PTV=CO×td.
The PiCCO® system uses the “downslope time” based on the finding that in serial mixing chambers with identical flow, the exponential decrease of the TPTD curve is determined by the largest chamber. By contrast, in the EV1000/VolumeView® td has been replaced by a “proprietary function” of the maximum ascending and descending slopes of the TPTD curve. With the exact mathematical algorithm of the EV1000/VolumeView® remaining occult, it is difficult to assess the impact of femoral indicator injection on the measurement of PTV in the EV1000/VolumeView®.
However, in both PiCCO and EV1000/VolumeView® the injection of the indicator bolus via a femoral catheter increases MTT and GEDVI.
Consequently, two recent studies using the PiCCO® device demonstrated mean bias values for GEDVIfem compared with GEDVIjug of 241 and 141 ml/m2, respectively (Schmidt et al., 2007; Saugel et al., 2010). Both values exceed the difference between the upper and lower normal values (normal range: 680–800 ml/m2). In other words, decreased TPTD values measured via a jugular venous catheter might exceed the upper normal level when simultaneously measured via femoral TPTD. This emphasizes the need for correction and awareness of this potential confounder.
One option could be to correct MTT for the time required for the indicator to flow from the femoral injection site to the right atrium as demonstrated by Calbet and Boushel (2015). This time has been termed “time from the femoral vein to the right atrium” (TFVRA). TFVRA can be determined as time between injection of agitated indicator and the appearance of air bubbles in the right atrium visualized by ultrasound (Calbet and Boushel, 2015).
Saugel et al. (2010) using the PiCCO® device provided a first empiric attempt for individual correction formulae. However, these formulae, so far, neither have been re-evaluated nor applied for the EV1000/VolumeView® device.
Our data using the EV1000/VolumeView® device confirm the findings of the above-mentioned study using the PiCCO® device, also including a substantial reduction of the bias of femoral indicator injection-derived GEDVI by the correction formula provided by the authors: regarding GEDVI, we found a significant overestimation by GEDVIfem with a bias of (+122±61) ml/m2 compared to simultaneous GEDVIjug. With regard to the normal range of GEDVI of 680–800 ml/m2, this is more than 100% of the difference of the upper and the lower normal value. This bias of (+122±61) ml/m2 was significantly reduced by 72% to 34.0 ml/m2 by the formula suggested by Saugel et al. (2010). As a result of correction according to this formula, corrected GEDVIfem was not significantly different from GEDVIjug. These findings resembling the data reported for the PiCCO® (Schmidt et al., 2007; Saugel et al., 2010) are particularly remarkable, since, compared to the PiCCO®, the EV1000/VolumeView® uses a slightly different algorithm to calculate GEDVI (Bendjelid et al., 2010). Compared to the marked impact on GEDVI (22.1% overestimation), PVPI (18.3% underestimation), and GEF (12.3% underestimation), the bias induced by femoral TPTD regarding CI of +0.38 L/(min·m2) seems to be less pronounced. In the patient investigated, the bias was about 9% of mean CIjug and 19% of the difference of the upper and lower normal levels of CI (2.5–4.5 L/(min·m2)). Similarly to GEDVI, the application of the correction formula significantly reduced the bias regarding CI. By contrast, femoral TPTD did not result in a significant difference ofEVLWIfem and EVLWIjug.
4.2 Clinical applications
Physicians using femoral access for TPTD should be aware of a marked bias particularly regarding GEDVI. Correction formulae should be further investigated and may be a more straightforward solution than establishing specific normal values for femoral indicator injection-derived TPTD parameters. As a consequence of the above-mentioned data the manufacturer of the PiCCO® device (Pulsion Medical Systems, Feldkirchen, Germany) implemented a new software requiring the information about the central venous catheter site (femoral or jugular/subclavian), and some kinds of correction cases of femoral injection can be assumed for GEDVI, but not for PVPI (Berbara et al., 2014).
Interestingly, a recent study also demonstrated substantial impact of misplacement of subclavian vein central venous catheters into the jugular vein (Yu et al., 2016). Catheter misplacement also resulted in marked overestimation of GEDVI.
4.3 Limitations
Despite the conclusive findings in our study in accordance with previous data it has to be clearly stated that we analyzed a limited number of repeated measurements originating from a single patient. Nevertheless, our findings are likely to be generalizable with regard to the previous data with the PiCCO® device including a total of more than 100 measurements in 41 patients (Schmidt et al., 2007; Saugel et al., 2010) and the obvious physical rationale that the thermodilution volume is artificially increased by inferior cava.
Randomization of the site of initial injection site (jugular or femoral) for different TPTDs would have avoided any bias, since GEDVI, CI, and EVLWI measured by TPTD at the second site might be slightly altered due to the impact on haemodynamics induced by the measurements using the first injection site. Since TPTD also calibrates pulse contour derived CI, for ethical reasons we performed TPTD on the gold standard site (jugular vein) after TPTD on the site with a potential confounding.
Furthermore, only duplicate and not three consecutive injections were performed for each measurement. The recommendations of the manufacturers suggest injectate volumes of 15 or 20 ml for patients with a bodyweight above 50 kg. To provide maximum accuracy also using a venous access with potential confounding (TPTD via femoral vein) we chose the higher amount of injectate volume. However, to avoid a direct impact of the injection volume on haemodynamics (potential increase in GEDVI and CI) and also considering the large number of thermodilutions (a total of 40 injections within 10 h), we performed only duplicate injection for each measurement.
5 Conclusions
Similarly as demonstrated for the PiCCO®, the use of femoral access for indicator injection results in markedly altered values provided by the EV1000/VolumeView®, particularly for GEDVI. The correction formulae suggested for the PiCCO®, markedly reduced the bias regarding GEDVI and CI induced by femoral TPTD. Further databases including heterogeneous populations of patients have to be analyzed.
Compliance with ethics guidelines
Wolfgang HUBER, Veit PHILLIP, Josef HÖLLTHALER, Caroline SCHULTHEISS, Bernd SAUGEL, and Roland M. SCHMID declare that they have no conflict of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). Informed consent was obtained from all patients for being included in the study.
References
Bendjelid, K., Giraud, R., Siegenthaler, N., et al., 2010. Validation of a new transpulmonary thermodilution system to assess global end-diastolic volume and extravascular lung water. Crit. Care, 14(6):R209. http://dx.doi.org/10.1186/cc9332
Bendjelid, K., Marx, G., Kiefer, N., et al., 2013. Performance of a new pulse contour method for continuous cardiac output monitoring: validation in critically ill patients. Br. J. Anaesth., 111(4):573–579. http://dx.doi.org/10.1093/bja/aet116
Berbara, H., Mair, S., Beitz, A., et al., 2014. Pulmonary vascular permeability index and global end-diastolic volume: are the data consistent in patients with femoral venous access for transpulmonary thermodilution: a prospective observational study. BMC Anesthesiol., 14:81. http://dx.doi.org/10.1186/1471-2253-14-81
Bland, J.M., Altman, D.G., 1999. Measuring agreement in method comparison studies. Stat. Methods Med. Res., 8(2):135–160.
Calbet, J.A., Boushel, R., 2015. Assessment of cardiac output with transpulmonary thermodilution during exercise in humans. J. Appl. Physiol., 118(1):1–10. http://dx.doi.org/10.1152/japplphysiol.00686.2014
Cho, Y.J., Koo, C.H., Kim, T.K., et al., 2015. Comparison of cardiac output measures by transpulmonary thermodilution, pulse contour analysis, and pulmonary artery thermodilution during off-pump coronary artery bypass surgery: a subgroup analysis of the cardiovascular anaesthesia registry at a single tertiary centre. J. Clin. Monit. Comput., online first. http://dx.doi.org/10.1007/s10877-015-9784-6
Critchley, L.A., Critchley, J.A., 1999. A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J. Clin. Monit. Comput., 15(2):85–91. http://dx.doi.org/10.1023/A:1009982611386
Deshpande, K.S., Hatem, C., Ulrich, H.L., et al., 2005. The incidence of infectious complications of central venous catheters at the subclavian, internal jugular, and femoral sites in an intensive care unit population. Crit. Care Med., 33(1):13–20, discussion 234–235. http://dx.doi.org/10.1097/01.CCM.0000149838.47048.60
Goepfert, M.S.G., Reuter, D.A., Akyol, D., et al., 2007. Goal-directed fluid management reduces vasopressor and catecholamine use in cardiac surgery patients. Intensive Care Med., 33(1):96–103. http://dx.doi.org/10.1007/s00134-006-0404-2
Gowardman, J.R., Robertson, I.K., Parkes, S., et al., 2008. Influence of insertion site on central venous catheter colonization and bloodstream infection rates. Intensive Care Med., 34(6):1038–1045. http://dx.doi.org/10.1007/s00134-008-1046-3
Jozwiak, M., Silva, S., Persichini, R., et al., 2013. Extravascular lung water is an independent prognostic factor in patients with acute respiratory distress syndrome. Crit. Care Med., 41(2):472–480. http://dx.doi.org/10.1097/CCM.0b013e31826ab377
Kiefer, N., Hofer, C.K., Marx, G., et al., 2012. Clinical validation of a new thermodilution system for the assessment of cardiac output and volumetric parameters. Crit. Care, 16(3):R98. http://dx.doi.org/10.1186/cc11366
Kuzkov, V.V., Kirov, M.Y., Sovershaev, M.A., et al., 2006. Extravascular lung water determined with single transpulmonary thermodilution correlates with the severity of sepsis-induced acute lung injury. Crit. Care Med., 34(6):1647–1653. http://dx.doi.org/10.1097/01.CCM.0000218817.24208.2E
Malbrain, M.L., de Potter, T.J., Dits, H., et al., 2010. Global and right ventricular end-diastolic volumes correlate better with preload after correction for ejection fraction. Acta Anaesthesiol. Scand., 54(5):622–631. http://dx.doi.org/10.1111/j.1399-6576.2009.02202.x
Michard, F., Alaya, S., Zarka, V., et al., 2003. Global end-diastolic volume as an indicator of cardiac preload in patients with septic shock. Chest, 124(5):1900–1908. http://dx.doi.org/10.1378/chest.124.5.1900
Renner, J., Gruenewald, M., Brand, P., et al., 2007. Global end-diastolic volume as a variable of fluid responsiveness during acute changing loading conditions. J. Cardiothorac. Vasc. Anesth., 21(5):650–654. http://dx.doi.org/10.1053/j.jvca.2007.05.006
Saugel, B., Umgelter, A., Schuster, T., et al., 2010. Transpulmonary thermodilution using femoral indicator injection: a prospective trial in patients with a femoral and a jugular central venous catheter. Crit. Care, 14:R95. http://dx.doi.org/10.1186/cc9030
Schmidt, S., Westhoff, T.H., Hofmann, C., et al., 2007. Effect of the venous catheter site on transpulmonary thermodilution measurement variables. Crit. Care Med., 35(3): 783–786. http://dx.doi.org/10.1097/01.CCM.0000256720.11360.FB
Sun, Y., Lu, Z.H., Zhang, X.S., et al., 2015. The effects of fluid resuscitation according to PiCCO on the early stage of severe acute pancreatitis. Pancreatology, 15(5):497–502. http://dx.doi.org/10.1016/j.pan.2015.06.006
Yu, W.Q., Zhang, Y., Zhang, S.Y., et al., 2016. Impact of misplaced subclavian vein catheter into jugular vein on transpulmonary thermodilution measurement variables. J. Zhejiang Univ.-Sci. B (Biomed. & Biotechnol.), 17(1):60–66. http://dx.doi.org/10.1631/jzus.B1500167
Acknowledgements
The EV1000/VolumeView® was provided for scientific use by Edwards Lifesciences, Irvine, USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
ORCID: Wolfgang HUBER, http://orcid.org/0000-0001-9086-7908
Rights and permissions
About this article
Cite this article
Huber, W., Phillip, V., Höllthaler, J. et al. Femoral indicator injection for transpulmonary thermodilution using the EV1000/VolumeView®: do the same criteria apply as for the PiCCO®?. J. Zhejiang Univ. Sci. B 17, 561–567 (2016). https://doi.org/10.1631/jzus.B1500244
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B1500244
Key words
- Hemodynamic monitoring
- Transpulmonary thermodilution
- Central venous catheter
- Femoral vein
- Jugular vein
- Global end-diastolic volume
- EV1000/VolumeView®
- PiCCO®