Abstract
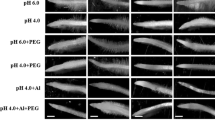
A transgenic barley line (LSY-11-1-1) with overexpressed Phalaris coerulescens thioredoxin gene (PTrx) was employed to measure the growth, protein oxidation, cell viability, and antioxidase activity in barley roots during germination on the presence of 2 mmol/L AlCl3 on filter paper. The results show that (1) compared with the non-transgenic barley, LSY-11-1-1 had enhanced root growth, although both were seriously inhibited after AlCl3 treatment; (2) the degree of protein oxidation and loss of cell viability in roots of LSY-11-1-1 were much less than those in roots of non-transgenic barley, as reflected by lower contents of protein carbonyl and Evans blue uptakes in LSY-11-1-1; (3) activities of catalase (CAT), glutathione peroxidase (GPX), ascorbate peroxidase (APX), and glutathione reductase (GR) in LSY-11-1-1 root tips were generally higher than those in non-transgenic barley root tips, although these antioxidase activities gave a rise to different degrees in both LSY-11-1-1 and non-transgenic barley under aluminum stress. These results indicate that overexpressing PTrx could efficiently protect barley roots from oxidative injury by increasing antioxidase activity, thereby quenching ROS caused by AlCl3 during germination. These properties raise the possibility that transgenic barley with overexpressed PTrx may be used to reduce the aluminum toxicity in acid soils.
Similar content being viewed by others
References
Asada, K., 1984. Chloroplasts: formation of active oxygen and its scavenging. Methods Enzymol., 105:422–429. [doi: 10.1016/S0076-6879(84)05059-X]
Balmer, Y., Vensel, W.H., Tanaka, C.K., Hurkman, W.J., Gelhaye, E., Rouhier, N., Jacquot, J.P., Manieri, W., Schürmann, P., Droux, M., et al., 2004. Thioredoxin links redox to the regulation of fundamental processes of plant mitochondria. PNAS, 101(8):2642–2647. [doi:10.1073/pnas.0308583101]
Boscolo, P.R.S., Menossi, M., Jorge, R.A., 2003. Aluminum-induced oxidative stress in maize. Phytochemistry, 62(2): 181–189. [doi:10.1016/S0031-9422(02)00491-0]
Bradford, M.M., 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem., 72(1–2):248–254. [doi:10.1016/0003-2697(76)90527-3]
Broin, M., Rey, P., 2003. Potato plants lacking the CDSP32 plastidic thioredoxin exhibit over-oxidation of the BAS1 2-Cys peroxiredoxin and increased lipid peroxidation in thylakoids under photooxidative stress. Plant Physiol., 132(3):1335–1343. [doi:10.1104/pp.103.021626]
Cakmak, I., Horst, W.J., 1991. Effect of aluminum on lipid peroxidation, superoxide dismutase, catalase, and peroxides activities in root tips of soybean (Glycine max). Physiol. Plantarum, 83(3):463–468. [doi:10.1034/j.1399-3054.1991.830320.x]
Delisle, G., Champoux, M., Houde, M., 2001. Characterization of oxalate oxidase and cell death in Al-sensitive and tolerant wheat roots. Plant Cell Physiol., 42(3):324–333. [doi:10.1093/pcp/pce041]
Ellman, G.D., 1959. Tissue sulfhydryl groups. Arch. Biochem. Biophys., 82(1):70–77. [doi:10.1016/0003-9861(59)90090-6]
Goodwin, S.B., Sutter, T.R., 2009. Microarray analysis of Arabidopsis genome response to aluminum stress. Biol. Plantarum, 53(1):85–99. [doi:10.1007/s10535-009-0012-4]
Jung, B.G., Lee, K.O., Lee, S.S., Chi, Y.H., Jang, H.H., Kang, S.S., Lee, K., Lim, D., Yoon, S.H., Yun, D.J., et al., 2002. A Chinese cabbage cDNA with high sequence identity to phospholipid hydroperoxide glutathione peroxidases encodes a novel isoform of thioredoxin-dependent peroxidase. J. Biol. Chem., 277(15):12572–12578. [doi:10.1074/jbc.M110791200]
Kochian, L.V., 1995. Cellular mechanisms of aluminum toxicity and resistance in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol., 46(1):237–260. [doi:10.1146/annurev.pp.46.060195.001321]
Laloi, C., Mestres-Ortega, D., Marco, Y., Meyer, Y., Reichheld, J.P., 2004. The Arabidopsis cytosolic thioredoxin h5 gene induction by oxidative stress and its W-box-mediated response to pathogen elicitor. Plant Physiol., 134(3):1006–1016. [doi:10.1104/pp.103.035782]
Lemaire, S.D., Collin, V., Keryer, E., Issakidis-Bourguet, E., Lavergne, D., 2003. Chlamydomonas reinhardtii: a model organism for the study of the thioredoxin family. Plant Physiol. Biochem., 41(6–7):513–521. [doi:10.1016/S0981-9428(03)00079-2]
Levine, R.L., Garland, D., Olive, C.N., Amici, A., Climent, I., Lenz, A.G., Ahn, B.W., Shaltiel, S., Stadtman, E.R., 1990. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol., 186:464–478. [doi:10.1016/0076-6879(90)86141-H]
Li, X.M., Nield, J., Hayman, D., Langridge, P., 1995. Thioredoxin activity in the C terminus of Phalaris S protein. Plant J., 8(1):133–138. [doi:10.1046/j.1365-313X.1995.08010133.x]
Li, Y.C., Ren, J.P., Cho, M.J., Zhou, S.M., Kim, Y.B., Guo, H.X., Wong, J.H., Niu, H.B., Kim, H.K., Morigasaki, S., et al., 2009. The level of expression of thioredoxin is linked to fundamental properties and applications of wheat seeds. Mol. Plant, 2(3):430–441. [doi:10.1093/mp/ssp025]
Livingstone, D.R., Lips, F., Garcia Martinez, P., Pipe, P.K., 1992. Antioxidant enzymes in the digestive gland of the common mussel Mytilus edulis. Mar. Biol., 112(2): 265–276. [doi:10.1007/BF00702471]
Maron, L.G., Kirst, M., Mao, C., Milner, M.J., Menossi, M., Kochian, L.V., 2008. Transcriptional profiling of aluminum toxicity and tolerance responses in maize roots. New Phytol., 179(1):116–128. [doi:10.1111/j.1469-8137.2008.02440.x]
Meyer, Y., Verdoucq, L., Vignols, F., 1999. Plant thioredoxins and glutaredoxins: identity and putative roles. Trends Plant Sci., 4(10):388–394. [doi:10.1016/S1360-1385(99)01475-2]
Mittler, R., 2002. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci., 7(9):405–410. [doi:10.1016/S1360-1385(02)02312-9]
Pan, J.W., Zhu, M.Y., Chen, H., 2001. Aluminum-induced cell death in root-tip cells of barley. Environ. Exp. Bot., 46(1): 71–79. [doi:10.1016/S0098-8472(01)00083-1]
Rey, P., Cuiné, S., Eymery, F., Garin, J., Court, M., Jacquot, J.P., Rouhier, N., Broin, M., 2005. Analysis of the proteins targeted by CDSP32, a plastidic thioredoxin participating in oxidative stress responses. Plant J., 41(1): 31–42. [doi:10.1111/j.1365-313X.2004.02271.x]
Richards, K.D., Schott, E.J., Sharma, Y.K., Davis, K.R., Gardner, R.C., 1998. Aluminum induces oxidative stress genes in Arabidopsis thaliana. Plant Physiol., 116(1): 409–418. [doi:10.1104/pp.116.1.409]
Sato, K., Shin-I, T., Seki, M., Shinozaki, K., Yoshida, H., Takeda, K., Yamazaki, Y., Conte, M., Kohara, Y., 2009. Development of 5006 full-length CDNAs in barley: a tool for accessing cereal genomics resources. DNA Res., 16(2): 81–89. [doi:10.1093/dnares/dsn034]
Schaedle, M., Bassham, J.A., 1977. Chloroplast glutathione reductase. Plant Physiol., 59(5):1011–1012. [doi:10.1104/pp.59.5.1011]
Schenk, H., Klein, M., Erdbrugger, W., Droge, W., Schulze-Osthoff, K., 1994. Distinct effects of thioredoxin and antioxidants on the activation of transcription factors NF-κB and AP-1. PNAS, 9l(5):1672–1676.
Shao, H.B., Jiang, S.Y., Li, F.M., Chu, L.Y., Shao, M.A., Li, F., 2007a. Some advances in plant stress physiology and their implications in the systems biology era. Colloids Surf. B Biointerfaces, 54(1):33–36. [doi:10.1016/j.colsurfb.2006.05.011]
Shao, H.B., Chu, L.Y., Lu, Z.H., Kang, C.M., 2007b. Primary antioxidant free radical scavenging and redox signaling pathways in higher plant cells. Int. J. Biol. Sci., 4(1):8–14.
Shao, H.B., Chu, L.Y., Shao, M.A., 2008. Calcium as a versatile plant signal transducer under soil water stress. Bioessays, 30(7):634–641. [doi:10.1002/bies.20770]
Shao, H.B., Jaleel, C.A., Manivannan, P., Panneerselvam, R., Shao, M.A., 2009. Understanding water deficit stress-induced changes in the basic metabolism of higher plants—biotechnologically and sustainably improving agriculture and the ecoenvironment in arid regions of the globe. Crit. Rev. Biotechnol., 29(2):131–151. [doi:10.1080/07388550902869792]
Tamás, L., BudÍková, S., Šimonovičová, M., Huttova, J., Siroka, B., Mistrik, I., 2006. Rapid and simple method for Al-toxicity analysis in emerging barley roots during germination. Biol. Plantarum, 50(1):87–93. [doi:10.1007/s10535-005-0079-5]
Tsukamoto, S., Morita, S., Hirano, E., Yokoi, H., Masumura, T., Tanaka, K., 2005. A novel cis-element that is responsive to oxidative stress regulates three antioxidant defense genes in rice. Plant Physiol., 137(1):317–327. [doi: 10.1104/pp.104.045658]
Vieira Dos Santos, C., Rey, P., 2006. Plant thioredoxins are key actors in the oxidative stress response. Trends Plant Sci., 11(7):329–334. [doi:10.1016/j.tplants.2006.05.005]
Wang, G.L., Fang, H.J., 1998. Principle and Technique of Plant Genetic Engineering. Science Press, Beijing (in Chinese).
Wong, J.H., Cai, N., Balmer, Y., Tanaka, C.K., Vensel, W.H., Hurkman, W.J., Buchanan, B.B., 2004. Thioredoxin targets of developing wheat seeds identified by complementary proteomic approaches. Phytochemistry, 65(11): 1629–1640. [doi:10.1016/j.phytochem.2004.05.010]
Yamamoto, Y., Kobayashi, Y., Matsumoto, H., 2001. Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol., 125(1):199–208. [doi:10.1104/pp.125.1.199]
Author information
Authors and Affiliations
Corresponding authors
Additional information
The two authors contributed equally to this work
Project supported by the National Natural Science Foundation of China (No. 30871530), the Science and Technology Transformation Plan of Henan Province (No. 0636000005), One Hundred-Talent Plan of Chinese Academy of Sciences (CAS), CAS-local Government Cooperative Project, the CAS/SAFEA International Partnership Program for Creative Research Teams, and CAS Young Scientists Fellowship (No. 2009Y2B211), China
Rights and permissions
About this article
Cite this article
Li, Qy., Niu, Hb., Yin, J. et al. Transgenic barley with overexpressed PTrx increases aluminum resistance in roots during germination. J. Zhejiang Univ. Sci. B 11, 862–870 (2010). https://doi.org/10.1631/jzus.B1000048
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B1000048