Abstract
For conservation interventions of historic masonry generally lime-based mortars such as pure air lime mortars, lime-pozzolan mortars, natural hydraulic lime mortars and ternary mortars (lime-pozzolan-cement) are used. The main reason is that their hygric and mechanical (strength and ductility) performance are easy to adapt to most existing historic masonry (compatibility requirements). Although the basic appropriateness of lime-based mortars for restoration is undisputed, there are also some limitations in the application of these mortars. In this report a review is given of the theoretical backgrounds and further of laboratory research developments in the field of lime-based binders and mortars over the past decades. Furthermore, practical experiences in positive and negative sense (damage cases) are elaborated. Drawbacks and points of attention are being dealt with, which are essential for a durable application of lime-based mortars for the conservation of historic masonry. In general, it is concluded that points of attention should be addressed through a thorough evaluation of their potential and through testing of their suitability. For these mortars, even more than for modern cement-based mortars, specifically, environmental exposure conditions and application conditions should be considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Although probably mud and gypsum mortars were the first mortar applications ever used in construction history, also the use of lime mortars, using a binder based on calcined limestone, goes far back in human history. The Greeks were perhaps the first to use (air) lime mortars for masonry and plasters. Probably the Greeks were also the first to use pozzolanic additions to lime mortars, a technology that became mainly known thanks to the Romans, who used the earth from Pozzuoli in their mortars that allowed the construction of harbor works all over the Mediterranean, thus contributing to the expansion of the Roman Empire. Still nowadays many examples exist of historic buildings with lime mortars that are well preserved under specific conditions. This shows clearly that lime mortars can possess an excellent durability.
In the last decades, many studies have been reported to reveal the specific characteristics that contributed to the considerable service life of these mortars. The selection of the raw materials used according to the functional role that mortars had in the structure, (e.g. the use of fine aggregates in renders/plasters in relation to coarse ones used for bedding mortars), the special application techniques (e.g. for renders, successive layers with differentiated properties in order to prevent moisture to penetrate into the structure), the type of binders used for the production of the old mortars, the proportioning and the type and size of the aggregates are among the basic parameters evaluated [1, 2]. From these analyses, it comes forward that the binding system is the most influential parameter in the long-term strength of the lime-based mortar mixtures.
Based on this concept, repair materials have been designed and applied in many different cases, and a critical degree of knowledge has been reached and divulgated with dedicated studies [3].
The formulation and application of compatible and durable repair mortars is the ideal scenario in each restoration project. Compatibility is achieved through functional and aesthetic harmonization of the repair mortar with the original material, while durability concerns the long-term behavior of the repair mortars in the environment where each structure is placed. The functional harmonization can be achieved through the comparable porosity, permeability and strength among other important properties. Parameters such as salt presence, freeze–thaw cycles, action of water and wind should be considered when it comes to design a durable mortar [4].
Within the framework of the activities of the RILEM technical committee 277-LHS, this paper aims to review the durability aspects deriving through the study of old lime-based mortars that should be taken into account for compatible restoration mortars. Different practical cases were used as example. The report, based on a comprehensive literature review, outlines the beneficial properties of these mortars, indicates their restrictions, and gives clues for their durable application.
The examples discussed in this paper reflect the situation in large parts of the world, but cannot claim completeness. Some additional literature references are given, that could give a wider scope [78, 79].
The structure of this TC report includes three main sections. The first one is firstly (chapter 2.1) devoted to the categorization of the lime-based mortars. The mortars are classified through the numerous combinations of lime with different hydraulic binders: pure air lime, lime-pozzolan, ternary binding systems (lime, pozzolan and cement) and natural hydraulic lime mortars have been identified.
Secondly in this first section (chapter 2.2) the mechanisms explaining the binding and hardening actions of these lime-based mortars are discussed with a focus to the internal parameters and external factors that affect their durability. Drying, hydration, pozzolanic reaction and carbonation are the most relevant mechanisms accounting for the hardening and binding action of lime-based mortars [5,6,7,8,9,10]. The report describes how these mechanisms are strongly influenced by the composition of the mortars (hydraulic nature of the lime, presence or not of pozzolan, composition and fineness of the pozzolans) [11,12,13] and by the curing conditions. The effects of other external factors that take place in real applications, such as suction by the substrate, frost damage or salt decay, are discussed as a function of the binder composition and the microstructure of the mortars.
The second section (chapter 3) provides information about the occurrence of these mechanisms (binding and hardening) and their prevalence during real applications. The potential as repair materials and drawbacks (problematic scenarios) of lime-based mortars are presented, all of them from a practical point of view aiming to help practitioners to select the best mixes and their optimal application and curing conditions, as well as to anticipate durability issues.
Finally, in the third section (chapter 4), different damage cases from practice are also shown with a view to further illustrating the potential shortcomings. Real cases of cracks formation due to shrinkage, frost damage causing layering in renders, loss of coherence and sanding of trass-lime repointing mortars, sulfate-induced and salt-crystallization damages, aesthetic alterations (Liesegang patterns formation, efflorescence), as well as leaching and encrustation are presented. The main factors responsible for these durability issues are analyzed and preventive solutions are suggested to achieve a successful application of lime-based repair mortars.
As conclusion, the paper emphasizes that lime is a well-performing building material largely compatible with historic and monumental structures, and understanding its behavior is the key factor for achieving durable repair mortars. Suggestions are given on how to increase, in real applications, the durability of mortars and avoid premature decay.
2 Background on lime-based mortars (L-b-M)
2.1 Categorization of lime-based mortars (L-b-M) and binders (L-b-B)
Most of historic masonries have been constructed with the use of L-b-M which played different functional roles in the old structures such as for bedding, flooring, plastering, and rendering [14]. A plethora of binders or combinations of them (generally available locally) provided adequate strength to meet the performance requirements of the ancient structural systems. Besides the binders with pure air lime, other combinations are:
-
(i)
L-b-B’s made by firing limestone containing slate or other siliceous material to produce different types of Natural Hydraulic Lime (NHL according to [15]).
-
(ii)
The addition of calcium silicates in pure air lime could be made by adding Portland cement to the lime or limestone filler enriching hydraulicity, hardening rate and strength such as masonry cements, which commonly contain Portland cement, hydrated lime and limestone filler. For repairing historic structures, the Portland cement percentage should not exceed 30% of the total binder content in order the mortar made with it to retain the porosity and moisture transport properties of the old mortar [16].
-
(iii)
Another large category of L-b-M concerns the combination of lime with natural pozzolans or artificial pozzolans such as calcined clays, fly ashes or slags and silica fume. Some of the aforementioned by-products possess self-hardening properties (calcareous fly ashes, blast furnace slag, ladle furnace slag).
Natural pozzolans are siliceous or siliceous and aluminous materials which themselves possess little or no cementitious properties but in finely divided form and, in the presence of moisture, chemically react with calcium hydroxide at ordinary temperature to form compounds possessing cementing/binding properties [17]. An indicative list of some natural pozzolans is given in Table 1 [18,19,20].
Clay minerals or shale by heating to 600–900ο C are transformed to pozzolans (calcined clays and shales). For example, pulverized clay rich in kaolin after calcination produces a highly reactive pozzolan (metakaolin). The reactivity of pozzolanic materials differs greatly depending mainly on chemical and mineralogical composition (amorphous silica content or glass), as well as fineness.
-
iv)
Apart from binary combinations (lime + pozzolan), the use of ternary systems (lime:pozzolan:cement) proved successful even in the case of lime-based grouts [21]. These L-b-B’s improve early strength, adhesion or other properties for higher performance and longevity. It could be said that from the 90’s and onwards these ternary binding systems are widely used in restoration projects.
In L-b-M the hydraulic components contribute to higher strength, resistance to moisture and better resistance to weathering. The 28-d compressive strength values ranged for CL90 from 0.5 to 2.0 MPa, NHL3 from 3.5 to 8 MPa, CL90-natural pozzolan (1:1) from 3.0 to 5.5 MPa [1]. The open porosity ranged from 40 to 23%, the highest value corresponding to the lowest strength value. The most often used L-b-B for repairing historic structures and their combinations for strength enhancement are shown in Table 2.
However, good performance of the repair mortars for historic structures is not necessarily related to strength level. Properties such as adhesion, shrinkage, absorption, water retention, drying, are mainly influenced by other characteristics of the mortar’s components.
2.2 Factors affecting the binding and hardening mechanisms of lime-based mortars applied in restoration
2.2.1 Pure air lime mortars
In either calcitic or dolomitic air lime mortars, slaked lime (Ca(OH)2, portlandite) is the main compound of the fresh binder. For limes with the presence of dolomite in the raw material, the presence of Mg(OH)2, brucite, has to be also considered. In these mortars, the hardening starts by the loss of the mixing water, i.e., the drying process [25, 26]. The excess of water that allows the characteristic plastic state achieved by the fresh mortars can be removed in air lime mortars by (i) evaporation and (ii) absorption by the substrate.
Concerning evaporation, climatic factors must be considered to guarantee the appropriate drying process. Final strengths and other hardened properties of air lime mortars are improved at moderate temperature and humidity conditions (for example, at lab scale, RH of 70 ± 5% has proved to be effective) [27]. High RH conditions (for example, 90%) lead to blockage of the pore network hindering the CO2 access and thus retarding carbonation and therefore are not recommended [27,28,29]. Extremely low RH conditions involve a quick water loss, causing shrinkage of the mortars and debonding [30].
With respect to the effect of the substrate, the pores of the substrate can induce a suction pressure, forcing the flow of water from the large pores of the air lime mortar to the smaller ones of the substrate [31]. Considering that air lime mortars have a high percentage of large pores, this suction phenomenon is very frequent and can lead to microstructural modification and, in some cases, to cracking and possibly debonding [32].
Carbonation is the main responsible factor for setting and hardening of air lime mortars [33]. Figure 1 depicts a scheme of the process. Significant carbonation takes place after a certain drying level has been reached (with RH percentages ranging from 40 to 80%). Chemically, the carbonation reaction can be summarized as follows [34]:
The thickness of the applied mortar strongly influences the rate of carbonation. For new repair air lime mortars, final carbonation values ranging from 80 to 92% were found [10]. This issue should be considered especially for new bedding mortars and grouts, in which a relevant part of the mortars is confined within the masonry structure. The limited carbonation can negatively affect the durability and performance of these mortars; therefore, the access of CO2 should be guaranteed by an appropriate pore size distribution of the hardened specimens [35].
In the case of the use of dolomitic limes, MgO, periclase, presents a slower rate of hydration as compared with CaO [36]. The control of the process of calcination and slaking is critical [36].
Among the most relevant factors influencing the durability of air lime mortars, pore size distribution and strength of the binding matrix can be quoted [37]. Air lime mortars are liable to suffer damage due to freezing–thawing cycles, a consequence of their porosity, water absorption and friable character of the binding matrix (that is, a matrix with tendency to easily break into small fragments under mechanical actions) [37,38,39]. To overcome these problems, the use of appropriate mixing water/lime and lime/aggregate ratios should be addressed [37], together with a wise choice of the period of the year to apply lime mortars outdoors (avoiding particularly extremely dry and hot climatic conditions, periods of heavy rains or extremely cold, frosty periods) and, above all, an appropriate tailoring of mortars’ pore system (meaning that the pore system can be controlled to a certain extent regulating parameters such as binder/aggregate and water/binder ratios and type and size of the aggregates) [14].
Different approaches have been elaborated to avoid frost damage to lime-based mortars through porosity optimization. One is the use of air entraining agents, as described in [40], possibly replacing the deteriorated part with an air-entrained mortar, to create macro pores or coarse pores that could reduce the risk of frost damage. A second theory is to make the mortar as compact as possible. If the porosity is low and formed in a way that the saturation almost never rises above the critical degree of saturation, frost damage will seldom or never occur. In Sweden there are many remaining medieval renders that have shown an excellent frost resistance: those lime mortars are always compact with a high content of binder and almost no air pores nor capillary pores [41]. The design of air lime mortars for repairing may also include the use of water repellent admixtures to increase their durability [39, 42, 43]. In view of acidic attack due to aggressive pollutants, air lime mortars can exhibit a long-lasting behaviour due to their alkaline character. Providing that the carbonation is not complete, the remaining unreacted Ca(OH)2 protects the mortar against SO2, NOx and other forms of acidic attack [37].
2.2.2 Lime-pozzolan mortars
Pozzolanic reaction consists in a series of chemical processes among the reactive phases of pozzolan, lime and water, leading to a recombination of alumino-silicate material and Ca(OH)2 in aqueous solution to form hydrated reaction products (calcium silicate and calcium aluminate hydrates) with marked binding properties related to their crystalline structure [44]. Pozzolanic reaction processes can be subdivided into three reaction stages (Fig. 2) [45], namely initial dissolution period, induction period and main reaction period.
Scheme of the pozzolanic reaction process: a Initial state (portlandite crystals depicted as hexagonal polygons, pozzolanic particle depicted as a large polygonal shape with non-reactive mineral constituents in black); b Initial dissolution period (C-S–H crystals depicted as grey lines, C-A-H crystals depicted as elongated textured polygons); c Induction period (red dashed arrows indicating water diffusion through osmosis); d Main reaction period
Pozzolanic reaction products are rather similar despite the high variability of raw materials, and phase assemblages are affine to those resulting from hydration of Portland cement. The silicate fraction combines with calcium ions precipitating C–S–H phases, a series of minerals of the tobermorite group with variable Ca/Si ratios, related both to the mix design and the pozzolan’s activity and composition [46]. Furthermore, an increased polymerization of the silicate groups generally occurs upon aging [47, 48], favouring a further increase in strength and durability of the binding mixtures.
On the other hand, alumina should be incorporated within C–S–H, favouring the formation of Al-tobermorite, whose cross-linked structure related to the Al3+ bonding environments is responsible for a relevant increase in strength and cohesion of the binding mixtures, also favouring durability through alkalis sequestration promoted by its marked cation exchange capacity [49]. When not incorporated within the structure of hydrated calcium silicates, alumina may be combined directly with calcium ions to form calcium aluminate hydrates, also defined as AFm phases [50].
In the hardened mortar, in the presence of sulfate ions, precipitation of secondary ettringite and monosulfoaluminate (kuzelite) could occur [51], with detrimental influences in terms of durability related to the triggering of sulphate attack phenomena, possibly leading also to the destruction of hydrated calcium silicates through the formation of expansive thaumasite. Low environmental temperatures may favour this process. Furthermore, C4AH13 undergoes carbonation processes upon air exposure or when abundant carbonate phases are present within the system (either as anthropogenic calcite or calcareous aggregates), transforming into hemicarboaluminate and/or monocarboaluminate [50].
The main factor contributing to the excellent properties of mortars with lime-pozzolanic binders (both from a mechanical perspective when compared with pure air lime mortars, and durability perspective when compared with modern cementitious materials) is their low microporosity. This is mainly due to their slow reaction kinetics, producing limited temperature rise during exothermic reaction processes [52]. Furthermore, they are generally characterized by self-healing properties related to the continuous precipitation of hydrated compounds through delayed dissolution of the pozzolanic matrices in presence of moisture [53], thus considering their permanence in water-saturated condition a positive factor. From a mechanical point of view, such effects are not observable through classic uniaxial compressive and tensile strength tests [53,54,55]. It is instead better assessed through strain-controlled tests, such as the determination of fracture energy values [53].
Adequate water/binder ratio and optimized early curing conditions are fundamental to ensure low total porosity and, therefore also contribute to air lime-pozzolan mortars’ durability [56]. The amount of water must be kept to an optimized value ensuring both a correct stoichiometric amount, and an adequate workability for the final fresh mortar. The correct value is mainly influenced by the type of pozzolan, especially in relation to its specific surface area [57] and further by the substrate and type of application. Furthermore, early curing condition must consider an excess of available water, to be favoured by wetting the mortar surface at least during the first week of maturation, providing at the same time enough moisture for the pozzolanic reaction, and ensuring CO2 transport for further carbonation reaction, whose synergic and competitive effect guarantees an adequate level of early strength in the pozzolanic systems [58]. If insufficient moisture is provided during early curing, the pozzolanic reaction may be heavily slowed down due to lack of reactants, favouring pervasive carbonation of the air lime, and thus irreversibly limiting a further development of pozzolanic phases after subtraction of free lime from the reactive system.
Apart from these main factors, additional parameters influencing both performance and durability of lime-pozzolan mortars also include intrinsic reactivity of both lime and pozzolan, and their proportioning on mortars formulation [59,60,61]. In particular, the percentage of the pozzolanic addition is important, since a too large amount of the additive may lead to an excessive drying shrinkage [62]. Furthermore, mortars application technology plays a fundamental role in the optimal maturation of the fresh mortar [34]. To obtain a thorough assessment of lime-pozzolan mortars performance, these should be tested both in laboratory and onsite conditions [63], with a focus also on long-term performance monitoring, in order to take into account all possible modifications in reactivity and reaction kinetics of such complex composite materials according to the specific environmental factors of each construction site.
2.2.3 NHL mortars
In these mortars, setting and hardening take place via two major processes: hydration of the hydraulic components and carbonation of the Ca(OH)2. On average, repair NHL mortars can achieve ca. 50% of their final strength during the first 28 curing days [64,65,66,67]. The hydration of C2S influences notably the strength development of NHL mortars at long term (6–12 months), together with a significant effect of the portlandite carbonation.
Optimal hardening conditions should allow both hydration and carbonation. The suggested strategy includes a 2-stage process:
-
(a)
To enhance the short-term hydration, which leads to fast setting and hardening of the mortar, high relative humidity conditions are appropriate [6, 7]. Like in cement mortars, prior to the application, the spraying of the substrate with water is recommended, to avoid an intense suction of water, detrimental for the hydration of the hydraulic components [30]. The wetting of the NHL mortars in real applications can be considered as a good practice, to foster the short-term hydration.
-
(b)
To improve the carbonation, curing at medium and long term should combine a certain reduction of the relative humidity (to not hinder the access of CO2) with enough water available to hydrate the remaining anhydrous calcium silicates [27]. RH values of ca. 80% in the inner part of the mortar could be adequate.
In practice the two stages will occur alternating in time; a period where mostly hydration is taking place at moist environmental conditions followed by a period where mostly carbonation is occurring at dry environmental conditions.
A much higher strength (for example, values of compressive strength above 2 MPa) has been reported on NHL mortars cured for 6 months in natural marine environment (humid conditions), close to the Atlantic Ocean, and humid laboratory conditions in comparison to the same mortars cured in laboratory at standard 65% relative humidity conditions (compressive strength values of just 1 MPa) [67]. To explain this fact, the authors indicated that the NHL hydration reaction–and thus the mechanical strength—was fostered due to the high humidity access. From the results of the mineralogical study, the authors explained that the main difference was the slower larnite (the anhydrous calcium silicate Ca2SiO4) hydration kinetics under the standard lab curing conditions (relatively low RH of 65%), which would account for the lower mechanical resistances observed.
Medium and long-term durability are better in NHL mortars than in some air lime-pozzolan mortars because of the large presence of aluminate phases in the later [6]. Calcium aluminate hydrates are not stable in the presence of portlandite, thus leading to a strength decrease and to structural changes that could damage the mortar [68].
Conversely, salt-decay susceptibility has been found to be strongly dependent on the significant presence of fine pores (below 0.01 microns of diameter) [69]. In this case, NHL mortars would be, due to the presence of very fine pores, which are related to the microstructure of the calcium silicate hydrates [70,71,72], prone to deterioration by salt crystallization. Furthermore, the presence of C–S–H in these mortars may induce the formation of thaumasite, under conditions of relatively low temperatures and a source of sulfates. The swelling of thaumasite has been revealed detrimental for the structural integrity of these mortars [73].
3 Lime-based mortars for restoration: potential and limitations in practice
Restoration mortars should be compatible with the pre-existent structures, while being efficient and durable enough to ensure long term stability of the intervention. For conservation interventions in lime-based masonry walls, a limited number of mortar solutions exist, such as: (i) pure air lime mortars; (ii) air lime-pozzolans mortars; (iii) Natural Hydraulic Lime (NHL) mortars. The solutions (i) and (ii) are the most similar to the original ones. Air lime has been used for millennia: in former times more as lime putty and, nowadays, mostly industrially produced as hydrated air lime in powder form. NHL has often proven to be a compatible binder for repair of historic masonry. Lime based mortars have important advantages, although they are also accounted for some drawbacks, in the restoration of historic masonry.
3.1 Air lime mortars
Many ancient constructions, all over the world, are composed of masonry with air lime mortars, thus proving the high durability and fitness for use of this material [74,75,76,77,78,79]. Due to their low modulus of elasticity, air lime mortars can follow the movements of the structures with minor cracking, also ensuring optimal adhesion. Furthermore, the high porosity and water vapour permeability of the mortars allow quick drying of walls, with evaporation of internal moisture to the exterior, thus contributing to a healthy internal environment and to the good condition of the walls. The slow hardening mechanism by carbonation ensures an improvement of performance along time and contributes, together with the self-healing properties, related with dissolution–recrystallization cycles of calcium carbonate, to a high durability, if destructive actions (by nature or by human activity) do not occur (Fig. 3).
In ancient pure air lime mortars the air lime could be applied as a putty, resulting from hydration of the quicklime with an excess of water, or by the hot lime technology when the quicklime, roughly crushed in small particles, was directly mixed with humid sand or humid earth.
Proportions air lime:sand around 1:1 to 1:3 in volume [34], with well graded sand, are adequate for masonry mortars and rendering/plastering mortars, having proved good applicability and performance, as referred in literature. These proportions corresponding to 1:3 to 1:10 in weight for contemporary common materials, although it is possible to find mortars richer in lime in old constructions, especially in important buildings. In some cases, sieved earth, composed by sand, silt and clay, was used as aggregate instead of sand.
Nowadays, air lime mortars are often produced with industrial air lime, slaked with a minimum of water, and commercialised as a powder. This process may ensure better quality control of the lime production and of the lime mortar mixing. However, care should be taken to check that the Ca(OH)2 has not partially carbonated, in contact with air and moisture, before being used. In a putty, the water accumulated above the paste protect it from CO2 contact, avoiding carbonation before use. When the putty is used, little additional water may be needed to achieve adequate workability [80]. However, when applied by a non-specialist worker, the mortars obtained by this process may be more porous and prone to cracking; this is probably due to the use of a higher water content to get a consistency similar to mortars prepared with lime in powder form, as they have a different rheology. The hot lime technique has even more practical difficulties for non-experienced workers: the proportion quick lime:sand is not directly convertible in hydrated lime:sand, and the complete slaking of the quicklime is difficult to be assured before the mortar application [81].
Air lime mortars have an important drawback as they need specific conditions and time for carbonation to take place: they must be protected against heavy rain in the first weeks after application to allow time for carbonation; they should be skilfully applied, and mortars with good workability must be used; however, the mixing water should be controlled to avoid too high porosity and low strength. In fact air lime mortars should be applied with lower flow than hydraulic mortars, requiring skilled workers; humidity must be kept in the range 40–80% Relative Humidity (RH) for a long period, to guarantee a proper carbonation [82, 83]. Especially for parts of the masonry exposed to heavy rain, a substantial leaching of lime may occur during the first weeks after application, when uncarbonated air lime (Ca(OH)2) is partially soluble and does not form a cohesive matrix [84,85,86,87]. The reason is that, at the early stages, although a randomly oriented agglomeration of Ca(OH)2 particles has taken place (reversible agglomeration), the cohesion of the mortar is not strong enough that washing off through rain can be avoided. However, as drying and thus carbonation progresses, two effects will occur to improve the cohesion of the mortar: i) an irreversible agglomeration [88, 89] takes place through epitaxial orientation of Ca(OH)2 particles, leading to strong crystallographic bonding; ii) the solubility of the formed compound (CaCO3) is significantly lower than of Ca(OH)2. This will minimize the leaching of the mortar.
The slow progression of the carbonation process is shown in the following test results from practice: measurements in a render (exterior plaster exposed to the weather) have shown penetration depths of 8 mm in 2 months [90] and 5 mm in 4 weeks [91] obviously, the carbonation rate will decrease with depth.
To spread the carbonation over the depth of a render, a built up in thin coats may be applied. For coats not thicker than 15 mm, a time interval before the application of the next coat should be about 2 weeks (for practice 1 week will be the minimum acceptable time), to allow partial carbonation. It is observed in practice that partial carbonation may be enough to avoid leaching if the rain is not heavy.
It should be noted as well that uncarbonated air lime (Ca(OH)2) is susceptible to frost. From this it can be understood that, in cold regions, application of air lime mortars should be done well before the onset of winter.
3.2 Air lime-pozzolan mortars
In this sub-chapter a general introduction on the potential of lime-pozzolan mortars is given in comparison to lime-mortars. Subsequently it is reported that research and practical experience over the past decades has shown that depending on the type of pozzolan, the composition of the mortar and curing conditions the long-term durability is not always guaranteed. Basic reasons for the sometimes observed substantial strength loss of lime-pozzolan mortars over time are elaborated.
Air lime-pozzolan mortars have a long tradition of application in construction in many places of the world, especially in volcanic regions and, consequently, natural pozzolans derived from pyroclastic products. These materials are nowadays often milled and sieved in fine particles with high specific surface, and they provide hydraulic characteristics to mortars when mixed with air lime (or other sources of calcium hydroxide) in the presence of water. Lime-pozzolan mortars harden not only by carbonation but also by hydration. This also allows to make hydraulic structures, as well as durable buildings in rainy climates or very moist environments. Simultaneously, air lime-pozzolan mortars keep most of the good characteristics of pure air lime mortars, such as low modulus of elasticity and drying ability. Thus, the addition of pozzolans extends the potential field of application of air lime mortars.
Besides natural pozzolans, also artificial pozzolans have been used both in antiquity and in more recent times. Natural pozzolans are mostly used in regions where they exist in nature. In other regions, artificial pozzolans have been or can be applied instead, after checking their suitability. Examples are ceramic powder (from bricks and roof-tiles, for example) [92], metakaolin resulting from thermally treated kaolin [60], ashes of several plants (such as rice husk ash) and some industrial by-products such as silica fume and other reactive industrial residues [93]. Recently, research showed the effectiveness of nanosilica as promoter of pozzolanic reaction [13, 94]. An example of experimental applications of lime-metakaolin mortars is shown in Fig. 4.
3.2.1 Competition between hydration and carbonation
While solving some drawbacks of pure air lime mortars, pozzolanic mortars have important specificities that may jeopardize their good performance: one of the main risks for a durable performance of air lime-pozzolan mortars is the occurrence of the competition between the pozzolanic reaction and carbonation reaction, causing a decrease in strength or even loss of cohesion in the mortar [14].
The pozzolanic reaction of air lime with pozzolans, aiming at the combination of calcium hydroxide with amorphous silica and/or alumina compounds, needs the presence of water for enough time for the reaction to occur. The pozzolanic reaction depends on the composition of the pozzolans (nature and content of active phases), lime-pozzolan/water ratio, specific surface area and curing conditions. Another possible reaction for the calcium hydroxide is carbonation, which occurs with lower needs of water, and is hindered in saturation conditions. This means that pozzolanic reaction and carbonation are competitive reactions [95] and that the existent calcium hydroxide will be preferentially consumed by the reaction that has better conditions to develop faster. In fact, if the material is saturated with water for enough time, the pozzolanic reaction occurs and the free lime is consumed in forming hydraulic compounds. On the other hand, if the RH allows for carbonation to occur, making the pozzolanic reaction more difficult, calcium carbonate is formed instead of hydraulic compounds, and the pozzolan, without possibility to react, acts as a filler. The resulting mortar has a low mechanical strength, due to a low amount of effective binder. An example can be seen in Fig. 5, where several experimental panels of lime-based mortars were applied, including one with lime and metakaolin. In the first months the L/MK panel seemed to perform well, but some years later the result was the one shown in the figure, with severe erosion of the mortar; due to insufficient moisture to ensure a full reaction, part of the metakaolin acted as a filler and the resulting mortar was too weak. The phenomena of insufficient reaction lime/pozzolan due to early consumption of portlandite by carbonation, and of formation, in unfavourable curing conditions, of instable hydraulic compounds that degrade with time are addressed in [94, 95] and more extensively referred in the next section.
3.2.2 Strength loss of lime-pozzolan mortars over time
In practice, as well as in laboratory research, it was observed that some lime-pozzolan mortars may lose strength over time. Loss of strength of pozzolanic mortars over time is a phenomenon which more often occurs and is not limited to the cases treated below, but it does not happen to all pozzolans.
Metakaolin is one of the most used artificial pozzolans in conservation/restoration of lime-based mortars in various countries, due to the availability of the raw material (kaolin), which is burnt at about 600 °C to 900 °C and subsequently ground. Metakaolin is highly reactive with lime, whenever favourable curing conditions are provided for the reaction. In various studies [7, 96, 97] it was observed that lime-metakaolin pastes/mortars may lose strength on the long-term. The main reason put forward [7] is the instability with aging of the calcium aluminate hydrates (MK contains a high quantity of aluminium phases). The unstable hydrates were found by [7] for pastes with MK/Lime ratio between 0.5 and 0.25, cured at 23 °C and 95% RH, after 180 days until 1.5 year: the hydration product, strätlingite (a phase related to strength of the paste/mortar), reacted through carbonation forming a weaker phase, katoite. On the other hand, for MK/Lime much higher than 0.5, it often happens that the reaction of the MK is incomplete [97], due to low calcium hydroxide content, which is spent in the competitive carbonation reaction. As a result, the remaining MK acts as a filler and a low strength mortar/paste results. Thus, the proportion MK/Lime around 0.5 appears, from this study, as the most adequate, although in some cases smaller proportions of MK/Lime present good results (Fig. 4). Additionally, the proportion of total binder (Lime + MK) should also be high by comparison with the aggregate, to ensure good conditions for a complete reaction. [68] verified that the ratio binder: aggregate should be not less than 1:2 (in volume).
Cizer et al. [98] reports the results of a research program investigating ternary mortars containing rice husk ash (RHA), Portland cement (PC) and air lime as binders.
For two mortars where the binder part consists of 10% of Portland cement (mortar compositions PC:lime:RHA:sand of 1: 2: 7:30 (binder parts 1 + 2 + 7 = 10; So, PC-part equals 10% of the binder) and 1:4: 5:30 (binder parts 1 + 4 + 5 = 10; So, PC-part equals 10% of the binder) and cured under 20 °C, 60% RH) from 30 to 60 days the compressive and flexural strength of the mortars started to decrease.
From scanning electron microscopy with X-ray microanalysis (SEM/EDS) it was inferred that calcium carbonate crystals had a destructive effect on the initially hydrated cement phases, causing negative effects on the mechanical properties.
As a conclusion, lime-pozzolan mortars could give good response to restoration situations, being at the same time, compatible with historic structures, resistant to water and durable. However, to guarantee good results, it is important to characterize the pozzolan to use, to optimise the mix to prepare and to take into account the application and the weathering conditions.
3.3 Natural hydraulic lime (NHL) mortars
For low strength repair mortars, Natural Hydraulic Lime (NHL) turned out to be a useful relatively modern alternative as binder for restoration, with good compatibility with existing historic masonry. There is a relatively high variability in the properties of the raw material of NHL (natural material). In the standard EN 459 [15] three classes (NHL2, NHL3.5 and NHL5) are distinguished with large ranges for the compressive strength after 28 days. The produced NHL should be classified according to this standard.
NHL contains as hydrating phase predominantly dicalcium silicate (C2S), and calcium hydroxide as carbonating phase. Characteristic for NHL mortars (like for the lime-pozzolan mortars), compared to the modern cement-lime mortars, is the slow strength development. The reason for NHL is the slow hydration reaction of dicalcium silicate (C2S).
To give an idea about the strength development over time of NHL mortars and to compare them with some standard lime-cement mortars, the results of a test series carried out over a period of 2 years are presented in Fig. 6. The curing conditions were 20 °C and RH 90%, i.e., favourable hydration conditions. It can be deduced that the NHL mortars reach their final strength values after 0.5–1 year (depending on the type of NHL). For lime-cement mortar (with quick-hardening Portland cement) about 90% of the end values are reached after 1 month.
Slow strength development for the NHL mortars and quick hardening of the cement-lime mortars: NHL mortars, 1:2.5 (NHL: sand) in vol.; cement-lime mortars e.g. 1:1:6 (cement: lime: sand), in vol.; curing conditions 20 °C and RH 90% [99]
The slow strength development and the influence of the curing conditions has been demonstrated also by [67]. They found an increase of compressive strength on NHL3.5 mortars from 28 to 90 days, (relative quick strength development compared with Fig. 6) than followed by an almost stabilization up to 180 days.
Humid curing and maritime curing conditions improve compressive strength in comparison to 65% RH curing. This is logical as the hydrates in the binder are largely responsible for the final compressive strength.
In general it should be noted that NHL being a natural product is showing differences in strength development depending on the basic constituents and the production process of the material.
3.4 Some comparative quantification
In this sub-chapter attention is paid to strength values of lime-pozzolan mortars in the context of the effects of a variety of parameters which play a role with regard to strength development and long-term strength. As parameters are treated curing conditions, differences in reactivity of pozzolans,, effects of proportioning of mortar components, comparison of lime-pozzolan mortars with mortars containing other binders, fineness of the pozzolan.
3.4.1 Strength development of lime-natural pozzolan mortars
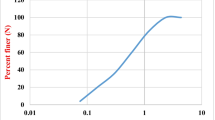
A highly reactive natural pozzolan of volcanic origin develops significant strength even at 28 days in comparison to corresponding pure lime mixtures, depending on the fineness of the grinding (Fig. 7). This pozzolan is available at the market and exhibits a high content in acid soluble silica (45–50%) and amorphous content (60–70% by XRPD patterns). The material (P) was ground at three levels of fineness and tested by preparing mortar mixtures with lime and siliceous sand 0–4 mm (L:P 1:1, B/A 1:3 by mass). The water added responded to a flow of 15 cm (according to EN 1015-3). Curing conditions were the following: moist curing > 90% RH for P1, P2, P3 mortars and dry curing 60–65%RH for L mortar [100].
Furthermore, as previously mentioned, moist curing conditions are essential for pozzolanic reaction, as showed in Fig. 8. Volcanic natural pozzolan (after grinding to 10% residue on 45 μm sieve) was used with lime powder (CL90) for the manufacture of the mortar, together with a standard CEN sand (B/A ratio 1:3 by mass). The water content was adjusted to have 14.5 cm flow (measured according to EN 1015-3).
3.4.2 Effects of curing conditions on the evolution of flexural strength over time
Research by Cizer et al. [58] on the strength development through hydration and carbonation of various lime-based mortars under different curing conditions and periods, provides useful comparative information (Fig. 9). Lime-Metakaolin, Lime-Rhenish trass, and two types of Natural Hydraulic Lime were chosen as binders. Binder/aggregate mass ratio was 1:4. The curing conditions were: dry curing at 20 °C and 60% RH, moist curing at 20 °C and 93% RH.
Flexural strengths values for Lime-Metakaolin (L-MK), Lime-Rhenish trass (L-RT), and two types of Natural Hydraulic Lime (HL1 and HL2) mortars, for 2 curing conditions: dry curing and moist curing (adapted from [58])
One important conclusion was that the flexural strength at 180 days for moist curing was for all the mortars double of that for dry curing (20 °C, 60% RH). In fact, moisture, especially for the pozzolanic reaction, is a requisite for a satisfactory strength development.
Although the Lime-metakaolin mortar shows a significant loss of strength over time under moist curing (flexural strength of 2.00 N/mm2 at 28 days dropped to 1.25 N/mm2 after 180 days), the end value is still double of the dry curing of L-MK: 0.60 N/mm2 and comparable with moist-cured lime-Rhenish trass: 1.30 N/mm2.
The 2 types of NHL showed significant differences in strength development, especially under moist curing.
3.4.3 Effects of proportioning of mortar components on the evolution of strength over time
Another study on lime-metakaolin mortars cured under moist conditions [68] shows the influence of the ratio binder/aggregate (b/a) and also the lime/metakaolin (L/MK) proportion, as represented in Fig. 10.
Flexural a) and compressive b) strengths of air lime-metakaolin mortars with ratio b/a 1:1, 1:2 and 1:3 (wt) and ratio MK/L 0, 0.3 and 0.5 (adapted from [68])
A volumetric ratio binder:aggregate around 1:1 and proportion MK/L of about 0.5 seem to favour the hydraulic reaction. However, mortars with such high binder proportion are difficult to apply due to high shrinkage and consequent cracking, besides presenting in general excessively high mechanical strength for conservation. Therefore, according to this study, the ratio 1:2 binder:aggregate could be a good compromise, with adequate mechanical characteristics.
Generalizing the aforementioned research means for practice that volumetric proportions around 1:0.5:3 (air lime:MK:sand) may be adequate, together with high humidity for the first two weeks and in some periods along the render life period. It should however be noticed that this is still a mix rather rich in binder and therefore tends to be susceptible to cracking due to shrinkage, so a careful application must be carried out.
3.4.4 A comparison of strength development over time between mortars of pure lime, lime: pozzolan, NHL 3.5 and lime:pozzolan:cement systems
Ground volcanic pozzolan (10% residue on 45 μm) was used as a component of binding system of mortar mixtures with siliceous sand 0–8 mm, B/A 1:2.5. The water content corresponded to flow 15 cm (flow table). The curing regime prescribed in [101] for each type of binder was kept for 90 days and then specimens were placed inside up to testing. Curing conditions were: for composition 1 dry curing 60–65%RH, for compositions 2,3,4,5 moist curing > 95% RH [102].
It seems that long term compressive strength for mixtures of lime with hydraulic components is substantially higher (8–12.5 MPa) than 28-d compressive strength ranged from (3.8 to 5.8 MPa) respectively. The higher values exhibited by ternary binding system in which cement was 15% of the binders. The inclusion of coarse aggregates seems to be positive to strength development (Fig. 11).
3.4.5 Evolution of compressive strength and porosity of lime-reactive natural pozzolan repair mortars over time
The long-term strength and porosity of lime-pozzolan repair mortars with different binder/aggregate ratio (R1 1:1, R3 1:3) has been studied by testing mechanical characteristics at 3, 12, 24 and 60 months [103]. The results were compared with pure lime (CL 90) mortars of comparable ratio. Indicative results of compressive strength are reported in Fig. 12. It seems that strength evolution is in general stabilized at the age of 12 months. The slight differences in strength with age (up or down) are related rather with microcracking due to drying shrinkage of specimens.
a Evolution of compressive strength (-s) and porosity (-p) in mortars of binder/aggregate ratio 1/1(R1); b Evolution of compressive strength (-s) and porosity (-p) in mortars of binder/aggregate ratio 1/3(R3). L: Hydrated lime CL90, P: Lime- natural ground pozzolan (1:1) (by mass), B: Lime:pozzolan:brick dust (1:0.5:0.5)
More even is the strength evolution in mortars with binder/aggregate ratio R1 1:1. The two lime-pozzolan compositions consisted of ground natural pozzolan of volcanic origin, which has been properly and thoroughly tested before its use. Specimens were cured for 3 months according to [101] for each type of binder: moist curing at > 95%RH for lime pozzolan combinations, dry curing at 60–65%RH and subsequent permanence in room conditions for pure lime.
Additionally, it could be said that the rate of strength development for the referred binders is higher from 28 to 90 days than for the period 90 days–1 or more years, where microcracks induced by drying shrinkage are more obvious.
Furthermore, to meet compatibility requirements, high compressive strength (i.e. > 6–7 MPa) is not often desirable. Therefore, trial mixes of compositions at lab and on site must be made.
3.5 Practical considerations
3.5.1 General
Considering the research results described before, some aspects must be taken into account in practice, to avoid bad results. As an example, in conditions of relatively dry weather, with wind accelerating drying, a mortar prepared with a mix of air-lime and pozzolan should not be used for laying of masonry or as a render/plaster, as it could result in a poor and friable mortar, subjected to easy erosion, with a matrix mainly of calcium carbonate, the pozzolanic material acting as a filler.
Practice and research [104, 105] have shown that hydration of a pozzolan may as well occur under relatively dry curing conditions if the pozzolan is a reactive one (e.g. pozzolan from Santorini, or metakaolin). This means that mortars may perform well after initial moist curing during 2 weeks upon application, followed by curing under relatively dry climatic conditions.
Under the dry conditions mentioned, an option could be to provide moistening for a relatively long period of time (but even that could be insufficient–see [97]–due to deterioration of the hydraulic compounds formed), or simply make the option to use pure air lime mortar, which performs well in a relatively dry environment. In a wall with capillary rising water, or in a very humid climate with frequent rain, the lime-pozzolan mortar may be a particularly good solution, while air lime mortars would not perform well, remaining uncarbonated for a long time and with risks of partial solubilization of calcium hydroxide. To ensure good conditions for hydraulic mortars based on air lime and pozzolan, besides keeping high RH for a long period, the need for high proportions of lime (lime/MK = 2 and (Lime + MK)/aggregate = 2, in volume) has been verified, for both carbonation and hydration.
[104] suggests (under lab conditions) a period of at least 28 days of moist curing conditions for adequate hydration reactions and sufficient strength development.
In practice, in case of the application of lime-pozzolan mortars in renders and repointing mortars a period of a week is often the maximum of time available for moist curing, depending on climatic conditions of each area. This means that complete hydration and consequently maximal strength development will not occur.
For strength development of NHL mortars especially hydration of the dicalcium silicate is needed; this means that humid curing conditions over a period directly upon application is basic for a good start of the hydration process.
Given the slow strength development, in cold climates, rendering and (re)pointing should be done well before or after freezing weather. In practice it is often not clear when a NHL (or lime-pozzolana) mortar has sufficient strength to overcome a first freeze–thaw period, although wetting of the masonry after brick laying for a longer period of time has been carried out. To avoid eventual frost damage, Portland cement or blast furnace cement may be added to the NHL to obtain sufficient early strength (ternary mortar). Blast furnace cement also improves the resistance to sulfate salts and seawater in the mortar [106, 107].
Comparing NHL mortars with lime-pozzolan mortars, an important difference is that in NHL mortars some part of calcium is incorporated in the dicalcium silicate structure and the other is available as free lime, while in lime-pozzolan mortars lime and pozzolan are separate phases. As in NHL mortars the hydraulic components are present from the beginning, their hydration will not be endangered by a premature carbonation.
Field observations on the hardening of repointing mortar has shown that moisture supply over a long period of time determines the depth of the occurring hydration products in a joint. This indicates that the hydraulic hardening process of NHL is in fact a process of ‘hydraulic penetration’ into the (depth of) the repointing; this contrasts with modern cement binders where the hydraulic hardening is an overall process in the mix. Consequently, this ‘hydraulic penetration’ is analogue in terms of a penetration process, to the carbonation process of air-hardening.
From this it can as well be understood that the degree of hydration, and the hydration penetration depth of NHL repointing, is depending on the orientation of a facade in relation to the preferential rain direction, in case that moist curing is not provided.
In practice, it is demonstrated that NHL mortars show a low risk of lime leaching [40]. Several possible reasons can be provided: the main cause for this appears to be that during hydration C2S produces 3.2 times less Ca(OH)2 than C3S, the main calcium silicate of cement. It can be assumed that Ca(OH)2 from the hydration reaction is an effective staining source as it is released as ions: the finest particles possible to leach out. Moreover, air lime present in NHL-binder is rather coarse: comparison of specific surface area of NHL (± 10 000 cm2/g) with modern air-limes (up to 50 000 cm2/g); consequently, this as well is a reason why low leaching risk is to be expected from a NHL-binder.
3.5.2 Experience from practice
In Greece, local natural pozzolan is used in repairing Historical Structures from the 1990’s. Their application is accompanied by technical specifications concerning characteristics of pozzolan and other constituents as well as instructions for good application curing conditions. For example, pozzolan is prescribed with pozzolanic reactivity with lime, fineness, content in soluble salts while for aggregates granulometric gradation, soluble salt content are also prescribed.
In the case of repair works of the Galerius Palace in Thessaloniki, most of the interventions with lime-natural pozzolan mortars took place in 1997 and 2000.
In 2018, ten samples of repair mortars (bedding and plasters) were taken and thoroughly analysed by determining porosity, specific gravity, compressive strength, soluble salt content, mineralogical composition and microscopical characteristics. The intention was to evaluate the behaviour of repair mortars which have been designed and proposed by Lab. of Building Materials 15–20 years before (see Fig. 13) after the analysis of a great number of old mortar samples (about 60) to understand compatibility requirements.
The mineralogical analysis of repair mortars showed the existence of hydrates (calcium silicate and calcium aluminates) and carbonates. The microcracking was limited and mortars remained sound. Compressive strength ranged from 2.00 to 5.40 MPa. The 28d strength of trial laboratory mixes (15–20 years before) ranged from 2.5 to 4.5 MPa (moist curing conditions) and porosity from 26 to 30%, while specific gravity ranged from 1.57 to 1.80 [108].
In general, it could be said that the use of the lime-natural pozzolan binding system was successful.
4 Damage to lime-based repair mortars
Summarizing the previous paragraphs, the following risks and or drawbacks of lime-based mortars can be listed.
Air lime mortars:
-
Prone to cracking, due to drying shrinkage; especially at risk are putty lime mortars, when unskilled professionals would add too much water in order to achieve a consistency similar to the one of cement mortars;
-
Lime leaching can occur in an early stage (first weeks), when carbonation is still insufficient and the water retention in the mixture is low;
-
Uncarbonated lime is susceptible to frost, which is to be avoided by seasonal application and protection of fresh masonry and render/plaster against low temperatures;
-
Frost damage may occur related to an unfavorable pore size distribution, which can however be avoided in different ways.
Lime-pozzolan mortars:
-
The competition between pozzolanic and carbonation reaction may, in case carbonation is prevailing, due to too low availability of water, lead to insufficient strength development and a weak mortar;
-
Some lime pozzolan combinations may show a loss of strength on the long term, although remaining considerably stronger than a comparable pure air lime mortar;
-
Shrinkage cracking may occur, mainly due to high binder-aggregate ratios;
-
The presence of sulfate in surrounding materials can lead to the formation of secondary ettringite, kuzelite or thaumasite, and hence to swelling.
NHL mortars:
-
The fine porosity, that is inherent to these mortars, may favor salt crystallization damage;
-
In the presence of sulfate, and mainly at lower temperatures, thaumasite may form, giving rise to cracks or deformation.
Some of the here mentioned risks will be described hereafter.
Amongst early problems that may arise, cracking of mortars is certainly important (Fig. 14). Shrinkage cracks can occur in all types of mortars and can be attributed to:
-
Excessive binder content;
-
Poorly optimized grain size distribution of aggregate;
-
Inadequate curing.
Although an important role of sand is to restrict cracking tendency and increase the mass stability [109], the phenomenon cannot completely be avoided, causing different problems as recorded through microscopic analysis under different magnification (Fig. 15). Cracks of different geometry are formed either within the binder or in the binder-aggregate interface when there are favorable conditions.
Early shrinkage cracks, formed during the setting and hardening of mortars, are caused by the drying of the mortar. Drying can occur both through evaporation from the surface and water absorption by the substrate [110].
However, a great advantage of lime mortars consists in the fact that early shrinkage cracks can still be taken care of by simply re-elaborating the surface and closing the cracks. This advantage is related to the slow hardening of lime mortars. Therefore, re-elaboration of lime renders and plasters should be included in lime mortar works.
Also lime leaching can be an important issue in the early life of lime mortars; this phenomenon is perhaps less evident in renders, but it can be important in the case of pointing mortars, due to the resulting esthetical degradation of the masonry. This is illustrated by Fig. 16.
This negative effect can be limited by protecting the fresh (uncarbonated) mortar against moisture sources like driving rain or percolating water.
Another problem that may occur in fresh lime mortars is frost damage, when frost occurs at the moment the mortar is still weak and only insufficiently carbonated. It is wise to avoid the application of lime mortars in the period from late autumn to early spring. Also in this case, protection (against too much wetting) may be a measure to be taken.
Many damages have to do with moisture, eventually combined with frost or salts. Interesting is that both frost (see for example [111]) and salt damage may look alike. This is illustrated by Fig. 17.
Typical forms of layering in renders and plasters. Separate render layers coming off due to freezing temperatures in a saturated and porous render (a), [110]; similar damage, due to salt crystallization in a plaster, applied on a wall with capillary rising damp (b)
The cause of the damage may sometimes be deducted from the environmental conditions, but in many cases, it will be necessary to perform additional investigations, including (little) destructive sampling to assess moisture and salt content and distribution over height and depth.
Hereafter several cases of damage from practice will be described in more detail.
4.1 Weak trass-lime repointing mortars
In lime-pozzolana mortars the competition between pozzolanic and carbonation reaction may lead to insufficient strength, in case carbonation is prevailing due to too low availability of water.
In Fig. 18 damage to the repointing of a church tower is shown. The mortar is a trass-lime mortar. The damage occurred within a few years after restoration. There was a severe loss of material; the damage type can be described as loss of coherence and sanding of the mortar.
Further investigations and tests concerned the exact composition of the mortar, its porosity, and the degree of carbonation. Petrography on thin sections and wet chemical analysis were performed.
The pointing mortar had a composition of 1:1:6 (trass:lime:sand) by volume.
It was further found that the lime had carbonated almost completely and over the full depth of the pointing. However, hardly any reaction with the trass had taken place.
Trass is a pozzolanic material and is only reactive in the presence of Ca(OH)2 and water. A quick drying of this type of mortar should be avoided. If this essential curing condition is not considered, a low cohesion and consequently a low durability will result.
In this case indeed the lime reacted only with carbon dioxide from the air. Lime converted into calcium carbonate is no longer available for the trass-lime reaction. As the trass did not react, it acts just as a filler and a very weak mortar results.
4.2 Sulfate damage of NHL bedding mortars
Salts, such as sulfates present in the masonry, may induce damage to mortars. A severe situation occurs when a reaction can take place between the salt and components of the mortar. For example, in case of hydraulic lime or trass lime mortars in the presence of sulfate, thaumasite and ettringite may be formed [112, 113].
The expansive reaction in the mortar may lead to different, often severe forms of damage to the masonry. The damage type depends, among others, on the location in the wall section where the expansive reaction takes place; especially when the process takes place deeper in the section of the wall, while the mortar near the surface is not affected, the resulting damage is at the first sight difficult to understand (Fig. 19). It can, for example, consist of cracks in several directions or lead to spalling of the masonry surface.
The sulfate may derive from the atmosphere (SO2) or from the brick (due to low firing of sulfate-containing clays). Thaumasite (CaCO3.CaSiO3.CaSO4.15H2O) is a swelling compound that can be formed due to a reaction of mortar components with calcium sulfate and water. The conditions for this reaction are a high sulfate content together with a high moisture content.
Severe sulfate damage can be also observed in the case of metakaolin-air lime mortars: the presence of aluminum-containing phases is detrimental for the endurance against sulfate attack as they promote the formation of expansive compounds [114]. If Mg2+ ions are also present, the leaching of C–S–H phases occur, and thus the mortar loses structural integrity [115].
In order for the described degradation process to occur, the mortar needs to contain calcium carbonate and calcium (mono)silicate. High water content is necessary for the reaction to take place and also serves for the transport of sulfate. Figure 20 shows the result of a test in laboratory, illustrating the effect of thaumasite formation [73].
Mortar prisms after 15 weeks in sulfate solution. Natural Hydraulic Lime based (NHL) specimens with clear expansion and deformation and Hydrated lime based (CL) without any expansion, from [72]. HL = natural hydraulic lime (St Astier), to the left; L = hydrated lime based, to the right; picture on the right = detail HL
In practice situations, it is therefore advisable to first try to take away at least one of the essential conditions, c.q. one of the sources, such as moisture or sulfate.
4.3 Sulfate damage in NHL-repointing mortars
In 2012 a church tower was repointed with a natural hydraulic lime mortar (composition: NHL3.5-Sand ratio, 1:2.5, by vol.).
During an inspection in 2013 a beginning of damage was observed of the repointing mortar cast in between underfired red clay bricks. Between 2013 to 2016 this type of damage spread in the repointing mortar over the south facade of the tower, always between underfired red clay bricks (underfired bricks may, depending on the composition of the clay, contain a high amount of sulfate, as these bricks are not fired at sufficiently high temperatures, for evaporation of sulfates to take place). This type of bricks was only found in the south facade; on the other orientations, this brick type is not present, and no damage could be observed. In several studies in Sweden, it was found that the sulfate damage is expected in south and west facades to a much larger extent than in east or north facades, if sulfates are occurring on all sides of a building. Both the main rain and wind orientation and the sun play an important role: wetting–drying cycling and with this recrystallisation-deliquescence cycling are under these conditions more frequent on south and west facades [116].
It may be assumed that the damage is a form of sulfate attack. From another case, gypsum and syngenite (a double sulfate of sodium and potassium) were found after sulfate attack in NHL mortars (exposed in a SO2 chamber at high relative humidity, [37, 117]). Efflorescences, detachments and similar problems were identified, as in the first described case.
The following advice was given: i) to replace the underfired bricks by well fired ones; ii) if no immediate funds would have been available for a replacement, to repoint the damaged joints with mortar containing (partly) a salt resistant binder, and consider to replace the underfired bricks after 5 years (Fig. 21).
The used mortar composition was: BFS cement-NHL 3.5-Sand in a ratio 1:3:10, by volume. This choice showed to be successful, as showed in Fig. 22.
4.4 Salt damage to render, after repair
Air lime mortars may degrade with time, due to several actions and need repair. The general idea could be to repair with mortars of lower permeability and higher strength, to provide better protection of masonry. However, incompatibility is a major cause of degradation and the use of mortars apparently with “better” characteristics may accelerate degradation instead of avoiding it.
The present case study illustrates accelerated damage by capillary rising moisture and salts crystallization in an old wall, increased by the application of a cement render.
In the presence of moisture and salts in a substrate, repair mortars, depending on their composition, can sometimes worsen an existing degradation process.
On the walls of an eighteenth century historic building, local restoration works were carried out, at the end of the nineteenth century and during the twentieth century. During these interventions, the old renders were kept, although with local, very distinguishable repairs (Figs. 23, 24).
The original render consists of two layers (Fig. 25): a base coat of 1.5–2.5 cm thick, composed of air lime and siliceous sand with a small amount of clay, in the proportion 1:5 to 1:5.5 (lime:sand, in wt); and a finishing coat 0.5–1.0 cm thick, of similar composition, but without clay, showing local repairs. In the East façade, which is the most degraded, the lower part of the render is partially covered with a thin repair coat of a cementitious mortar (Fig. 26).
The main types of degradation observed on the most degraded façade consist of crumbling and loss of adhesion of the render, in a zone of the wall in between 1.40 and 1.80 m from the soil.
The measurements of humidity show that between 1.40 and 1.80 m, the moisture content is clearly higher than in other zones without anomalies.
Presence of salts (chlorides and sulfates) was detected in large amounts in the affected zones. The origin of the salts may be in the use of cementitious repair mortars next to salty water from the soil, related to the nearby Ocean.
The degradation is most probably caused by the combination of capillary water from the soil, together with the use of incompatible cementitious render coats on the façade, most probably even re-applied after the damage first occurred.
The damage results from introducing (more) soluble salts and reducing the evaporation through the render, leading to a higher drying front. Salt crystallization in and behind the render consequently leads to loss of adhesion and crumbling of the render.
4.5 Liesegang phenomenon in the carbonation process of lime mortars
The texture presented in detail in Fig. 27b, on a rendered wall of a historic building from the XVI century, located near Lisbon (Valflores Palace, Fig. 27a), is quite common on old lime renders and can be considered an esthetical damage.
A sample collected of the damaged render facing East even shows a deep circular erosion following the banded pattern (Fig. 28).
This common phenomenon is explained by [118], who states it can occur both in high- and low-quality air lime renders, exposed to different environmental conditions. The occurrence of these irregular circular patterns is probably due to the presence of a drying process that superimposes on the normal diffusion process along the carbonation front. Balksten and Strandberg-de Bruijn [116] suggest that the Liesegang phenomenon is a leading mechanism in air lime mortar carbonation: the reaction of CO2 dissolved in water with Ca(OH)2 in the uncarbonated mortar, produces amorphous CaCO3 (ACC), an unstable product that will convert to calcite, the most stable polymorph of CaCO3. Therefore, the damage can be seen as showing a partially very well carbonated render, that should be repaired but not replaced.
4.6 Aesthetic damage by efflorescence on brickwork repaired with lime-based mortars
The Palace of Galerius complex (4th Century AD) covers an area of 8000 m2 in the center of Thessaloniki, Greece, and consists of masonry ruins that suffered from severe deterioration due to weathering (due to pollutants and biological agents) and previous interventions (1970’s) based on cement. The masonries have been built either with alternating courses of stone (gneiss) and brickwork or totally in brickwork (Fig. 29).
The characteristics of old bricks and mortars were determined. The compressive strength of the bricks ranged from 10 to 20 MPa and their capillary water absorption was between 15.3 and 16.3 wt%. Some samples showed a high salt content, mostly nitrates and sulfates. Two types of bedding mortar were recognized, containing coarse aggregates up to 16 mm.
Both types of mortar were based on lime and clayish material with pozzolanic properties. The proportion of the binder varied from place to place. Type A prevailed. In Type B (showing a reddish hue) brick dust was used in the binding system and crushed brick as aggregate. For type A, the compressive strength ranged from 2.5 to 4.0 MPa and the porosity from 29 to 38%. For type B, the compressive strength was 1.0–5.0 MPa and the porosity 15–22%. A number of samples showed a high content in soluble salts; in several locations this concerned a mix of different salts. The content of Cl− ranged from 0.63% to 1.80% wt. of binder, of NO3− from 1.72% to 5.5% wt. of binder and SO42− from 1.37% to 4.39% wt. of binder.
The high salt concentration was related to the cistern and baths operation and the use of the area for refugee accommodation in the past.
Taking into account the situation, the proposed repair materials (bricks and mortars) were checked to contain the lowest possible salt content. The proposed compositions of bedding mortars were based on ternary systems of lime-putty—ground pozzolan and white cement.
In 2018 a survey of repaired masonries of the central buildings of Galerius Palace was made to assess damages occurring from the period of the main restoration project (2000–2006) up to 2019. Generally, masonries seemed to be in a very good state of conservation.
Some deterioration phenomena were encountered in specific places such as microcracks near vertical joints, discoloration of facades due to oxidation products of gneiss stones and biological growth and efflorescence, near to the ground level (Figs. 30 and 31).
The analysis concerned: Differential Thermal Analysis (DTA), determination of porosity, absorption, compressive strength, soluble salt content and microscopic analysis. Indicative values are shown in Table 3.
The salts were mostly concentrated on bricks as white powder and consisted mainly of sulfates.
At some places of the Galerius Palace complex the thick masonry walls as well as the foundation soil seem to have been contaminated with salts. It is positive that repair mortars have a high porosity and efflorescence could occur without disintegrating the mortars.
4.7 Leaching and encrustation
Leaching of lime is a phenomenon that may cause considerable esthetical damage to repaired sections in historic masonry. The example described, shows that it is not always easy to avoid this problem.
Figure 32 shows a severe encrustation at the battlements of a motte castle in Leiden, Netherlands.
The mortar, probably dating back from a previous restoration at the end of the nineteenth century showed a strong leaching and encrustation. The mortar is lime-based and contains both Portland cement and trass. Most probably this damage is closely related to the occurrence of frost damage to the mortar in the upper parts of the battlements; the weakening by frost action made the mortar prone to leaching. When at the beginning of the twenty-first century a (new) restoration of the battlements had become necessary, it was decided to repair and reconstruct also parts of the surrounding masonry. However, even after re-building parts of the masonry, using a mortar intended to avoid leaching, by adding a pozzolan, the phenomenon re-appeared in exposed places, like the part shown in Fig. 33: water can penetrate via the horizontal wall walk. The mortar composition used, was 0.5:2.5:2.5:12 (CEM I:lime:trass:sand by vol.).
Although it is generally assumed that addition of a pozzolan could avoid leaching, either the quantity of pozzolan or the reaction conditions were not sufficient to obtain this goal.
Apart from avoiding water percolation through fresh masonry it is important to use the right mortar mix and take well care of the necessary execution conditions. This means to use a mortar containing a (highly) reactive pozzolan, in order to bind the calcium hydroxide from the binder (both from lime and from cement hydration); further, for the pozzolanic reaction to occur, a good curing is necessary (keep masonry wet for the first 15 days) [119].
5 Conclusions
For the repair of historic masonry, lime-based mortars such as pure air lime mortars, lime-pozzolan mortars, natural hydraulic lime mortars and ternary mortars are often used. The main reason is that their hygric and mechanical (strength and ductility) performance are easy to adapt to most of the existing historic masonry (compatibility requirements).
Although the basic appropriateness of lime-based mortars for restoration is undisputed, there are also some drawbacks in the application of these mortars, that should be taken into account in order to prevent problems.
Research efforts and practical experience have shown that for an optimal use of the lime-based mortars the following points of attention should be considered:
-
The strength development of lime-based mortars is slow, which may entail vulnerability of the young mortar (e.g. risk of frost damage, lime leaching); an advantage is the easy treatment of early shrinkage cracks;
-
For optimal strength development of lime-pozzolan and NHL-mortars, moist curing conditions directly upon application of the mortar are required for a longer period of time depending on the environmental conditions, enabling the formation of hydrated phases (in practice the curing period under humid conditions is often not long enough to obtain a complete hydration);
-
Competition between hydration and carbonation may impair the strength of lime-pozzolan mortars (in this field there still is insufficient knowledge on the importance of this aspect);
-
The negative influence of continuous dry conditions on the durability of lime-pozzolan mortars may be significant and still no adequate test is available to judge and predict this behavior;
-
As many pozzolans are natural materials, properties and performance may vary significantly from type to type. So, it is important to perform a thorough characterization of the pozzolans before using them in a repair mortar;
-
The properties of the various NHL types are less varying than for natural pozzolans through production process and classification by existing standards;
-
Under specific conditions expansive and soluble salts and frost may cause damage to lime-based mortars; before application moisture and salt content of the masonry should best be investigated and mortar mix could be adapted accordingly;
-
Ternary binding systems may solve several of the drawbacks mentioned above (e.g. accelerating early strength development, preventing hydration-carbonation competition effects), provided that they are compatible with the pre-existing materials;
Most of the drawbacks and points of attention mentioned, can be addressed through a thorough evaluation of the potential of lime-based mortars in relation to the expected exposition conditions and by testing their suitability, preferably using test panels in practice. Further, adverse application conditions should be avoided.
References
Papayianni I, Papadimitriou D (2019) Lime based Mortars–Relationships between composition parameters and mechanical strength. In: Álvarez, J.I., Fernández, J.M., Navarro, I. et al (eds.) Proceedings of the 5th Historic Mortars Conference. Paris, France, RILEM Publications SARL, pp 1316–1324
Schueremans L, Cizer Ö, Janssens E, Serré G, Balen V, Koenraad (2011) Characterization of repair mortars for the assessment of their compatibility in restoration projects: research and practice. Constr Build Mater 25(12):4338–4350
Groot C, Ashall G, Hughes J (2004) Characterisation of old mortars with respect to their repair - final report of RILEM TC 167-COM. RILEM, Paris. ISBN: 978–2–912143–56–3, e-ISBN: 2912143675
Maurenbrecher P, Groot C (2016) Repair mortars for historic masonry-state-of-the-art report of RILEM technical committee TC 203-RHM. e-ISBN: 978–2–35158–163–6
Arizzi A, Cultrone G (2014) The water transfer properties and drying shrinkage of aerial lime-based mortars: an assessment of their quality as repair rendering materials. Environ Earth Sci 71:1699–1710
Gameiro A, Santos Silva A, Veiga R et al (2013) Phase and microstructural characterization of Lime-MK blended mixes. Mater Sci Forum 730–732:135–140
Santos Silva A, Gameiro A, Grilo J et al (2014) Long-term behavior of lime-metakaolin pastes at ambient temperature. Appl Clay Sci 88–89:49–55
Navrátilová E, Rovnaníková P (2016) Pozzolanic properties of brick powders and their effect on the properties of modified lime mortars. Constr Build Mater 120:530–539
Van Balen K (1991) Karbonatatie van kalkmortel en haar invloed op historische strukturen (Carbonation of lime mortars and its influence on historical structures). Dissertation, Katholieke Universiteit Leuven
Lawrence RMH, Mays TJ, Walker P et al (2006) Determination of carbonation profiles in non-hydraulic lime mortars using thermogravimetric analysis. Thermochim Acta 444:179–189
Duran A, Navarro-Blasco I, Fernández JM et al (2014) Long-term mechanical resistance and durability of air lime mortars with large additions of nanosilica. Constr Build Mater 58:147–158
Ergenç D, Fort R (2018) Accelerating carbonation in lime-based mortar in high CO2 environments. Constr Build Mater 188:314–325
Stefanidou M, Tsardaka EC, Pavlidou E (2017) Influence of nano-silica and nano-alumina in lime-pozzolan and lime-metakaolin binders. Mater Today Proc 4:6908–6922
Alvarez JI, Veiga R, Martínez-Ramírez S et al (2021) RILEM TC 277-LHS report: a review on the mechanisms of setting and hardening of lime-based binding systems. Mater Struct 54:63
EN 459–1 (2015) Building lime - Part 1: Definitions, specifications and conformity criteria
Papayianni I (2009) Design and manufacture of repair mortars for interventions on monuments and historical buildings. In: Groot, C. (ed.) Proceedings of RILEM International Workshop Repair Mortars for Historic Masonry. Paris, France, RILEM Publications SARL, pp 292–304
Malhotra VM, Mehta PK (1996) Pozzolanic and cementitious materials. Gordon and Breach Science Publishers, London, UK
Massazza F (2006) Pozzolana and pozzolanic cements. Chapter 10. In: Hewlett PC (ed) Lea’s chemistry of cement and concrete, 4th edn. Elsevier, Amsterdam, Netherlands, pp 471–635
Ramenzanianpour AA, Kazemian N, Sedighi S et al (2019) Study of durability of mortars with natural pozzolans under carbonation. J New Approach Civil Eng 3(3):63–75
Nijland TG, Larbi JA, de Rooij MR et al (2007) Use of Rhenish trass in marine concrete. A microscopic and durability perspective. HERON 52(4):269–287
Toumbakari E (2002) Lime-pozzolan-cement grouts and their structure effects on composite masonry walls. PhD dissertation, Katholieke Universiteit Leuven
EN 197–1 (2011) Cement - Part 1: Composition, specifications and conformity criteria for common cements
EN 196–5 (2011) Methods of testing cement - Part 5: pozzolanicity test for pozzolanic cement
ASTM C618–02, Standard specification for coal fly ash and raw or calcined natural pozzolan for use as a mineral admixture in concrete, ASTM International, West Conshohocken, PA, 2002, www.astm.org
Torres I, Veiga R, Freitas V (2018) Influence of substrate characteristics on behavior of applied mortar. J Mater Civil Eng 30:04018254
Arandigoyen M, Alvarez JI (2006) Blended pastes of cement and lime: Pore structure and capillary porosity. Appl Surf Sci 252:8077–8085
Pozo-Antonio JS (2015) Evolution of mechanical properties and drying shrinkage in lime-based and lime cement-based mortars with pure limestone aggregate. Constr Build Mater 77:472–478
Cizer O, Van Balen K, Elsen J, Van Gemert D (2008) Carbonation Reaction Kinetics of Lime Binders Measured Using XRD. In: Bacciocchi R, Costa G, Polettini A, Pomi R (eds) Conference on accelerated carbonation for environmental and materials engineering, pp 139–148
Carcasses M, Petit JY, Ollivier JP (1998) Gas permeability of mortars in relation to the microstructure of interfacial transition zone. In: Katz, A., Bentur, A., Alexander, M. & Arliguie, G. (eds). second international conference on the interfacial transition zone in cementitious composites, pp 85–92
Veiga R (2000) Influence of application conditions on the cracking susceptibility of renderings. Concr Sci Eng 2:134–140
Eiras JN, Popovics JS, Borrachero MV et al (2015) The effects of moisture and micro-structural modifications in drying mortars on vibration-based NDT methods. Constr Build Mater 94:565–571. https://doi.org/10.1016/j.conbuildmat.2015.07.078
Veiga R, Abrantes V (1998) Improving the cracking resistance of rendering mortars. Influence of composition factors. Int J Housing Sci 22:245–254
Lawrence R (2006) A study of carbonation in nonhydraulic lime mortars. Dissertation, University of Bath
Veiga MR (2017) Air lime mortars: What else do we need to know to apply them in conservation and rehabilitation interventions? A review. Constr Build Mater 157:132–140
Lanas J, Alvarez JI (2003) Masonry repair lime-based mortars: factors affecting the mechanical behavior. Cem Concr Res 33:1867–1876
Lanas J, Alvarez J (2004) Dolomitic limes: evolution of the slaking process under different conditions. Thermoch Acta 423:1–12
Lanas J, Sirera R, Alvarez JI (2006) Study of the mechanical behaviour of masonry repair lime-based mortars cured and exposed under different conditions. Cem Concr Res 36:961–970
Török Á, Szemerey-Kiss B (2019) Freeze-thaw durability of repair mortars and porous limestone: compatibility issues. Prog Earth Planet Sci 6(1):42
Nunes C, Slížková Z (2016) Freezing and thawing resistance of aerial lime mortar with metakaolin and a traditional water-repellent admixture. Constr Build Mater 114:896–905
Groot C, Maurenbrecher P (2016): Rilem TC 203-RHM repair mortars historic masonry, State of the art report, Ch3. From problem to intervention: the decision process
Balksten K (2010) Understanding historic mortar and their variations – a condition for performing restorations with traditional materials. Historic Mortars Conference HMC-2010, Prague, Czech Republic
Izaguirre A, Lanas J, Alvarez JI (2010) Ageing of lime mortars with admixtures: durability and strength assessment. Cem Concr Res 40:1081–1095
Nunes C, Slizkova Z (2014) Hydrophobic lime-based mortars with linseed oil: characterization and durability assessment. Cem Concr Res 61–62:28–39
Artioli G, Secco M, Addis A (2019) The vitruvian legacy: mortars and binders before and after the Roman world. In: Artioli G, Oberti R (eds) EMU Notes in Mineralogy, vol 20. The Contribution of Mineralogy to Cultural Heritage. European Mineralogical Union, London, pp 151–202
Snellings R, Mertens G, Elsen J (2012) Supplementary cementitious materials. Rev Mineral Geochem 74(1):211–278
Richardson IG (2008) The calcium silicate hydrates. Cem Concr Res 38:137–158
Massazza F, Testolin M (1983) Trimethylsilylation in the study of pozzolana-containing pastes. Il Cemento 80:49–62
Brough AR, Dobson CM, Richardson IG, Groves GW (1995) A study of the pozzolanic reaction by solid-state 29Si nuclear magnetic resonance using selective isotopic enrichment. J Mater Sci 30:1671–1678
Jackson MD, Mulcahy SR, Chen H, Li Y, Li Q, Cappelletti P, Wenk HR (2017) Phillipsite and Al-tobermorite mineral cements produced through low-temperature water-rock reactions in Roman marine concrete. Am Mineral 102:1435–1450
Matschei T, Lothenbach B, Glasser FP (2007) The AFm phase in Portland cement. Cem Concr Res 37:118–130
Taylor HFW (1997) Cement chemistry. Thomas Telford, London, UK
Brune P (2011) The mechanics of Imperial Roman concrete and the structural design of the vaulted monuments. Univ of Rochester, Rochester, NY
Jackson MD (2014) Sea-water concretes and their material characteristics. In: Oleson JP (ed) Building for Eternity. Oxbow Books, Philadelphia, USA, pp 141–188
Jackson MD, Chae SR, Mulcahy SR, Meral C, Taylor R, Li P, Emwas AH, Moon J, Yoon S, Vola G, Wenk HR, Monteiro PJM (2013) Unlocking the secrets of Al-tobermorite in Roman seawater concrete. Am Mineral 98:1669–1687
Jackson MD, Landis EN, Brune PF, Vitti M, Chen H, Li Q, Kunz M, Wenk HR, Monteiro PJM, Ingraffea AR (2014) Mechanical resilience and cementitious processes in Imperial Roman architectural mortar. Proc Natl Acad Sci USA 111(52):18484–18489
Papayianni I, Stefanidou M (2006) Strength–porosity relationships in lime–pozzolan mortars. Constr Build Mater 20:700–705
Walker R, Pavia S (2011) Physical properties and reactivity of pozzolans, and their influence on the properties of lime–pozzolan pastes. Mater Struct 44:1139–1150
Cizer O, Van Balen K, Van Gemert D (2010) Competition between hydration and carbonation in hydraulic lime and lime-pozzolana mortars. Adv Mater Res 133–134:241–246
Arizzi A, Viles H, Cultrone G (2012) Experimental testing of the durability of lime-based mortars used for rendering historic buildings. Constr Build Mater 28(1):807–818
Faria Rodrigues F (2009) Resistance to salts of lime and pozzolan mortars. In: RILEM Proceedings pro 067 –international RILEM workshop on repair mortars for historic masonry, C.Groot, Ed., RILEM Publications, pp 99–110
Arizzi A, Cultrone G (2018) Comparing the pozzolanic activity of aerial lime mortars made with metakaolin and fluid catalytic cracking catalyst residue: a petrographic and physical-mechanical study. Constr Build Mater 184:382–390
Fusade L, Viles H, Wood C, Burns C (2019) The effect of wood ash on the properties and durability of lime mortar for repointing damp historic buildings. Constr Build Mater 212:500–513
Veiga R, Velosa A, Magalhães A (2009) Experimental applications of mortars with pozzolanic additions: characterization and performance evaluation. Constr Build Mater 23:318–327
Lanas J, Perez Bernal JL, Bello MA et al (2004) Mechanical properties of natural hydraulic lime-based mortars. Cem Concr Res 34:2191–2201
Kalagri A, Karatasios I, Kilikoglou V (2014) The effect of aggregate size and type of binder on microstructure and mechanical properties of NHL mortars. Constr Build Mater 53:467–474
Figueiredo C, Lawrence M, Bal, R (2016). Mechanical properties of standard and commonly formulated NHL mortars used for retrofitting. In S. Emmitt, & K. Adeyeye (Eds.). integrated design conference ID@50: building our future
Grilo J, Santos Silva A, Faria P, Gameiro A, Veiga R, Velosa A (2014) Mechanical and mineralogical properties of natural hydraulic lime-metakaolin mortars in different curing conditions. Constr Build Mater 51:287–294
Gameiro A, Santos-Silva A, Faria P, Grilo J, Branco T, Veiga R, Velosa A (2014) Physical and chemical assessment of air lime-metakaolin mortars: influence of binder:aggregate ratio. Cement Concr Compos 45:264–271
Gulotta D, Goidanich S, Tedeschi C, Toniolo L (2015) Commercial NHL-containing mortars for the preservation of historical architecture. Part 2: durability to salt decay. Constr Build Mater 96:198–220
Santos AR, Veiga MR, Santos Silva A, Brito J (2020) Mortars as rehabilitation elements: the influence of the pore structure (in Portuguese). In: ENCORE 2020 – 4º Encontro sobre Conservação e Reabilitação de Edifícios, Lisbon, LNEC, 3–6 Nov. 2020, 491-502. ISBN 978-972-49-2313-0. https://doi.org/10.34638/yzys-hn57
Isebaert A, De Boever W, Descamps F, Dils J, Dumon M, De Schutter G, Van Ranst E, Cnudde V, Van Parys L (2016) Pore-related properties of natural hydraulic lime mortars: an experimental study. Mater Struct 49:2767–2780. https://doi.org/10.1617/s11527-015-0684-5
Maravelaki-Kalaitzaki (2007) Hydraulic lime mortars with siloxane for waterproofing historic masonry. Cem Concr Res 37:283–290
Van Hees RPJ, Wijffels T, van der Klugt LJAR (2003) Thaumasite swelling in historic mortars. Field observations and laboratory research. Cement Concr Compos 25:1165–1171
Papayianni I, Stefanidou M (2007) The influence of mixture design parameters on the long term strength of lime-based mortars, In: Gorun Arun (ed) Studies on Historical Heritage SHH07 Atalya September, Turkey, pp355–362
Santos Silva A, Cruz T, Paiva MJ, Candeias A, Adriano P, Schiavon N, Mirão J (2011) Mineralogical and chemical characterization of historical mortars from military fortifications in Lisbon harbour (Portugal). Environ Earth Sci 7(63):1641–1650
Alvarez JI, Navarro I, Martin A, García Casado PJ (2000) A study of the ancient mortars in the north tower of Pamplona’s San Cernin church. Cem Concr Res 30:1413–1419
Maurenbrecher P, Groot C (Editors) (2016) Repair Mortars for Historic Masonry, State of the Ministry of Culture, Ephorate of Antiquities of the town of Thessaloniki (2015), The Restoration of the remnants of the Galerius complex in Thessaloniki (1994–2014), Volume B’, The restoration works (Thessaloniki, 2015)
Rani D, Bais S, Santhanam M (2016) Characterisation of incised plaster works of historic monuments in Delhi, India. In: Proc. of 4th historic mortars conference (HMC-2016), Santorini, Greece, pp 10–12 October 2016
Mohammed Haneefa K, Divya Rani S, Ramasamy R, Santhanam M (2019) Microstructure and geochemistry of lime plaster mortar from a heritage structure. Constr Build Mater 225(2019):538–554
Margalha G, Veiga R, Santos Silva A, de Brito J, Bal R, Allen G (2013) Microstructural changes of lime putty during aging. J Mater Civ Eng 25(10):1524–1532
Margalha G, Veiga R, Santos Silva A, Brito J (2011) Traditional methods of mortar preparation: the hot lime mix method. Cement Concr Compos 33:796–804
Van Balen K, Van Gemert D (1994) Modelling lime mortar carbonation. Mater Struct 27:393–398
Oliveira M (2016) A multi–physics approach applied to masonry structures with non–hydraulic lime mortars (Ph.D. thesis), Minho University
Martínez-García C et al (2020) Carbonation evolution of lime putty coatings with mussel shell aggregate. Constr Build Mater 264:120165
Lawrence RM, Mays TJ, Rigby SP et al (2007) Effects of carbonation on the pore structure of non-hydraulic lime mortars. Cem Concr Res 37:1059–1069
Arandigoyen M, Pérez Bernal JL, Bello López MA, Alvarez JI (2005) Lime-pastes with different kneading water: pore structure and capillary porosity. Appl Surf Sci 252(5):1449–1459
Arandigoyen M, Bicer-Simsir B, Alvarez JI, Lange DA (2006) Variation of microstructure with carbonation in lime and blended pastes. Appl Surf Sci 252(20):7562–7571
Ruiz-Agudo E, Rodriguez-Navarro C (2010) Microstructure and rheology of lime putty. Langmuir 26(6):3868–3877
Rodriguez-Navarro C et al (2005) Nanostructure and irreversible colloidal behavior of Ca(OH)2: implications in cultural heritage conservation. Langmuir 21:10948–10957
Waldum AM (2002) Historic materials and their diagnostic, State of the Art for masonry monuments in Norway. norwegian building research institute. Ariadne 9 Workshop on Historic Materials and their Diagnostics
Ratcliffe T, Orton J (1998) Success with lime renders. Interim discussion article of the Society Protection Ancient Buildings (SPAB), UK
Matias G, Faria P, Torres I (2014) Lime mortars with ceramic wastes: characterization of components and their influence on the mechanical behaviour. Constr Build Mater 73:523–534
Veiga MR, Velosa A, Magalhães AC (2009) Experimental Applications of mortars with pozzolanic additions. Characterization and performance evaluation. Constr Build Mater 23(1):318–327
Fernández JM, Duran A, Navarro-Blasco I et al (2013) Influence of nanosilica and a polycarboxylate ether superplasticizer on the performance of lime mortars. Cem Concr Res 43:12–24
Cizer O, Van Balen K, Elsen J, Van Gemert D (2012) Real-time investigation of reaction rate and mineral phase modifications of lime carbonation. Constr Build Mater 35:741–751
Cizer Ö, Van Balen K, Van Gemert DA (2010) Competition between hydration and carbonation in hydraulic lime and lime-pozzolana mortars. Adv Mat Res 133–134:241–247
Gameiro A, Santos Silva A, Veiga MR, Velosa A (2012) Hydration products of lime–metakaolin pastes at ambient temperature with ageing. Thermochim Acta 535:36–41
Cizer Ö, Van Balen K, Elsen J, Van Gemert D (2006) Carbonation and hydration of calcium hydroxide and calcium silicate binders with rice husk ash. In: 2nd symposium on advances in concrete through science and engineering, pp 11–13 September, Quebec City, Canada
St. Astier, Hydraulicity and properties of St Astier Natural Hydraulic Lime http://www.stastier.co.uk/nhl/info/hydraul.htm
Papayianni I (2019) Hydraulic mortars of antiquity characterization and evaluation Cutting Edge technologies. In: Ancient Greece, Oxbow books, Eds Panagiotaki M., Tomazos I., Papadrimitakopoulos F
EN 459–2 (2021): Building lime - Part 2: Test methods
Papayianni I, Pachta V, Berberidou E, Kalogirou M (2019) Investigating differences in the performance of lime-based mortars, 5th Historic Mortars Conference. Pamplona, Spain
Papayianni I, Stefanidou M (2007) Durability aspects of ancient mortars of the archaeological site of Olynthos. J Cult Heritage 2(8):193–196
Cizer Ö, Van Balen K, Van Gemert D., & Elsen, J (2007) Carbonation and hydration of mortars with calcium hydroxide and calcium silicate binders. Paper presented at the Sustainable Construction Materials and Technologies-International Conference on Sustainable Construction Materials and Technologies, pp 611–621
Monteiro J, Silva V, Faria P (2021) Effect of type of curing and metakaolin replacement on air lime mortars for the durability of masonries. Infrastructures 6:143. https://doi.org/10.3390/infrastructures6100143
Roy DM and Idorn GM (1982) Hydration, structure and properties of blast furnace slag cement, mortars and concretes. ACI Mater J 79:444–457
Hooton RD, Emery JJ (1990) Sulfate resistance of a canadian slag cement. ACI Mater J 87:547–555
Papayianni I, Stefanidou M, Pachta V, Konopisi S, Athanasiou F, Sarantidou M (2019) Evaluation of lime-based mortars for repairing the galerius palace (4th cent. AD) during restoration project (1994–2014), In: Proc. of RILEM SMSS Conference, Rovinj 2019
Stefanidou M, Papayianni I (2006) The role of aggregates on the structure and properties of lime mortars. Cement Concr Compos 27:914–919
Eriksson J, Lindqvist JE (2019) Lime render, shrinkage cracks and craftsmanship in building restoration. J Cult Herit 37:73–81
Balksten K (2007) Traditional lime mortar and plaster – Reconstruction with emphasis on durability. Chalmers, Göteborg
Hartshorn S (1998) Thaumasite, a brief guide for engineers. Concrete Sept 1998, pp 24–27
Van Balen K, Toumbakari EE, Blanco-Varela MT, Aguilera J, Puertas F, Paloma A, Sabbioni C, Riontini C, and Zappia G (2003), Environmental deterioration of ancient and modern hydraulic mortar (EDAMM). Protection and conservation of European cultural heritage, research report european commission, N°15, Luxemburg: Office for Official Publications of the European Communities, EUR 19863, 202 p., ISBN 62–894–3169–5.
González-Sánchez JF, Fernández JM, Navarro-Blasco Í, Alvarez JI (2021) Improving lime-based rendering mortars with admixtures. Constr Build Mater 271:121887
Aziez MN, Bezzar A (2017) Magnesium sulphate attacks on mortars - influence of temperature, type of sand and type of cement. J Eng Sci Technol Rev 10:41–50
Balksten K, Strandberg-de Bruijn P (2021) Understanding deterioration due to salt and ice crystallization in scandinavian massive brick masonry. Heritage 4(1):349–370
Lanas J, Sirera R, Alvarez JI (2005) Compositional changes in lime-based mortars exposed to different environments, Thermochim. Acta 429:219–226
Delgado Rodrigues J (2020) Practical evidence and experimental demonstration of the Liesegang phenomenon in the carbonation process of lime mortars in exposed masonries. J Cult Herit 45:169–179
Van Hees R, Members RILEM TC 203 (2016) Chapter 3, From problems to intervention: the decision process. In: C Groot & P Maurenbrecher eds, RILEM report 45, Repair Mortars for Historic Masonry, state of the art report of RILEM TC 203-RHM, pp 16–43
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This review was prepared by Caspar Groot, Rosario Veiga, Ioanna Papayianni, Rob van Hees, Michele Secco, Jose I. Alvarez, Paulina Faria and Maria Stefanidou within RILEM TC LHS-277 ‘‘Specifications for testing and evaluation of lime-based repair materials for historic Structures’’ and further reviewed and approved by all members of the RILEM TC 277-LHS. TC Chair: Ioanna Papayianni. TC Deputy Chair: Jan Valek. Members: Jose I. Alvarez, Beril Bicer-Simsir, Violeta Bokan-Bosiljkov, Claudia Brito De Carvalho Bello, Arnaldo Manoel Carneiro, Paulina Faria, Liberato Ferrara, Caspar Groot, Davide Gulotta, John J. Hughes, Ioannis Ioannou, Heejeong Kim, Loucas Kyriakou, Cristiana Lara Nunes, Pagona Maravelaki, Sagrario Martinez-Ramirez, Didem Oktay, Vasiliki Pachta, Andreja Padovnik, Ioanna Papayianni, Chiara Pasian, Sara Pavia, Giovanni Pesce, Ulrike Peter, Meera Ramesh, Divya Rani, Sriram Pradeep Saridhe, Michele Secco, Maria Stefanidou, Petra Štukovnik, Cristina Tedeschi, Magdalini Theodoridou, Lucia Toniolo, Jan Valek, Rob van Hees, Maria Rosario Veiga.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Groot, C., Veiga, R., Papayianni, I. et al. RILEM TC 277-LHS report: lime-based mortars for restoration–a review on long-term durability aspects and experience from practice. Mater Struct 55, 245 (2022). https://doi.org/10.1617/s11527-022-02052-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1617/s11527-022-02052-1