Abstract
To address the low energy density and potential safety issues of modern lithium-ion batteries (LIBs), all-solid-state lithium batteries (ASSLBs) with solid-state electrolytes (SSEs) have emerged as a highly promising option. Among different SSEs, inorganic electrolytes (IEs) are the most probable to replace organic liquid electrolytes because of their relatively high lithium ionic conductivity and wide cell voltage window. Nevertheless, IEs encounter challenges such as elevated interfacial resistance, limited ionic conductivity at room temperature, and air instabilities. Different hybrid solid electrolytes, including organic–inorganic hybrid solid electrolytes (OIHSEs) and inorganic composite electrolytes (ICEs), have been developed to overcome these difficulties. While OIHSEs have been reviewed thoroughly, ICEs have been reviewed rarely, despite their crucial role in the advancement of ASSLBs. This review focuses on the synthesis methodologies, structures, compositions, and electrochemical performance of ICEs, providing a comprehensive overview of the present state-of-art ICEs, with constructive conclusions and perspectives for different ICEs on varied purposes. Ultimately, this review aims to shed light on potential research directions for the designing and building of practical ASSLBs with varying ICEs, thereby promoting the energy storage application of ASSLBs.
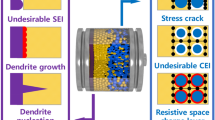
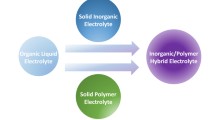
Graphical abstract
The recent advances in “Inorganic composite electrolytes for all-solid-state lithium batteries” were reviewed, with an emphasis on their compositions, synthesis techniques, electrochemical performances, and applications. Several research directions are offered to design and manufacture viable ICEs.

Highlights
-
This review provides a comprehensive overview of the present state-of-art inorganic composite electrolytes, with constructive conclusions and perspectives for different ICEs on varied purposes.
-
This review focuses on the synthesis methodologies, structures, compositions, and electrochemical performance of inorganic composite electrolytes in ASSLBs.
Discussion
The implementation of all-solid-state lithium batteries emerges as a pivotal remedy for addressing the safety concerns and energy density limitations inherent in conventional lithium-ion batteries. The decision between inorganic electrolytes and hybrid solid electrolytes, specifically the relatively unexplored inorganic composite electrolytes, necessitates thorough deliberation and underscores the importance of interdisciplinary collaboration. The utilization of theoretical computations and the development of durable and environmentally friendly energy solutions are imperative for augmenting the efficacy and security of all-solid-state batteries, while also promoting the adoption of sustainable energy technologies in diverse societies.























Reproduced with permission from Ref. [31]. Copyright © 2019 Wiley–VCH.



Similar content being viewed by others
Data availability
Not applicable.
Code availability
No code was written or used.
References
A. Manthiram, X. Yu, S. Wang, Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2(4), 1–16 (2017). https://doi.org/10.1038/natrevmats.2016.103
J.M. Tarascon, M. Armand, Issues and challenges facing rechargeable lithium batteries. Nature 414(6861), 359–367 (2001). https://doi.org/10.1142/9789814317665_0024
B. Scrosati, J. Garche, Lithium batteries: status, prospects and future. J. Power. Sources 195(9), 2419–2430 (2010). https://doi.org/10.1016/j.jpowsour.2009.11.048
L. Fan, S. Wei, S. Li, Q. Li, Y. Lu, Recent progress of the solid-state electrolytes for high-energy metal-based batteries. Adv. Energy Mater. 8(11), 1702657 (2018). https://doi.org/10.1002/aenm.201702657
O.V. Yarmolenko, A.V. Yudina, K.G. Khatmullina, Nanocomposite polymer electrolytes for the lithium power sources (a review). Russ. J. Electrochem. 54, 325–343 (2018). https://doi.org/10.1134/S1023193518040092
J. Wu, Z. Rao, Z. Cheng, L. Yuan, Z. Li, Y. Huang, Ultrathin, flexible polymer electrolyte for cost-effective fabrication of all-solid-state lithium metal batteries. Adv. Energy Mater. 9(46), 1902767 (2019). https://doi.org/10.1002/aenm.201902767
M. Jia et al., Fluorinated bifunctional solid polymer electrolyte synthesized under visible light for stable lithium deposition and dendrite-free all-solid-state batteries. Adv. Funct. Mater. 31(27), 2101736 (2021). https://doi.org/10.1002/adfm.202101736
R. Chen, W. Qu, X. Guo, L. Li, F. Wu, The pursuit of solid-state electrolytes for lithium batteries: from comprehensive insight to emerging horizons. Mater. Horizons. 3(6), 487–516 (2016). https://doi.org/10.1039/C6MH00218H
M.V. Reddy, C.M. Julien, A. Mauger, K. Zaghib, Sulfide and oxide inorganic solid electrolytes for all-solid-state li batteries: a review. Nanomaterials 10(8), 1606 (2020). https://doi.org/10.3390/nano10081606
W.L. Huang, N. Zhao, Z.J. Bi, C. Shi, X.X. Guo, L.Z. Fan, C.W. Nan, Can we find solution to eliminate Li penetration through solid garnet electrolytes? Mater. Today Nano. 10, 100075 (2020). https://doi.org/10.1016/j.mtnano.2020.100075
C. Chen, K. Wang, H. He, E. Hanc, M. Kotobuki, L. Lu, Processing and properties of garnet-type Li7La3Zr2O12 ceramic electrolytes. Small 19(12), 2205550 (2023). https://doi.org/10.1002/smll.202205550
S. Tang, W. Guo, Y. Fu, Advances in composite polymer electrolytes for lithium batteries and beyond. Adv. Energy Mater. 11(2), 2000802 (2021). https://doi.org/10.1002/aenm.202000802
C. Wang et al., Garnet-type solid-state electrolytes: materials, interfaces, and batteries. Chem. Rev. 120(10), 4257–4300 (2020). https://doi.org/10.1021/acs.chemrev.9b00427
Z. Liu, W. Fu, E.A. Payzant, X. Yu, Z. Wu, N.J. Dudney, J. Kiggans, K. Hong, A.J. Rondinone, C. Liang, Anomalous high ionic conductivity of nanoporous β-Li3PS4. J. Electrochem. Soc. 135(3), 975–978 (2013). https://doi.org/10.1021/ja3110895
G. Özsin, K.B. Dermenci, S. Turan, Thermokinetic and thermodynamics of Pechini derived conditions. J. Therm. Anal. Calorim. 146, 1405–1420 (2021). https://doi.org/10.1007/s10973-020-10462-y
J.C. Bachman et al., Inorganic solid-state electrolytes for lithium batteries: mechanisms and properties governing ion conduction. Chem. Rev. 116(1), 140–162 (2015). https://doi.org/10.1021/acs.chemrev.5b00563
Q. Liu, Z. Geng, C. Han, Y. Fu, S. Li, Y. He, F. Kang, B. Li, Challenges and perspectives of garnet solid electrolytes for all solid-state lithium batteries. J. Power. Sources 389, 120–134 (2018). https://doi.org/10.1016/j.jpowsour.2018.04.019
M. Rosen, M. Finsterbusch, O. Guillon, D. Fattakhova-Rohlfing, Free standing dual phase cathode tapes–scalable fabrication and microstructure optimization of garnet-based ceramic cathodes. J. Mater. Chem. A. 10(5), 2320–2326 (2021). https://doi.org/10.1039/d1ta07194g
M. Dirican, C. Yan, P. Zhu, X. Zhang, Composite solid electrolytes for all-solid-state lithium batteries. Mater. Sci. Eng. R Reports. 136, 27–46 (2019). https://doi.org/10.1016/j.mser.2018.10.004
K. Chen, M. Huang, Y. Shen, Y. Lin, C.W. Nan, Enhancing ionic conductivity of Li0.35La0.55TiO3 ceramics by introducing Li7La3Zr2O12. Electrochim. Acta 80, 133–139 (2012). https://doi.org/10.1016/j.electacta.2012.06.115
P. Yao et al., Review on polymer-based composite electrolytes for lithium batteries. Front. Chem. 7, 522 (2019). https://doi.org/10.3389/fchem.2019.00522
T. Famprikis, P. Canepa, J.A. Dawson, M.S. Islam, C. Masquelier, Fundamentals of inorganic solid-state electrolytes for batteries. Nat. Mater. 18(12), 1278–1291 (2019). https://doi.org/10.1038/s41563-019-0431-3
E. Rangasamy, G. Sahu, J.K. Keum, A.J. Rondinone, N.J. Dudney, C. Liang, A high conductivity oxide-sulfide composite lithium superionic conductor. J. Mater. Chem. A. 2, 4111–4116 (2014). https://doi.org/10.1039/c3ta15223e
J. Liang, J. Luo, Q. Sun, X. Yang, R. Li, X. Sun, Recent progress on solid-state hybrid electrolytes for solid-state lithium batteries. Energy Storage Mater. 21, 308–334 (2019). https://doi.org/10.1016/j.ensm.2019.06.021
N. Meng, X. Zhu, F. Lian, Particles in composite polymer electrolyte for solid-state lithium batteries: a review. Particuology. 60, 14–36 (2021). https://doi.org/10.1016/j.partic.2021.04.002
Z.D. Hood, H. Wang, Y. Li, A.S. Pandian, M.P. Paranthaman, C. Liang, The “filler effect”: a study of solid oxide fillers with β-Li3PS4 for lithium conducting electrolytes. Solid State Ionics J. 283, 75–80 (2015). https://doi.org/10.1016/j.ssi.2015.10.014
J. Zhu et al., Enhanced ionic conductivity with Li7O2Br3 phase in Li3OBr anti-perovskite solid electrolyte. Am. Inst. Phys. 109(1), 101904 (2016). https://doi.org/10.1063/1.4962437
E. Zhao, F. Ma, Y. Guo, Y. Jin, Stable LATP/LAGP double-layer solid electrolyte prepared via a simple dry-pressing method for solid state lithium ion batteries. RSC Adv. 6(95), 92579–92585 (2016). https://doi.org/10.1039/c6ra19415j
Y. Tian et al., Li6.75La3Zr1.75Ta0.25O12@amorphous Li3OCl composite electrolyte for solid state lithium-metal batteries. Energy Storage Mater. 14, 49–57 (2018). https://doi.org/10.1016/j.ensm.2018.02.015
F. Han, J. Yue, X. Zhu, C. Wang, Suppressing Li dendrite formation in Li2S-P2S5 solid electrolyte by LiI incorporation. Adv. Energy Mater. 8(18), 1703644 (2018). https://doi.org/10.1002/aenm.201703644
F. Mo et al., Inside or outside: origin of lithium dendrite formation of all solid-state electrolytes. Adv. Energy Mater. 9(40), 1902123 (2019). https://doi.org/10.1002/aenm.201902123
Q. Zhang, J.P. Mwizerwa, H. Wan, L. Cai, X. Xu, X. Yao, Fe3S4@Li7P3S11 nanocomposites as cathode materials for all-solid-state lithium batteries with improved energy density and low cost. J. Mater. Chem. A. 5(45), 23919–23925 (2017). https://doi.org/10.1039/c7ta07972a
Q. Zhang, Z. Ding, G. Liu, H. Wan, J.P. Mwizerwa, J. Wu, X. Yao, Molybdenum trisulfide based anionic redox driven chemistry enabling high-performance all-solid-state lithium metal batteries. Energy Storage Mater. 23, 168–180 (2019). https://doi.org/10.1016/j.ensm.2019.05.015
L. Cai, Q. Zhang, J.P. Mwizerwa, H. Wan, X. Yang, X. Xu, X. Yao, Highly crystalline layered VS2 nanosheets for all-solid-state lithium batteries with enhanced electrochemical performances. ACS Appl. Mater. Interfaces 10(12), 10053–10063 (2018). https://doi.org/10.1021/acsami.7b18798
S.Y. Jung, R. Rajagopal, K.S. Ryu, Synthesis and electrochemical performance of (100–x)Li7P3S11-xLi2OHBr composite solid electrolyte for all-solid-state lithium batteries. J. Energy Chem. 47, 307–316 (2020). https://doi.org/10.1016/j.jechem.2020.02.018
J. Hüttl et al., Ultra-low LPS/LLZO interfacial resistance–towards stable hybrid solid-state batteries with Li-metal anodes. Energy Storage Mater. 40, 259–267 (2021). https://doi.org/10.1016/j.ensm.2021.05.020
Z. Lai, W. Feng, X. Dong, X. Zhou, Y. Wang, Y. Xia, Lithium dendrites suppressed by low temperature in-situ anti-perovskite coated garnet solid-state electrolyte. J. Power. Sources 500, 229982 (2021). https://doi.org/10.1016/j.jpowsour.2021.229982
Y.S. Park, J.M. Lee, E.J. Yi, J.W. Moon, H. Hwang, All-solid-state lithium-ion batteries with oxide/sulfide composite electrolytes. Materials (Basel) 14(8), 1998 (2021). https://doi.org/10.3390/ma14081998
C. Zou et al., Ionic conductivity and interfacial stability of Li6PS5Cl–Li6.5La3Zr1.5Ta0.5O12 composite electrolyte. J. Solid State Electrochem. 25(10–11), 2513–2525 (2021). https://doi.org/10.1007/s10008-021-05004-x
Z. Deng et al., Bilayer halide electrolytes for all-inorganic solid-state lithium-metal batteries with excellent interfacial compatibility. ACS Appl. Energy Mater. 14(43), 48619–48626 (2022). https://doi.org/10.1021/acsami.2c12444
S.K. Jung, H. Gwon, G. Yoon, L.J. Miara, V. Lacivita, J.S. Kim, Pliable lithium superionic conductor for all-solid-state batteries. ACS Energy Lett. 6(5), 2006–2015 (2021). https://doi.org/10.1021/acsenergylett.1c00545
R. Xu et al., Room temperature halide-eutectic solid electrolytes with viscous feature and ultrahigh ionic conductivity. Adv. Sci. 9(35), 2204633 (2022). https://doi.org/10.1002/advs.202204633
A.H. Dao et al., Improvement of the ionic conductivity on new substituted borohydride argyrodites. Solid State Ion. 339, 114987 (2019). https://doi.org/10.1016/j.ssi.2019.05.022
H. Morimoto, H. Yamashita, M. Tatsumisago, T. Minami, Mechanochemical synthesis of new amorphous materials of 60Li2S·40SiS2 with high lithium ion conductivity. J. Am. Ceram. Soc. 82(5), 1352–1354 (1999). https://doi.org/10.1111/j.1151-2916.1999.tb01923.x
M. Tatsumisago, S. Hama, A. Hayashi, H. Morimoto, T. Minami, New lithium ion conducting glass-ceramics prepared from mechanochemical Li2S–P2S5 glasses. Solid State Ion. 154, 635–640 (2002). https://doi.org/10.1016/S0167-2738(02)00509-X
J. Zhu et al., A multilayer ceramic electrolyte for all-solid-state Li batteries. Angew. Chemie Int. Ed. 60(7), 3781–3790 (2021). https://doi.org/10.1002/anie.202014265
J. Gao, J. Zhu, X. Li, J. Li, X. Guo, H. Li, W. Zhou, Rational design of mixed electronic-ionic conducting Ti-doping Li7La3Zr2O12 for lithium dendrites suppression. Adv. Funct. Mater. 31(2), 2001918 (2021). https://doi.org/10.1002/adfm.202001918
K. Chen, M. Huang, Y. Shen, Y. Lin, C.W. Nan, Improving ionic conductivity of Li0.35La0.55TiO3 ceramics by introducing Li7La3Zr2O12 sol into the precursor powder. Solid State Ion. 235, 8–13 (2013). https://doi.org/10.1016/j.ssi.2013.01.007
Z. Zhang, L. Zhang, C. Yu, X. Yan, B. Xu, L. Wang, Lithium halide coating as an effective intergrain engineering for garnet-type solid electrolytes avoiding high temperature sintering. Electrochim. Acta 289, 254–263 (2018). https://doi.org/10.1016/j.electacta.2018.08.079
H. Duan et al., Building an air stable and lithium deposition regulable garnet interface from moderate-temperature conversion chemistry. Angew. Chemie Int. Ed. 132(29), 12167–12173 (2020). https://doi.org/10.1002/ange.202003177
W. Kou et al., Highly conductive thin lamellar Li7La3Zr2O12/Li3InCl6 composite inorganic solid electrolyte for high-performance all-solid-state lithium battery. J. Memb. Sci. 687, 122080 (2023). https://doi.org/10.1016/j.memsci.2023.122080
Y. Gao et al., Amorphous dual-layer coating: enabling high Li-ion conductivity of non-sintered garnet-type solid electrolyte. Adv. Funct. Mater. 31(15), 2009692 (2021). https://doi.org/10.1002/adfm.202009692
H. Xu, Y. Li, A. Zhou, N. Wu, S. Xin, Z. Li, J.B. Goodenough, Li3N-modified garnet electrolyte for all-solid-state Li-metal batteries operated at 40 °C. Nano Lett. 18(11), 7414–7418 (2018). https://doi.org/10.1021/acs.nanolett.8b03902
H. Nagata, J. Akimoto, Hybrid oxide solid electrolyte of crystalline garnet and highly deformable glass for all-solid-state lithium-ion batteries. J. Power. Sources 539, 231596 (2022). https://doi.org/10.1016/j.jpowsour.2022.231596
Y. Niu et al., Constructing stable Li-solid electrolyte interphase to achieve dendrite-free solid-state battery: a nano-interlayer/Li pre-reduction strategy. Nano Res. 15(8), 7180–7189 (2022). https://doi.org/10.1007/s12274-022-4362-y
S.G. Ling, J.Y. Peng, Q. Yang, J.L. Qiu, J.Z. Lu, H. Li, Enhanced ionic conductivity in LAGP/LATP composite electrolyte. Chin. Phys. B 27(3), 038201 (2018). https://doi.org/10.1088/1674-1056/27/3/038201
H. Onishi, S. Takai, T. Yabutsuka, T. Yao, Synthesis and electrochemical properties of LATP-LLTO lithium ion conductive composites. Electrochemistry 84(12), 967–970 (2016). https://doi.org/10.5796/electrochemistry.84.967
F. Song, T. Yamamoto, T. Yabutsuka, T. Yao, S. Takai, Synthesis and characterization of LAGP-based lithium ion-conductive composites with an LLTO additive. J. Alloys Compd. 853, 157089 (2021). https://doi.org/10.1016/j.jallcom.2020.157089
F. Song, T. Yamamoto, T. Yabutsuka, T. Yao, S. Takai, Synthesis and characterization of lithium-ion conductive LATP-LaPO4 composites using La2O3 nano-powder. Materials (Basel). 14(13), 3502 (2021). https://doi.org/10.3390/ma14133502
C. Seidl, Investigation of the compatibility of different solid electrolytes for solid-state batteries. Manag. Chem. Technol. (2020)
S.Y. Jung, R. Rajagopal, K.S. Ryu, Synthesis and electrochemical performance of (100–x)Li7P3S11-xLi3SI composite solid electrolyte for all-solid-state lithium batteries. J. Ind. Eng. Chem. 95, 350–356 (2021). https://doi.org/10.1016/j.jiec.2021.01.009
M. Park, R. Rajagopal, K.S. Ryu, Electrochemical performance of the mixed solid electrolyte (100–x)Li3SI-xLi6PS5Cl (x = 0, 10, 20, and 30) for all-solid-state lithium batteries. J. Power. Sources 501, 230031 (2021). https://doi.org/10.1016/j.jpowsour.2021.230031
B.R. Shin, Y.J. Nam, D.Y. Oh, D.H. Kim, J.W. Kim, Y.S. Jung, Comparative study of TiS2/Li-In all-solid-state lithium batteries using glass-ceramic Li3PS4 and Li10GeP2S12 solid electrolytes. Electrochim. Acta 146, 395–402 (2014). https://doi.org/10.1016/j.electacta.2014.08.139
S. Ujiie, A. Hayashi, M. Tatsumisago, Structure, ionic conductivity and electrochemical stability of Li2S-P2S5–LiI glass and glass–ceramic electrolytes. Solid State Ion. 211, 42–45 (2012). https://doi.org/10.1016/j.ssi.2012.01.017
S. Ujiie, A. Hayashi, M. Tatsumisago, Preparation and ionic conductivity of (100–x)(0.8Li2S0,2P2S5)·xLiI glass-ceramic electrolytes. J. Solid State Electrochem. 17, 675–680 (2013). https://doi.org/10.1007/s10008-012-1900-7
S. Ujiie, A. Hayashi, M. Tatsumisago, Preparation and electrochemical characterization of (100–x)(0.7Li2S0.3P2S5)·xLiBr glass-ceramic electrolytes. Mater. Renew. Sustain. Energy. 3, 3–18 (2014). https://doi.org/10.1007/s40243-013-0018-x
E. Rangasamy et al., An iodide-based Li7P2S8I superionic conductor. J. Am. Chem. Soc. 137(4), 1384–1387 (2015). https://doi.org/10.1021/ja508723m
N.H.H. Phuc, T. Yamamoto, H. Muto, A. Matsuda, Fast synthesis of Li2S–P2S5–LiI solid electrolytes precursors. Inorg. Chem. Front. 4(10), 1660–1664 (2017). https://doi.org/10.1039/C7QI00353F
N.H.H. Phuc, E. Hirahara, K. Morikawa, H. Muto, A. Matsuda, One-pot liquid phase synthesis of (100–x)Li3PS4–xLiI solid electrolytes. J. Power. Sources 365(10), 7–11 (2017). https://doi.org/10.1016/j.jpowsour.2017.08.065
S.J. Choi et al., Synthesis and electrochemical characterization of a glass-ceramic Li7P2S8I solid electrolyte for all-solid-state Li-ion batteries. J. Electrochem. Soc. 165(5), 957–962 (2018). https://doi.org/10.1149/2.0981805jes
T. Yamamoto, N.H.H. Phuc, H. Muto, A. Matsuda, Preparation of Li7P2S8I solid electrolyte and its application in all-solid-state lithium-ion batteries with graphite anode. Electron. Mater. Lett. 15, 409–414 (2019). https://doi.org/10.1007/s13391-019-00133-y
S. Choi, S. Lee, J. Yu, C.H. Doh, Y.C. Ha, Slurry-processed glass-ceramic Li2S-P2S5-LiI electrolyte for all-solid-state Li-ion batteries. ECS Trans. 77(1), 65–70 (2017). https://doi.org/10.1149/07701.0065ecst
L. Wu, G. Liu, H. Wan, W. Weng, X. Yao, Superior lithium-stable Li7P2S8I solid electrolyte for all-solid-state lithium batteries. J. Power. Sources 491, 229565 (2021). https://doi.org/10.1016/j.jpowsour.2021.229565
S. Yang et al., Studies on the inhibition of lithium dendrite formation in sulfide solid electrolytes doped with LiX (X = Br, I). Solid State Ion. 377, 115869 (2022). https://doi.org/10.1016/j.ssi.2022.115869
H. Zhang, Z. Yu, J. Cheng, H. Chen, X. Huang, B. Tian, Halide/sulfide composite solid-state electrolyte for Li-anode based all-solid-state batteries. Chinese Chem. Lett. 34, 108228 (2023). https://doi.org/10.1016/j.cclet.2023.108228
A. Yamauchi, A. Sakuda, A. Hayashi, M. Tatsumisago, Preparation and ionic conductivities of (100–x(0.75Li2S·0.25P2S5·xLiBH4 glass electrolytes. J. Power. Sources 244, 707–710 (2013). https://doi.org/10.1016/j.jpowsour.2012.12.001
A. Sakuda, A. Yamauchi, S. Yubuchi, N. Kitamura, Y. Idemoto, A. Hayashi, M. Tatsumisago, Mechanochemically prepared Li2S−P2S5−LiBH4 solid electrolytes with an argyrodite structure. ACS Omega 3(5), 5453–5458 (2018). https://doi.org/10.1021/acsomega.8b00377
A. Unemoto, H. Wu, T.J. Udovic, M. Matsuo, T. Ikeshoji, S. Orimo, Fast lithium-ionic conduction in a new complex hydride-sulphide crystalline phase. Chem. Commun. 52(3), 564–566 (2016). https://doi.org/10.1039/c5cc07793a
Y. Hu et al., Lithium ionic conduction in composites of Li(BH4)0.75I0.25 and amorphous 0.75Li2S.0.25P2S5 for battery applications. Electrochim. Acta 278, 332–339 (2018). https://doi.org/10.1016/j.electacta.2018.05.041
T. Zhang et al., Fast lithium ionic conductivity in complex hydride-sulfide electrolytes by double anions substitution. Small Methods. 5(8), 2100609 (2021). https://doi.org/10.1002/smtd.202100609
A. El Kharbachi et al., Pseudo-ternary LiBH4-LiCl-P2S5 system as structurally disordered bulk electrolyte for all-solid-state lithium batteries. Phys. Chem. Chem. Phys. 22(25), 13872–13879 (2020). https://doi.org/10.1039/d0cp01334j
D. Sveinbjörnsson et al., Effect of heat treatment on the lithium ion conduction of the LiBH4−LiI solid solution. J. Phys. Chem. C 117(7), 3249–3257 (2013). https://doi.org/10.1021/jp310050g
V. Gulino, M. Brighi, E.M. Dematteis, F. Murgia, C. Nervi, R. Černý, M. Baricco, Phase stability and fast ion conductivity in the hexagonal LiBH4-LiBr-LiCl solid solution. Chem. Mater. 31(14), 5133–5144 (2019). https://doi.org/10.1021/acs.chemmater.9b01035
C.H Kühl, N. Straße, Free sintering or hot pressing? A decision support. Diamond Tool Consult. 1–8 (2013).
J.S Konstanty, in: Applications of Powder Metallurgy to Cutting Tools, ed. I. Chang, Y. Zhao (Woodhead Publishing, 2013), pp. 555–585.
S. Ohta, C. Yada, T. Saito, H. Iba, LLZ oxide and LPS sulfide composite solid electrolyte for lithium ion battery. ECS Meet. Abstr. 230(5), 857–857 (2016). https://doi.org/10.1149/ma2016-02/5/857
K. Takada, Progress and prospective of solid-state lithium batteries. Acta Mater. 61, 759–770 (2013). https://doi.org/10.1016/j.actamat.2012.10.034
G. Goglio, A. Ndayishimiye, C. Elissalde, C. Randall, in: Cold Sintering and Hydrothermal Sintering, ed. M. Pomeroy (Elsevier, 2021), pp. 11–326
S. Cao et al., Modeling, preparation, and elemental doping of Li7La3Zr2O12 garnet-type solid electrolytes: a review. J. Korean Ceram. Soc. 56(2), 111–129 (2019). https://doi.org/10.4191/kcers.2019.56.2.01
A. Hayashi, K. Minami, M. Tatsumisago, High lithium ion conduction of sulfide glass-based solid electrolytes and their application to all-solid-state batteries. J. Non Cryst. Solids 355(37–42), 1919–1923 (2009). https://doi.org/10.1016/j.jnoncrysol.2008.12.020
D. Safanama, D. Damiano, R.P. Rao, Lithium conducting solid electrolyte Li1+xAlxGe2−x(PO4)3 membrane for aqueous lithium air battery. Solid State Ion. 262, 211–215 (2013). https://doi.org/10.1016/j.ssi.2013.11.031
D. Safanama, S. Adams, High efficiency aqueous and hybrid lithium-air batteries enabled by Li1.5Al0.5Ge1.5(PO4)3 ceramic anode-protecting membranes. J. Power. Sources 340, 294–301 (2017). https://doi.org/10.1016/j.jpowsour.2016.11.076
M. Tatsumisago, A. Hayashi, Sulfide glass-ceramic electrolytes for all-solid-state lithium and sodium batteries. Int. J. Appl. Glas. Sci. 5(3), 226–235 (2014). https://doi.org/10.1111/ijag.12084
S. Ito, M. Nakakita, Y. Aihara, T. Uehara, N. Machida, A synthesis of crystalline Li7P3S11 solid electrolyte from 1,2-dimethoxyethane solvent. J. Power. Sources 271, 342–345 (2014). https://doi.org/10.1016/j.jpowsour.2014.08.024
T. Thompson et al., Electrochemical window of the Li-ion solid electrolyte Li7La3Zr2O12. ACS Energy Lett. 2(2), 462–468 (2017). https://doi.org/10.1021/acsenergylett.6b00593
R. Xu et al., Artificial interphases for highly stable lithium metal anode. Matter. 1(2), 317–344 (2019). https://doi.org/10.1016/j.matt.2019.05.016
C. Wang et al., Mixed ionic-electronic conductor enabled effective cathode-electrolyte interface in all solid state batteries. Nano Energy 50, 393–400 (2018). https://doi.org/10.1016/j.nanoen.2018.05.062
H. Huo et al., Design of a mixed conductive garnet/Li interface for dendrite-free solid lithium metal batteries. Energy Environ. Sci. 13(1), 127–134 (2020). https://doi.org/10.1039/c9ee01903k
J. Qian et al., High rate and stable cycling of lithium metal anode. Nat. Commun. 6(1), 6362 (2015). https://doi.org/10.1038/ncomms7362
J. Zheng, B. Perry, Y. Wu, Antiperovskite superionic conductors: a critical review. ACS Mater. Au. 1(2), 92–106 (2021). https://doi.org/10.1021/acsmaterialsau.1c00026
S. Wenzel, T. Leichtweiss, D. Krüger, J. Sann, J. Janek, Interphase formation on lithium solid electrolytes—an in situ approach to study interfacial reactions by photoelectron spectroscopy. Solid State Ion. 278, 98–105 (2015). https://doi.org/10.1016/j.ssi.2015.06.001
R. Murugan, V. Thangadurai, W. Weppner, Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angew. Chem. Int. Ed. 46(41), 7778–7781 (2007). https://doi.org/10.1002/anie.200701144
R. Mercier, J.P. Malugani, B. Fahys, G. Robert, Superionic conduction in Li2S-P2S5-Lil-glasses. Solid State Ion. 5, 663–666 (1981). https://doi.org/10.1016/0167-2738(81)90341-6
X. Li, J. Liang, X. Yang, K.R. Adair, C. Wang, F. Zhao, X. Sun, Progress and perspectives on halide lithium conductors for all-solid-state lithium batteries. Energy Environ. Sci. 13(5), 1429–1461 (2020). https://doi.org/10.1039/c9ee03828k
Y. Pang, Y. Liu, J. Yang, S. Zheng, C. Wang, Hydrides for solid-state batteries: a review. Mater. Today Nano. 18, 100194 (2022). https://doi.org/10.1016/j.mtnano.2022.100194
J. Monnier, J. Zhang, F. Cuevas, M. Latroche, Hydrides compounds for electrochemical applications. Curr. Opin. Electrochem. 32, 100921 (2022). https://doi.org/10.1016/j.coelec.2021.100921
C. Liu, Z.Y. Wen, K. Rui, High ion conductivity in garnet-type F-doped Li7La3Zr2O12. J. Inorg. Mater. 30(9), 995–1001 (2015). https://doi.org/10.15541/jim20150163
Y. Zhu, X. He, Y. Mo, Origin of outstanding stability in the lithium solid electrolyte materials: insights from thermodynamic analyses based on first-principles calculations. ACS Appl. Mater. Interfaces 7(42), 23685–23693 (2015). https://doi.org/10.1021/acsami.5b07517
X. Ma, Y. Xu, Enhanced critical current density of garnet Li7La3Zr2O12 solid electrolyte by incorporation of LiBr. Electrochim. Acta 409, 139986 (2022). https://doi.org/10.1016/j.electacta.2022.139986
C. Wang et al., Interface-assisted in-situ growth of halide electrolytes eliminating interfacial challenges of all-inorganic solid-state batteries. Nano Energy 76, 105015 (2020). https://doi.org/10.1016/j.nanoen.2020.105015
X. Li et al., Air-stable Li3InCl6 electrolyte with high voltage compatibility for all-solid-state batteries. Energy Environ. Sci. 12(9), 2665–2671 (2019). https://doi.org/10.1039/c9ee02311a
D. Blanchard et al., Nanoconfined LiBH4 as a fast lithium ion conductor. Adv. Funct. Mater. 25(2), 184–192 (2015). https://doi.org/10.1002/adfm.201402538
Y.S. Choi, Y.S. Lee, K.H. Oh, Y.W. Cho, Interface-enhanced Li ion conduction in a LiBH4-SiO2 solid electrolyte. Phys. Chem. Chem. Phys. 18(32), 22540–22547 (2016). https://doi.org/10.1039/c6cp03563a
M. Matsuo, S. Orimo, Lithium fast-ionic conduction in complex hydrides: review and prospects. Adv. Energy Mater. 1(2), 161–172 (2011). https://doi.org/10.1002/aenm.201000012
R. Mohtadi, S. Orimo, The renaissance of hydrides as energy materials. Nat. Rev. Mater. 2(3), 16091 (2016). https://doi.org/10.1038/natrevmats.2016.91
F. Han et al., High electronic conductivity as the origin of lithium dendrite formation within solid electrolytes. Nat. Energy 4(3), 187–196 (2019). https://doi.org/10.1038/s41560-018-0312-z
J.A. Dawson, T. Famprikis, K.E. Johnston, Anti-perovskites for solid-state batteries: recent developments, current challenges and future prospects. J. Mater. Chem. A. 9(35), 18746–18772 (2021). https://doi.org/10.1039/d1ta03680g
Y. Zhao, L.L. Daemen, Superionic conductivity in lithium-rich anti-perovskites. J. Am. Chem. Soc. 134(36), 15042–15047 (2012). https://doi.org/10.1021/ja305709z
S. Li et al., Reaction mechanism studies towards effective fabrication of lithium-rich anti-perovskites Li3OX (X = Cl, Br). Solid State Ion. 284, 14–19 (2016). https://doi.org/10.1016/j.ssi.2015.11.027
A. Emly, E. Kioupakis, A. Van der Ven, Phase stability and transport mechanisms in antiperovskite Li3OCl and Li3OBr superionic conductors. Chem. Mater. 25(23), 4663–4670 (2013). https://doi.org/10.1021/cm4016222
X. Li et al., Water-mediated synthesis of a superionic halide solid electrolyte. Angew. Chemie Int. Ed. 131(46), 16579–16584 (2019). https://doi.org/10.1002/ange.201909805
Y. Nikodimos, W.N. Su, B.J. Hwang, Halide solid-state electrolytes: stability and application for high voltage all-solid-state Li batteries. Adv. Energy Mater. 13(3), 2202854 (2022). https://doi.org/10.1002/aenm.202202854
D. Park et al., Theoretical design of lithium chloride superionic conductors for all-solid-state high-voltage lithium-ion batteries. ACS Appl. Mater. Interfaces 12(31), 34806–34814 (2020). https://doi.org/10.1021/acsami.0c07003
J. Lou et al., Achieving efficient and stable interface between metallic lithium and garnet-type solid electrolyte through a thin indium tin oxide interlayer. J. Power. Sources 448, 227440 (2020). https://doi.org/10.1016/j.jpowsour.2019.227440
Y. Jin, P.J. McGinn, Al-doped Li7La3Zr2O12 synthesized by a polymerized complex method. J. Power. Sources 196(20), 8683–8687 (2011). https://doi.org/10.1016/j.jpowsour.2011.05.065
J. Saienga, S.W. Martin, The comparative structure, properties, and ionic conductivity of LiI+Li2S+GeS2 glasses doped with Ga2S3 and La2S3. J. Non Cryst. Solids 354(14), 1475–1486 (2008). https://doi.org/10.1016/j.jnoncrysol.2007.08.058
P. Knauth, Inorganic solid Li ion conductors: an overview. Solid State Ion. 180(14–16), 911–916 (2009). https://doi.org/10.1016/j.ssi.2009.03.022
T. Lapp, S. Skaarup, A. Hooper, Ionic conductivity of pure and doped Li3N. Solid State Ion. 11(2), 97–103 (1983). https://doi.org/10.1016/0167-2738(83)90045-0
W. Li et al., Li+ ion conductivity and diffusion mechanism in α-Li3N and β-Li3N. Energy Environ. Sci. 3(10), 1524–1530 (2010). https://doi.org/10.1039/c0ee00052c
K. Park, J.B. Goodenough, Dendrite-suppressed lithium plating from a liquid electrolyte via wetting of Li3N. Adv. Energy Mater. 7(19), 1700732 (2017). https://doi.org/10.1002/aenm.201700732
Y. Li et al., Robust pinhole-free Li3N solid electrolyte grown from molten lithium. ACS Cent. Sci. 4(1), 97–104 (2018). https://doi.org/10.1021/acscentsci.7b00480
F. Yonemoto, A. Nishimura, M. Motoyama, N. Tsuchimine, S. Kobayashi, Y. Iriyama, Temperature effects on cycling stability of Li plating/stripping on Ta-doped Li7La3Zr2O12. J. Power. Sources 343, 207–215 (2017). https://doi.org/10.1016/j.jpowsour.2017.01.009
M. Kotobuki, Y. Suzuki, H. Munakata, K. Kanamura, Y. Sato, K. Yamamoto, T. Yoshida, Effect of sol composition on solid electrode/solid electrolyte interface for all-solid-state lithium ion battery. Electrochim. Acta 56(3), 1023–1029 (2011). https://doi.org/10.1016/j.electacta.2010.11.008
M. Kotobuki, M. Koishi, Sol-gel synthesis of Li1.5Al0.5Ge1.5(PO4)3 solid electrolyte. Ceram. Int. 41(7), 8562–8567 (2015). https://doi.org/10.1016/j.ceramint.2015.03.064
C.R. Mariappan, C. Yada, F. Rosciano, B. Roling, Correlation between micro-structural properties and ionic conductivity of Li1.5Al0.5Ge1.5(PO4)3 ceramics. J. Power. Sources 196(15), 6456–6464 (2011). https://doi.org/10.1016/j.jpowsour.2011.03.065
C.J. Leo, B.V.R. Chowdari, G.V.S. Rao, Lithium conducting glass ceramic with Nasicon structure. Mater. Res. Bull. 37(8), 1419–1430 (2002). https://doi.org/10.1016/S0025-5408(02)00793-6
X. Xu, Z. Wen, X. Wu, X. Yang, Z. Gu, Lithium ion-conducting glass-ceramics of Li1.5Al0.5Ge1.5(PO4)3-xLi2O (x=0.0–0.20) with good electrical and electrochemical properties. J. Am. Ceram. Soc. 90(9), 2802–2806 (2007). https://doi.org/10.1111/j.1551-2916.2007.01827.x
M. Hou, F. Liang, K. Chen, Y. Dai, D. Xue, Challenges and perspectives of NASICON-type solid electrolytes for all solid-state lithium batteries. Nanotechnology 31(13), 132003 (2020). https://doi.org/10.1088/1361-6528/ab5be7
J.K. Feng, B.G. Yan, J.C. Liu, M.O. Lai, L. Li, All solid state lithium ion rechargeable batteries using NASICON structured electrolyte. Mater. Technol. 28(5), 276–279 (2013). https://doi.org/10.1179/1753555713Y.0000000085
Z. Wang, H. Xu, M. Xuan, G. Shao, From anti-perovskite to double anti-perovskite: tuning lattice chemistry to achieve super-fast Li+ transport in cubic solid lithium halogen-chalcogenides. J. Mater. Chem. A. 6(1), 73–83 (2018). https://doi.org/10.1039/C7TA08698A
S. Lorger, R. Usiskin, J. Maier, Transport and charge carrier chemistry in lithium oxide. J. Electrochem. Soc. 166(10), A2215–A2220 (2019). https://doi.org/10.1149/2.1121910jes
M. Chen, S. Adams, High performance all-solid-state lithium/sulfur batteries using lithium argyrodite electrolyte. J. Solid State Electrochem. 19, 697–702 (2014). https://doi.org/10.1007/s10008-014-2654-1
H.J. Deiseroth, S.T. Kong, H. Eckert, J. Vannahme, C. Reiner, T. Zaiß, M. Schlosser, Li6PS5X: a class of crystalline Li-rich solids with an unusually high Li+ mobility. Angew. Chemie Int. Ed. 47(4), 755–758 (2008). https://doi.org/10.1002/anie.200703900
W.D. Jung et al., Superionic halogen-rich Li-argyrodites using in situ nanocrystal nucleation and rapid crystal growth. ACS Appl. Mater. Interfaces 20(4), 2303–2309 (2020). https://doi.org/10.1021/acs.nanolett.9b04597
C. Yu, L. van Eijck, S. Ganapathy, M. Wagemaker, Synthesis, structure and electrochemical performance of the argyrodite Li6PS5Cl solid electrolyte for Li-ion solid-state batteries. Electrochim. Acta 215, 93–99 (2016). https://doi.org/10.1016/j.electacta.2016.08.081
S. Yubuchi, M. Uematsu, M. Deguchi, A. Hayashi, M. Tatsumisago, Lithium-ion-conducting argyrodite-type Li6PS5X (X = Cl, Br, I) solid electrolytes prepared by a liquid-phase technique using ethanol as a solvent. ACS Appl. Energy Mater. 1(8), 3622–3629 (2018). https://doi.org/10.1021/acsaem.8b00280
A. Hayashi, Y. Ishikawa, S. Hama, T. Minami, M. Tatsumisago, Fast lithium-ion conducting glass-ceramics in the system Li2S-SiS2-P2S5. Electrochem. Solid-State Lett. 6(3), 47–49 (2003). https://doi.org/10.1149/1.1540792
N. Kamaya et al., A lithium superionic conductor. Nat. Mater. 10(9), 682–686 (2011). https://doi.org/10.1038/nmat3066
Y. Seino, T. Ota, K. Takada, A. Hayashi, M. Tatsumisago, A sulphide lithium super ion conductor is superior to liquid ion conductors for use in rechargeable batteries. Energy Environ. Sci. 7(2), 627–631 (2014). https://doi.org/10.1039/c3ee41655k
R. Kanno, M. Murayama, Lithium ionic conductor thio-LISICON: the Li2S-GeS2-P2S5 system. J. Electrochem. Soc. 148(7), 477–479 (2001). https://doi.org/10.1149/1.1379028
D.C. Marcano et al., Improved synthesis of graphene oxide. ACS Nano 4(8), 4806–4814 (2010). https://doi.org/10.1021/nn1006368
Y. Mo, S.P. Ong, G. Ceder, First principles study of the Li10GeP2S12 lithium super ionic conductor material. Chem. Mater. 24(1), 15–17 (2012). https://doi.org/10.1021/cm203303y
Y. Tao, S. Chen, D. Liu, G. Peng, X. Yao, X. Xu, Lithium superionic conducting oxysulfide solid electrolyte with excellent stability against lithium metal for all-solid-state cells. J. Electrochem. Soc. 163(2), 96–101 (2015). https://doi.org/10.1149/2.0311602jes
Y. Haven, The ionic conductivity of Li-halide crystals. Recl. des Trav. Chim. des Pays-Bas. 69(12), 1471–1489 (1950). https://doi.org/10.1002/recl.19500691203
B.J.H. Jackson, D.A. Young, Ionic conduction in pure and doped single-crystalline lithium iodide. J. Phys. Chem. Solids 30(8), 1973–1976 (1969). https://doi.org/10.1016/0022-3697(69)90174-7
C.R. Schlaikjer, C.C. Liang, Ionic conduction in calcium doped polycrystalline lithium iodide. J. Electrochem. Soc. 118(9), 1447–1450 (1971). https://doi.org/10.1149/1.2408351
M.L.B. Rao, US Pat., 3455742, 15 Jul. (1969)
C.C. Liang, J. Epstein, G.H. Boyle, A high-voltage, solid-state battery system: II fabrication of thin-film cells. J. Electrochem. Soc. 116(10), 1452–1454 (1969). https://doi.org/10.1149/1.2411560
C.C. Liang, The self-discharge mechanism of the Li/Lil/Agl solid electrolyte cell. J. Electrochem. Soc. 118(6), 894–895 (1971). https://doi.org/10.1149/1.2408213
J.P. Malugani, G. Robert, Preparation and electrical properties of the 0.37Li2S-0.18P2S5–0.45LiI glass. Solid State Ion. 1(5–6), 519–523 (1980). https://doi.org/10.1016/0167-2738(80)90048-X
Y. Wang et al., Formation mechanism of Li7P3S11 solid electrolytes through liquid phase synthesis. Chem. Mater. 30(3), 990–997 (2018). https://doi.org/10.1021/acs.chemmater.7b04842
Z. Wang et al., Reaction mechanism of Li2S-P2S5 system in acetonitrile based on wet chemical synthesis of Li7P3S11 solid electrolyte. Chem. Eng. J. 393, 124706 (2020). https://doi.org/10.1016/j.cej.2020.124706
M. Takahashi et al., Investigation of the suppression of dendritic lithium growth with a lithium-iodide-containing solid electrolyte. Chem. Mater. 33(13), 4907–4914 (2021). https://doi.org/10.1021/acs.chemmater.1c00270
C. Wang, J. Liang, J.T. Kim, X. Sun, X. Sun, Prospects of halide-based all-solid-state batteries: from material design to practical application. Sci. Adv. 8(36), eadc9516 (2022). https://doi.org/10.1126/sciadv.adc9516
F. Han, Y. Zhu, X. He, Y. Mo, C. Wang, Electrochemical stability of Li10GeP2S12 and Li7La3Zr2O12 solid electrolytes. Adv. Energy Mater. 6(8), 1–9 (2016). https://doi.org/10.1002/aenm.201501590
M. Suyama, A. Kato, A. Sakuda, A. Hayashi, M. Tatsumisago, Lithium dissolution/deposition behavior with Li3PS4-LiI electrolyte for all-solid-state batteries operating at high temperatures. Electrochim. Acta 286, 158–162 (2018). https://doi.org/10.1016/j.electacta.2018.07.227
Y. Lu, Z. Tu, L.A. Archer, Stable lithium electrodeposition in liquid and nanoporous solid electrolytes. Nat. Mater. 13(10), 961–969 (2014). https://doi.org/10.1038/NMAT4041
L. Ma, M.S. Kim, L.A. Archer, Stable artificial solid electrolyte interphases for lithium batteries. Chem. Mater. 29(10), 4181–4189 (2017). https://doi.org/10.1021/acs.chemmater.6b03687
S. Wenzel, S.J. Sedlmaier, C. Dietrich, W.G. Zeier, J. Janek, Interfacial reactivity and interphase growth of argyrodite solid electrolytes at lithium metal electrodes. Solid State Ion. 318, 102–112 (2018). https://doi.org/10.1016/j.ssi.2017.07.005
L. Cheng, W. Chen, M. Kunz, K. Persson, N. Tamura, G. Chen, M. Doeff, Effect of surface microstructure on electrochemical performance of garnet solid electrolytes. ACS Appl. Mater. Interfaces 7(3), 2073–2081 (2015). https://doi.org/10.1021/am508111r
A. Sharafi, C.G. Haslam, R.D. Kerns, J. Wolfenstine, J. Sakamoto, Controlling and correlating the effect of grain size with the mechanical and electrochemical properties of Li7La3Zr2O12 solid-state electrolyte. J. Mater. Chem. A. 5(40), 21491–21504 (2017). https://doi.org/10.1039/C7TA06790A
L. Porz et al., Mechanism of lithium metal penetration through inorganic solid electrolytes. Adv. Energy Mater. 7(20), 1701003 (2017). https://doi.org/10.1002/aenm.201701003
S. Yu et al., Elastic properties of the solid electrolyte Li7La3Zr2O12 (LLZO). Chem. Mater. 28(1), 197–206 (2016). https://doi.org/10.1021/acs.chemmater.5b03854
O. Pecher et al., Atomistic characterisation of Li+ mobility and conductivity in Li7-xPS6-xIx argyrodites from molecular dynamics simulations, solid-state NMR, and impedance spectroscopy. Chem. Eur. J. 16(28), 8347–8354 (2010). https://doi.org/10.1002/chem.201000501
R. Miyazaki et al., Room temperature lithium fast-ion conduction and phase relationship of LiI stabilized LiBH4. Solid State Ion. 192(1), 143–147 (2011). https://doi.org/10.1016/j.ssi.2010.05.017
Z. Liu et al., Lithium migration pathways at the composite interface of LiBH4 and two-dimensional MoS2 enabling superior ionic conductivity at room temperature. Phys. Chem. Chem. Phys. 22(7), 4096–4105 (2020). https://doi.org/10.1039/c9cp06090a
A. El Kharbachi et al., MgH2–CoO: a conversion-type composite electrode for LiBH4-based all-solid-state lithium ion batteries. RSC Adv. 8(41), 23468–23474 (2018). https://doi.org/10.1039/c8ra03340d
H. Benzidi, M. Lakhal, A. Benyoussef, M. Hamedoun, M. Loulidi, A. El-kenz, O. Mounkachi, First principle study of strain effect on structural and dehydrogenation properties of complex hydride LiBH4. Int. J. Hydrogen Energy 42(30), 19481–19486 (2017). https://doi.org/10.1016/j.ijhydene.2017.06.068
K. Kisu, S. Kim, H. Oguchi, N. Toyama, S. Orimo, Interfacial stability between LiBH4-based complex hydride solid electrolytes and Li metal anode for all-solid-state Li batteries. J. Power. Sources 436, 226821 (2019). https://doi.org/10.1016/j.jpowsour.2019.226821
X. Bai, Y. Duan, W. Zhuang, R. Yang, J. Wang, Research progress in Li-argyrodite-based solid-state electrolytes. J. Mater. Chem. A. 8(48), 25663–25686 (2020). https://doi.org/10.1039/D0TA08472G
T.J. Udovic et al., Sodium superionic conduction in Na2B12H12. Chem. Commun. 50(28), 3750–3752 (2014). https://doi.org/10.1039/c3cc49805k
Z. Yao, S. Kim, K. Michel, Y. Zhang, M. Aykol, C. Wolverton, Stability and conductivity of cation- and anion-substituted LiBH4-based solid-state electrolytes. Phys. Rev. Mater. 2(6), 065402 (2018). https://doi.org/10.1103/PhysRevMaterials.2.065402
M. Matsuo, Y. Nakamori, S. Orimo, H. Maekawa, H. Takamura, Lithium superionic conduction in lithium borohydride accompanied by structural transition Lithium superionic conduction in lithium borohydride accompanied. Appl. Phys. Lett. 91(22), 224103 (2007). https://doi.org/10.1063/1.2817934
T.J. Udovic et al., Exceptional superionic conductivity in disordered sodium decahydro-closo-decaborate. Adv. Mater. 26(45), 7622–7626 (2014). https://doi.org/10.1002/adma.201403157
M. Matsuo, H. Takamura, H. Maekawa, H.W. Li, S. Orimo, Stabilization of lithium superionic conduction phase and enhancement of conductivity of LiBH4 by LiCl addition. Appl. Phys. Lett. 94(8), 084103 (2009). https://doi.org/10.1063/1.3088857
L.M. Arnbjerg et al., Structure and dynamics for LiBH4-LiCl solid solutions. Chem. Mater. 21(24), 5772–5782 (2009). https://doi.org/10.1021/cm902013k
L.H. Rude et al., Iodide substitution in lithium borohydride, LiBH4–LiI. J. Alloys Compd. 509(33), 8299–8305 (2011). https://doi.org/10.1016/j.jallcom.2011.05.031
H. Maekawa, M. Matsuo, H. Takamura, M. Ando, Y. Noda, T. Karahashi, S. Orimo, Halide-stabilized LiBH4, a room-temperature lithium fast-ion conductor. J. Am. Chem. Soc. 131(3), 894–895 (2009). https://doi.org/10.1021/ja807392k
J. Cuan, Y. Zhou, T. Zhou et al., Borohydride-scaffolded Li/Na/Mg fast ionic conductors for promising solid-state electrolytes. Adv. Mater. 31, 1803533 (2019). https://doi.org/10.1002/adma.201803533
T. Ikeshoji, Y. Ando, M. Otani, E. Tsuchida, S. Takagi, M. Matsuo, S. Orimo, Biased interface between solid ion conductor LiBH4 and lithium metal: a first principles molecular dynamics study. Appl. Phys. Lett. 103(13), 133903 (2013). https://doi.org/10.1063/1.4823503
F. Mo et al., Stable three-dimensional metal hydride anodes for solid-state lithium storage. Energy Storage Mater. 18, 423–428 (2019). https://doi.org/10.1016/j.ensm.2019.01.014
L.M. Riegger, R. Schlem, J. Sann, W.G. Zeier, J. Janek, Lithium-metal anode instability of the superionic halide solid electrolytes and the implications for solid-state batteries. Angew. Chemie Int. Ed. 60(12), 6718–6723 (2021). https://doi.org/10.1002/ange.202015238
M. Tatsumisago, Y. Akamatsu, T. Minami, Ionic conductivity of ZrF4-BaF2-MX (M = Li, Na; X= F, Cl) glasses. Solid State Ion. 31(1), 41–47 (1988). https://doi.org/10.1016/0167-2738(88)90286-X
A. El Kharbachi, E. Pinatel, I. Nuta, M. Baricco, A thermodynamic assessment of LiBH4. Calphad 39, 80–90 (2012). https://doi.org/10.1016/j.calphad.2012.08.005
Funding
This work was supported financially by the National Key R&D Program of China (No. 2018YFE0181300), the National Natural Science Foundation of China (21905035), Liaoning Revitalization Talents Program (XLYC1907093), and the Liaoning Natural Science Foundation (20180510043).
Author information
Authors and Affiliations
Contributions
MA: Conceptualization, Formal analysis, Writing—original draft; CSD: Editing; YZ: Conceptualization; YY: Conceptualization, Writing—reviewing and editing, Supervision, Funding acquisition.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval
We follow the ethical code of conduct by the MRS Energy and Sustainability.
Consent to participate
All authors consent to participate in this research article.
Consent for publication
All authors gave their consent for publication in the journal MRS Energy and Sustainability.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abitonze, M., Diko, C.S., Zhu, Y. et al. Recent progress on inorganic composite electrolytes for all-solid-state lithium batteries. MRS Energy & Sustainability (2024). https://doi.org/10.1557/s43581-023-00076-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1557/s43581-023-00076-w